Abstract
OBJECTIVE
To investigate the pharmacokinetics (PK), pharmacodynamics, and safety of single-dose RM-131 in type 2 diabetic patients with gastrointestinal cardinal symptoms (GCSI) and previously documented delayed gastric emptying (DGE).
RESEARCH DESIGN AND METHODS
In a randomized crossover study, 10 female patients received RM-131 (100 μg s.c.) or placebo and underwent scintigraphic gastric emptying (GE) and colonic filling at 6 h (CF6) of a solid-liquid meal administered 30 min postdosing. Adverse events, plasma glucose, and hormonal levels were assessed. GCSI daily diary (GCSI-DD) was completed during treatments. PK was assessed in this cohort and healthy volunteers (HVs).
RESULTS
At screening, HbA1c was 7.2 ± 0.4% (SEM) and total GCSI-DD score was 1.32 ± 0.21. RM-131 accelerated GE t1/2 of solids (P = 0.011); mean difference (Δ) in solid GE t1/2 was 68.3 min (95% CI 20–117) or 66.1%. There were numerical differences in GE lag time, CF6 solids, and GE t1/2 liquids (all P < 0.14). With a significant (P < 0.014) order effect, further analysis of the first treatment period (n = 5 per group) confirmed significant RM-131 effects on GE t1/2 (solids, P = 0.016; liquids, P = 0.024; CF6, P = 0.013). PK was similar in DGE patients and HVs. There were increases in 120-min blood glucose (P = 0.07) as well as 30–90-min area under the curve (AUC) levels of growth hormone, cortisol, and prolactin (all P < 0.02) with single-dose RM-131. Only light-headedness was reported more on RM-131.
CONCLUSIONS
RM-131 greatly accelerates the GE of solids in patients with type 2 diabetes and documented DGE. PK is similar in diabetic patients and HVs.
There is a need for effective pharmacological therapies for diabetic gastroparesis, a disease associated with morbidity, increased mortality (1,2), and impacts on health care utilization, hospitalizations, and quality of life (3,4). The cumulative 10-year incidence of gastroparesis has been estimated to affect 5.2% of people with type 1 diabetes and 1% of people with type 2 diabetes, in contrast to ∼0.2% of community control subjects (5). Longitudinal studies show that despite improvement in glycemic control, once established, delayed gastric emptying (DGE) persists for at least a decade (6). This affects morbidity of the disease, so that even though patients do not necessarily die of the gastroparesis, they have greater morbidity and mortality.
The treatment of diabetic gastroparesis is suboptimal (7); the only approved drug is metoclopramide (8), which may be associated with significant neurologic and endocrine side effects. Regulatory agencies have recommended treatment of this life-long condition with no more than 3 months of metoclopramide. In some countries, other medications, such as substituted benzamides (e.g., cisapride or itopride) or dopaminergic agents (e.g., domperidone), are approved and used to provide symptom relief. Unapproved alternatives are erythromycin (most effective in the acute setting) (9), intrapyloric injection of botulinum toxin (despite the only randomized trials showing no evidence of efficacy) (10,11), venting gastrostomy (12) with enteral feeding, and gastric electrical stimulation (GES). A systematic analysis of the published literature (13) noted that the greatest efficacy of GES was observed in open-label treatment trials. The most recent controlled trial of GES showed no evidence of efficacy in the crossover phase of the trial (14). Some patients are so incapacitated that gastrectomy is recommended (15).
Ghrelin is the natural ligand for the growth hormone secretagogue-1a (GHS-1a) receptor and a potential target for treatment of impaired gastric motility and energy balance (16). In pharmacodynamic (PD) studies using ultrasonography, ghrelin accelerated gastric emptying (GE) of nutrient liquids in patients with diabetes (17); ghrelin accelerated GE of solids (reviewed in reference 16) when administered at pharmacological doses. Although ghrelin induced inhibition of gastric accommodation, there was no increase in upper gastrointestinal symptoms with pharmacological doses of ghrelin (18). In addition, in a study that used a dose of exogenous ghrelin that stimulated growth hormone (GH) release in the physiological range in human subjects, there were no significant effects of intravenous bolus synthetic human ghrelin (0.33 μg/kg) on gastric accommodation, emptying, or aggregate postprandial symptoms (19). The ghrelin analog TZP-101 in diabetic gastroparesis slightly reduced the GE t1/2 of solids by ∼20% (20). Thus, the potential for ghrelin receptor agonists to normalize GE in people with diabetes is far from clear.
RM-131 is a novel pentapeptide that acts as a potent ghrelin receptor agonist. RM-131 has similar characteristics to native ghrelin but 100-fold greater potency in reversing gastric ileus in animal models. In a rat model of postoperative ileus, RM-131 effectively reversed gastric ileus; in addition, RM-131 was highly effective in reversing postsurgical, opiate-induced gastric ileus in rats. In normal, nonsurgical primates, RM-131 also increased the rate of GE (data on file; Rhythm Pharmaceuticals). In healthy humans, the estimated half-maximal effective concentration (EC50) for RM-131 acceleration of GE t1/2 was ∼0.49 ng/mL (unpublished data).
The aim of this study was to investigate the PD and pharmacokinetic (PK) profiles, safety, and tolerability of a single dose of RM-131 in patients with type 2 diabetes and prior documentation of both symptoms and DGE.
RESEARCH DESIGN AND METHODS
Design
We conducted a randomized, double-blind, placebo–controlled, single-dose, two-period, crossover study during period 1, September to 30 November 2011, in 10 patients with type 2 diabetes and prior documentation of DGE. Patients with type 2 diabetes were chosen to establish a well-defined patient population, as disease prevalence and PD/PK profiles of RM-131 may differ among those with type 1 diabetes. Diagnosis of type 2 diabetic gastroparesis was defined by the presence of both the following criteria: 1) at least a 3-month past or current history of symptoms of gastroparesis (e.g., postprandial fullness, early satiety, bloating, postprandial nausea/vomiting, and epigastric or abdominal pain); and 2) previously documented DGE by scintigraphy using a radiolabeled egg meal or by GE breath test within the past 10 years. Specifically, identification of DGE was based on either 60% retained at 2 h or 10% retained at 4 h on the scintigraphic GE test.
Patients were enrolled and, during period 1, received a subcutaneous injection of RM-131 or placebo (5% mannitol), which had identical appearance. After a 7-day washout period, patients crossed over to receive the alternative therapy in period 2. Patients were randomly allocated to a treatment sequence by a computer-generated allocation schedule. Patients were allowed antiemetics and prokinetics (e.g., metoclopramide or erythromycin), but doses of these drugs had to be stable for at least 2 weeks prior to period 1 and remain stable during the study. Consistent with the ethical review that patients should not be withdrawn from medication that may have been contributing to their health, only 2 of the 10 patients were receiving another medication for gastroparesis during the conduct of the crossover study: one on metoclopramide and one on domperidone. The doses of these medications were constant throughout both phases of the study. The study was conducted on an outpatient basis in the Clinical Research Unit at the Mayo Clinic. The participants, all members of the research team, and the study statistician were blinded to the interventions.
Experimental protocol
After approval by the Mayo Clinic Institutional Review Board and after signed, written, informed consent, patient study eligibility was confirmed by medical history, physical examination (including vital signs and height and weight measurements), concomitant medication review, and clinical laboratory tests, including a 12-lead electrocardiogram (ECG). The presence of normal sinus arrhythmia was used as an indicator of normal cardiovagal function. Patients recorded gastrointestinal cardinal symptoms daily diary (GCSI-DD) entries for one full day during screening. Male and female patients 18–60 years of age, meeting specific inclusion and exclusion criteria, were eligible for enrollment. Although age between 18 and 60 years does not appear to influence GE (21), there is evidence that age >60 years delays GE of solids and 5% glucose drinks (22–25).
Patients were required to have a diagnosis of type 2 diabetic gastroparesis (see above), controlled type 2 diabetes (HbA1c <8.5%), previous exclusion of upper gastrointestinal mechanical obstruction, and BMI = 18–40 kg/m2. Women were required to be postmenopausal, surgically sterile, or not pregnant and using an acceptable form of birth control. Men were required to agree to abstinence throughout the study.
After screening, patients reported to the Clinical Research Unit in the fasted state on day 1 for period 1 dosing and for PD, PK, and safety evaluations. PD evaluations included upper GI motility evaluation by scintigraphy. After obtaining pretreatment samples, a single dose of RM-131 or placebo was injected subcutaneously. Thirty minutes after subcutaneous administration of the study drug, a standardized radiolabeled study meal was administered, which patients were asked to consume within 10 min. The study meal consisted of the following: 4 oz. of scrambled Egg Beaters that had been radiolabeled with 0.5–1.0 mCi of 99mTc sulfur colloid; 120 mL of water that had been radiolabeled with 100 μCi of 111In diethylene triamine pentaacetic acid; and two slices of white bread with strawberry jam. Gamma scans were obtained immediately after completion of the study meal through 6 h postmeal. Postinjection blood sampling for postdose GH, insulin (in patients not receiving exogenous insulin), prolactin, and cortisol levels were collected. PK was also collected through 6 h postdose. Patients were discharged from the Clinical Research Unit after the final scintigraphic scan (6 h postmeal). Safety evaluations included the measurement of vital signs and assessment for adverse events on day 1. Patients were also contacted by phone on day 2 to evaluate for adverse events. Concomitant medication review was conducted at screening and at every study visit.
After the washout period, patients returned to the Clinical Research Unit in the fasted state for period 2 day 1 dosing, according to the same procedures as in period 1. After the follow-up phone calls on period 2 day 2, patients were terminated from the study.
Patients were required to fast overnight on the day preceding study drug administration. They were allowed to take their usual morning study medications (except those for treatment of diabetes) with a sip of water on the morning of study drug administration. Oral agents for the treatment of diabetes were administered with the study meal. Patients on insulin were instructed to bring their insulin and glucometer to the study center on day 1, periods 1 and 2. Patients were instructed to self-administer their usual morning insulin injections, prorated based on meal caloric content. This self-administration occurred within 10 min after the consumption of the study meal.
The dose level of 100 μg RM-131 was chosen based on the safety, tolerability, PK profile, and PD profile (GE breath test) established in a phase 1 single-ascending dose study of healthy normal volunteers (data not shown).
Measurement of GE of solids and liquids and small bowel transit
Validated scintigraphy was used to evaluate GE and colonic filling at 6 h (CF6) of solids after a standardized meal (administered 30 min postdosing and consumed within 10 min). Patients underwent dual-phase (solid and liquid) GE scintigraphy on the days in which the study drug was administered (day 1, periods 1 and 2). The method has been described in detail elsewhere (26,27) and is documented in the Supplementary Data online.
Hormonal measurements
GH, prolactin, and cortisol levels were measured on day 1 of periods 1 and 2 immediately predose and 30, 45, 60, and 90 min and 2 and 4 h postdose. Given the PK properties of the drug (Cmax), we appraised the hormonal results as the AUC of the 30–90-min samples.
Serum insulin levels were assessed (in the six patients not receiving exogenous insulin) at 4 h postdosing with RM-131 or placebo. The blood sample collection for one measurement in each treatment group was missed due to technical errors: hemolysis of one sample and study coordinator error. Serum insulin was not assessed in the four subjects who were receiving exogenous insulin.
PK
PK samples from the 10 patients and 3 healthy, normal volunteers were collected over 6 h after dosing and analyzed for RM-131 concentrations. The lower limit of quantification was 0.1 ng/mL, and based on a prior study, the estimated EC50 value was ∼0.49 ng/mL (data on file; Rhythm Pharmaceuticals). Further details are provided in the Supplementary Data online.
Symptoms
On the day of screening and the 2 days after treatment, patients filled out the GCSI-DD (28) in order to assess their postprandial symptoms (29). This instrument is described more fully in the Supplementary Data online.
Safety and tolerability
Safety and tolerability were assessed by the evaluation of adverse events, vital signs, ECG, physical examination, routine hematology and chemistry laboratory tests, and blood glucose. Each patient was monitored for the development of any adverse events during clinic visits on day 1 of periods 1 and 2 and by telephone follow-up on day 2 of periods 1 and 2. Vitals signs were assessed predose and then postdose every 30 min for 6 h. Blood glucose was determined predose and 2 and 4 h postdose, and at other times when clinically indicated.
Statistical considerations
The primary end point of the study was the GE t1/2 for solids. Other end points are detailed in the Supplementary Materials and Methods. The intraindividual coefficient of variation of GE of solids t1/2 was 24%, justifying the sample size of 10 patients.
A two-tailed (α = 0.05) paired Student t test or Wilcoxon signed rank test (as warranted) were used to compare end points based on the two-period, two-treatment crossover design in 10 patients. Potential order effects were checked using a two-sample test (two-sided α = 0.05), that is, comparing the within-subject treatment differences between those who received placebo first versus those who received active treatment first. Since an order effect was detected for some end points, treatment effects were confirmed using a two (independent)-sample Student t test of period 1 data only to compare two independent groups of subjects (n = 5 per group) for these end points. From the observed subject-specific differences in the end-point responses, the percent difference between the two treatment groups, relative to the overall subject-specific mean values of the RM-131 and placebo values, was also calculated (see above). For the analysis of period 1 data, the percent difference in the primary end point (GE t1/2) was calculated as 100 times the difference in treatment group means divided by the overall subjects’ mean. Total adverse effects were compared using McNemar test (a test for paired binary data), which appraised the difference between placebo and RM-131 in the proportions experiencing adverse effects.
RESULTS
Patient characteristics at study entry and disposition
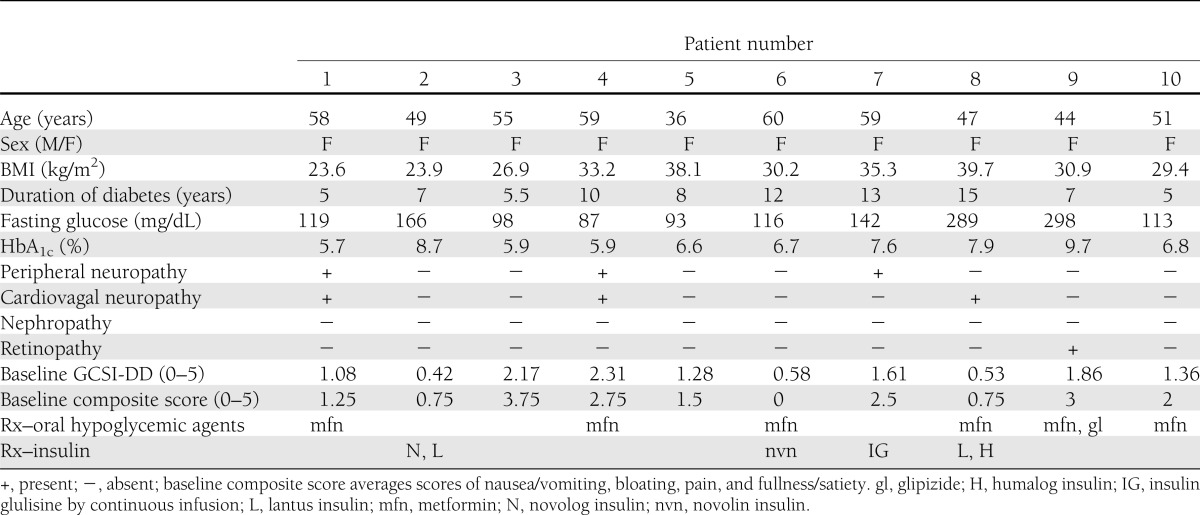
Table 1 provides clinical features of the 10 patients (all female) in the study, and details as well as patient disposition are provided in Supplementary Fig. 1.
Table 1.
Patient demographics, characteristics, and features associated with diabetes
Effects of RM-131 on transit
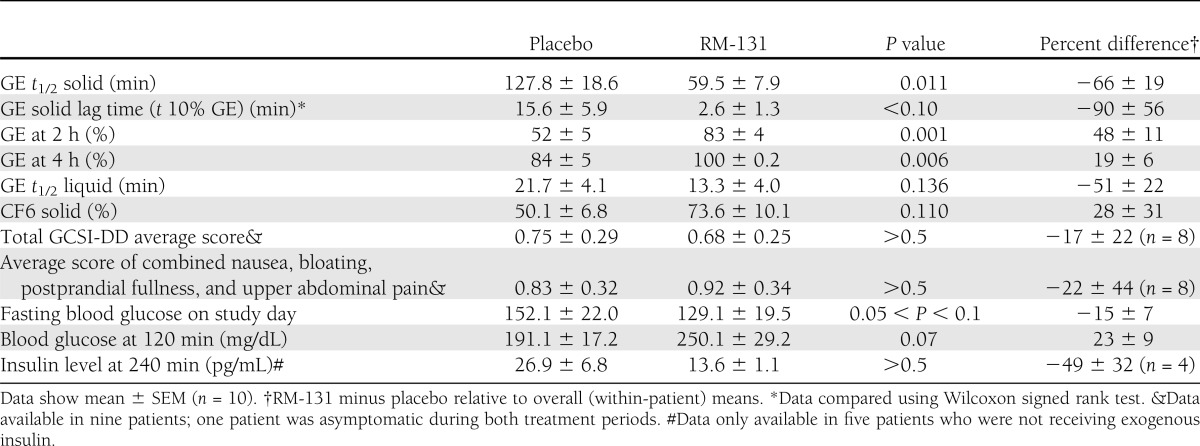
RM-131 was associated with a significant treatment effect on GE t1/2 of solids (P = 0.011) (Table 2 and Fig. 1, top), as well as secondary end points of GE at 2 and 4 h. For the entire group, the estimated treatment effect mean difference (Δ) in solid GE t1/2 was 68.3 min (95% CI 20–117), an average 66.1% decrease relative to overall mean. Supplementary Fig. 2 shows that GE t1/2 of solids was lower on RM-131 treatment compared with placebo in 9 of 10 patients. In the other patient, GE t1/2 of solids was actually accelerated on placebo and remained in the normal range on RM-131 treatment.
Table 2.
Summary of main transit, symptom, and glycemic indicators in all 10 patients in the randomized crossover study
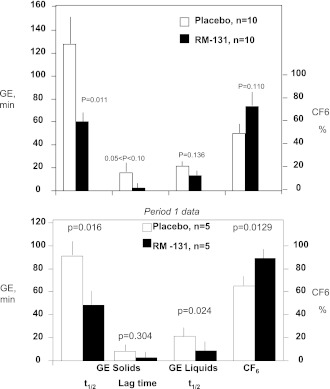
Figure 1.
Effect of RM-131 on main transit measurements (GE, minutes for solids and liquids, and CF6, percent). Top: Data in all 10 patients. Bottom: Analysis of data in period 1 only (n = 5 per group; see text for details). White bars, placebo; black bars, RM-131, 100 μg, treatment. Data are mean ± SEM. P values by the Student paired t test comparing RM-131 vs. placebo above each comparison (top) and by the Student unpaired t test comparing RM-131 vs. placebo (bottom). Published normal data (28) with this meal show t1/2 solid of median 83 min (IQR 64–103 min).
There were numerical differences in the lag time for GE solids, GE t1/2 of liquids, and CF6 of solids (Table 2); however, these were borderline significant (0.05 > P < 0.14). The estimated treatment effects, i.e., mean percent differences (Δ), for the lag time of GE solids, GE t1/2 liquid, and CF6 relative to the corresponding overall mean values were 90, 51, and 28%, respectively.
Order effect.
There was a significant (P < 0.014) order effect. The effect of RM-131 on solid GE t1/2 and CF6 was larger when the participant received, by randomization, RM-131 first. To explore the robustness of the primary analysis, a supportive analysis of period 1 alone was examined. This analysis confirmed that when assessing period 1 alone (i.e., two [independent]-sample Student t test, n = 5 per group), there were still significant drug effects for GE t1/2 (solids, P = 0.016; liquids, P = 0.024) and CF6 (P = 0.013) (Fig. 1, bottom). The estimated treatment effect on solid GE t1/2 (difference [Δ] in means) was 43 min (95% CI 10–75), a 61% difference in mean values relative to the overall mean (period 1 data).
Average symptom scores
This was a single-dose study and not designed or powered to assess symptoms. In addition, GCSI scores were low in the placebo treatment periods (0.75) (Table 2). Therefore, there were no significant effects (P > 0.5) on GCSI total or the selected composite score of nausea, bloating, early satiety (postprandial fullness), and pain with single-day treatment (Table 1).
Glucose and insulin levels
Fasting and 120-min postprandial blood glucose levels are shown in Table 2. Higher 120-min blood glucose (P = 0.07) reflected more rapid GE t1/2 with RM-131. There were no significant treatment effects on insulin in the five patients who were not receiving exogenous insulin treatment.
Hormonal effects of single-dose RM-131
Baseline hormone levels were not different on the two treatment days (data not shown). The expected single-dose posttreatment increases in the 30–90-min AUC (Supplementary Fig. 3) in GH, cortisol, and prolactin levels with RM-131 treatment were observed (all P < 0.02).
PK
Figure 2 shows plasma levels of RM-131. PK was similar in DGE patients and healthy volunteers (HVs); after single 100-μg s.c. dose, Cmax was ∼4 ng/mL in both patients with diabetes and healthy control subjects.
Figure 2.
PK of RM-131, 100 μg, in diabetic gastroparesis patients (yellow triangles) vs. a cohort of three male, nonobese HVs (at 100 μg [blue diamonds]) showing similar PK in diabetic patients and volunteers over the 6-h sampling scheme. The volunteers were studied separately in the RM-131 single ascending dose study (RM-131 Study 001). (A high-quality color representation of this figure is available in the online issue.)
Safety and tolerability of single-dose RM-131
RM-131 was generally well tolerated. Although the total number of adverse events (P = 0.016 using McNemar test) recorded was higher with RM-131 (Supplementary Table 1), there were no serious adverse effects and no obvious pattern to the adverse effects, and only light-headedness was reported more often on RM-131. All adverse effects resolved spontaneously. No clinically significant effects on physical examination, ECG parameters, vital signs, or routine hematology and chemistry laboratory tests were observed.
CONCLUSIONS
The current study is the first clinical investigation to demonstrate that, compared with placebo, a single subcutaneous injection of RM-131 compared with placebo significantly accelerated GE t1/2 of solids (the most accepted measurement of gastroparesis) (7,30) in patients with type 2 diabetes who had previously documented DGE and current symptoms suggestive of gastroparesis. Numerical improvements were also observed in mean GE tlag solid, mean GE t1/2 liquid, and mean CF6 with RM-131 compared with placebo; the study was not powered for these secondary end points. With placebo treatment, the mean GE t1/2 solid (127.8 ± 18.6 min) was >117 min, the 90th percentile observed in 123 HVs with the same radiolabeled meal (26), suggesting that at least 50% had delayed GE when tested. Results also confirm the prokinetic effects of RM-131; in a rat model of postoperative ileus, RM-131 effectively reversed gastric ileus with greater potency than human ghrelin. It was also highly effective in the reversal of postsurgical, opiate-induced gastric ileus and increased GE in primates (data on file; Rhythm Pharmaceuticals) and in healthy males in a single ascending dose study (by GE breath test; data on file; Rhythm Pharmaceuticals).
It is worth noting that these PD results demonstrate a magnitude of the effect on GE even with a single-dose study that has not been previously observed with other ghrelin agonists. Investigation of the ghrelin agonist TZP-101 by Wo et al. (31) demonstrated improvement in nausea/vomiting subscale scores as well as overall gastroparesis symptoms among patients with diabetes gastroparesis, but postdose GE data was not presented (31). Prior experience with TZP-101 demonstrated an ∼20–25% effect on GE in a subset analysis, in contrast to the ∼65% effect observed in the entire cohort in this study with RM-131 (20,32). In fact, the magnitude of this effect is similar to that previously observed in response to intravenous erythromycin (9). Thus, the average percent retained in the stomach at 60 min in 10 patients with diabetic gastroparesis was 85% on placebo and 21% on intravenous erythromycin for an estimated effect of 75% relative to placebo.
Assessment of RM-131 effects on endocrine PD parameters, including GH, prolactin, and cortisol levels, demonstrated expected postdrug increases after a single administration of the ghrelin analog. Indeed, these responses are similar to those observed with a single dose of acylated ghrelin (1.0 μg/kg) administered as a bolus intravenously (33). Ghrelin injection in humans has been found to stimulate ACTH, cortisol, prolactin, and GH secretion in humans (34); these actions are thought to be mediated through the hypothalamic-pituitary axis (35). In repeated-dose administration studies of RM-131, the effect on hormonal end points was substantially less after 7 days of administration, without any reduction in the efficacy of RM-131’s acceleration of GE (data on file; Rhythm Pharmaceuticals). However, long-term efficacy and safety studies are clearly necessary.
Consistent with prior data, RM-131 was associated with higher 120-min blood glucose compared with placebo (P = 0.07), reflecting the faster GE t1/2 with delivery of nutrients to the small bowel for absorption. There was no significant change in 4-h insulin levels. Such effects may also be mediated by direct effects on the regulation of glucose homeostasis (35). Erratic emptying from the stomach, resulting in a mismatch between the dose of insulin administered and the nutrients emptied and absorbed in the upper gut may lead to poor glycemic control, poor compliance with treatment due to fear of hypoglycemia, and deterioration of control (36). Hence, normalizing GE may result in improved glycemic control because of a more predictable appearance of glucose in the portal circulation, matching the action of the hypoglycemic therapy. More detailed clinical evaluation of the effects of RM-131 on glucose metabolism in diabetic patients is warranted in future studies.
A defined baseline symptom score was not a criterion for study participation. Participants were required to have a 3-month history of at least one of the following four cardinal symptoms: postprandial fullness/early satiety, bloating, nausea/vomiting, or epigastric/abdominal pain. No significant association with RM-131 was noted for total GCSI-DD score or composite score for nausea, bloating, or postprandial fullness and pain; the absence of a significant association in our study is not unexpected as there has been little evidence to demonstrate that GE is associated with symptom severity (37). Multiple additional mechanisms, including impaired accommodation and visceral hypersensitivity, may contribute to upper GI symptoms in patients with documented DGE (38). It is conceivable that RM-131 may improve symptoms, based on enhanced GE of solids. Although it is also conceivable that an increase in gastric tone, as described with exogenous ghrelin in healthy participants (18), might aggravate some postprandial symptoms, Ang et al. (18) did not record increased postprandial symptoms, despite reduced accommodation with ghrelin. Further characterization of the effects of RM-131 on gastric accommodation is needed. The management of diabetic dyspepsia may require the correction of GE hypersensitivity and impaired accommodation (30).
The safety and tolerability of RM-131 were consistent with other preliminary investigations of the medication. Although adverse events were more common with RM-131 than with placebo when taking any and all patient-reported events into consideration, no serious adverse effects were noted. The most frequently reported adverse event with RM-131 was light-headedness or dizziness, which was more commonly reported on RM-131 compared with placebo. No clinically significant laboratory abnormalities were recorded, no adverse events required clinical intervention, and all adverse events resolved spontaneously. The PK of RM-131 in DGE patients was very similar to a small cohort of HVs studied in the RM-131 single ascending dose study at the same dose. Cmax was ∼4 ng/mL, which is substantially higher than the estimated EC50 for GE t1/2 (0.49 ng/mL) from a PK-PD model that was constructed from data in the RM-131 multiple ascending dose study (data on file; Rhythm Pharmaceuticals).
This investigation identified the presence of a significant order effect where patients receiving RM-131 first had faster GE t1/2, whereas in patients receiving placebo first, this effect was much more modest. Although the treatment effect on GE t1/2 solid was observed with RM-131 when the placebo came first, this effect was not statistically significant. Such an order effect was also noted for CF6, which may have masked overall treatment effect. This effect may be a result of a significant placebo effect upon study initiation, which is a common phenomenon in gastrointestinal disease and can be observed in both subjective and objective markers of disease (39). However, an analysis of the period 1 GE data only, which provided an unbiased assessment of treatment effects, fully confirmed the significant drug effect for GE t1/2 solid, GE t1/2 liquid, and CF6. This period 1 assessment serves and demonstrates the efficacy of RM-131 in the acceleration of GE as measured by GE t1/2 solids, and the mean difference in GE t1/2 for RM-131 compared with placebo ranged from 61 (parallel group, n = 5) to 66% (crossover, n = 10).
Although we had not a priori excluded males from participation, the fact that the first 10 patients fulfilling eligibility criteria were women is advantageous since there is a significant sex-related difference in GE of solids. This is well illustrated in a recent paper from our research group (21) in a cohort consisting of 105 males and 214 females. Thus, sex (but not age or BMI) was significantly associated with GE t1/2 (P < 0.001; females, 127.6 ± 28.7 [SD] min; males, 109.9 ± 28.6 min). However, significant effects on the GE of solids in healthy males were demonstrated in a separate study (RM-131 Multiple Ascending Dose Study; data on file, Rhythm Pharmaceuticals), suggesting that the results reported in the current study would likely be generalizable between sexes.
A potential pitfall of this study is that patients’ symptoms were low to moderate (group mean score, 1.32) at baseline, as measured by average GCSI-DD total score, and even lower during the placebo treatments (group mean score, 0.75). The low level of symptom scores in the study at baseline (a “floor effect”) made it difficult to assess any effect of the medication on symptoms; the primary goals of the current study were to assess PK and PD effects. In addition, although gastroparesis often develops in patients who have had diabetes for at least 10 years (5,7), only 4 of 10 patients in our study had diabetes for at least that duration. It is possible that patients in our study represented those with milder disease. Applicability to patients with severe and symptomatic gastroparesis is limited and will require further investigation.
In summary, PD investigation of a single administration of RM-131 reveals that it is effective in greatly accelerating GE in patients with type 2 diabetes and DGE, similar to the known effects of intravenous erythromycin in patients hospitalized with gastroparesis (9). These observations support the need for further clinical investigation of this promising and novel pharmacological agent in the medium- and long-term for the treatment of symptoms in patients with diabetic gastroparesis. Future studies will also be necessary in patients with type 1 diabetes, which differs in disease prevalence of gastroparesis (5) and may also differ in responsiveness to treatment.
Acknowledgments
The work was supported by research grants from the National Institutes of Health (DK-067071 to M.C. and the Mayo Clinic Clinical and Translational Science Award RR0024150).
This study was supported by a research grant from Rhythm Pharmaceuticals. E.S., P.N., and K.G. are employees of Rhythm Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
A.S. performed research, recruited participants, and wrote the manuscript. M.C. conceptualized the study and wrote the protocol and manuscript. I.B. recruited participants and performed scintigraphic studies. D.B. performed scintigraphic studies and analysis. E.S. conceptualized the study, interpreted hormonal measurements, and studied the PK. P.N. studied the PK. K.G. conceptualized the study, interpreted hormonal measurements, and wrote the manuscript. S.A.S. recruited participants. A.V. recruited participants and monitored diabetic control. A.R.Z. performed database management and statistical analysis and wrote the manuscript. All authors approved the final submitted paper. M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Rhythm Pharmaceuticals was not involved in the design, conduct, collection, or interpretation of the data.
Footnotes
Clinical trial reg. no. NCT01394055, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1128/-/DC1.
References
- 1.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009;136:1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology 2009;137:445–452 [DOI] [PubMed] [Google Scholar]
- 3.Jones KL, Russo A, Berry MK, Stevens JE, Wishart JM, Horowitz M. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med 2002;113:449–455 [DOI] [PubMed] [Google Scholar]
- 4.Punkkinen J, Färkkilä M, Mätzke S, et al. Upper abdominal symptoms in patients with type 1 diabetes: unrelated to impairment in gastric emptying caused by autonomic neuropathy. Diabet Med 2008;25:570–577 [DOI] [PubMed] [Google Scholar]
- 5.Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol 2012;107:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care 1999;22:503–507 [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med 2007;356:820–829 [DOI] [PubMed] [Google Scholar]
- 8.McCallum RW, Ricci DA, Rakatansky H, et al. A multicenter placebo-controlled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care 1983;6:463–467 [DOI] [PubMed] [Google Scholar]
- 9.Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 1990;322:1028–1031 [DOI] [PubMed] [Google Scholar]
- 10.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther 2007;26:1251–1258 [DOI] [PubMed] [Google Scholar]
- 11.Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol 2008;103:416–423 [DOI] [PubMed] [Google Scholar]
- 12.Kim CH, Nelson DK. Venting percutaneous gastrostomy in the treatment of refractory idiopathic gastroparesis. Gastrointest Endosc 1998;47:67–70 [DOI] [PubMed] [Google Scholar]
- 13.O’Grady G, Du P, Lammers WJ, et al. High-resolution entrainment mapping of gastric pacing: a new analytical tool. Am J Physiol Gastrointest Liver Physiol 2010;298:G314–G321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol 2010;8:947–954; quiz e116 [DOI] [PubMed] [Google Scholar]
- 15.Watkins PJ, Buxton-Thomas MS, Howard ER. Long-term outcome after gastrectomy for intractable diabetic gastroparesis. Diabet Med 2003;20:58–63 [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 2009;6:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut 2005;54:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang D, Nicolai H, Vos R, et al. Influence of ghrelin on the gastric accommodation reflex and on meal-induced satiety in man. Neurogastroenterol Motil 2009;21:528–533, e8–e9 [DOI] [PubMed] [Google Scholar]
- 19.Cremonini F, Camilleri M, Vazquez Roque M, et al. Obesity does not increase effects of synthetic ghrelin on human gastric motor functions. Gastroenterology 2006;131:1431–1439 [DOI] [PubMed] [Google Scholar]
- 20.Ejskjaer N, Vestergaard ET, Hellström PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther 2009;29:1179–1187 [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2 July 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brogna A, Loreno M, Catalano F, et al. Radioisotopic assessment of gastric emptying of solids in elderly subjects. Aging Clin Exp Res 2006;18:493–496 [DOI] [PubMed] [Google Scholar]
- 23.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Harding PE, Shearman DJ. Changes in gastric emptying rates with age. Clin Sci (Lond) 1984;67:213–218 [DOI] [PubMed] [Google Scholar]
- 24.O’Donovan D, Hausken T, Lei Y, et al. Effect of aging on transpyloric flow, gastric emptying, and intragastric distribution in healthy humans—impact on glycemia. Dig Dis Sci 2005;50:671–676 [DOI] [PubMed] [Google Scholar]
- 25.Kao CH, Lai TL, Wang SJ, Chen GH, Yeh SH. Influence of age on gastric emptying in healthy Chinese. Clin Nucl Med 1994;19:401–404 [DOI] [PubMed] [Google Scholar]
- 26.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95:1456–1462 [DOI] [PubMed] [Google Scholar]
- 27.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology 2006;131:1717–1724 [DOI] [PubMed] [Google Scholar]
- 28.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther 2009;30:670–680 [DOI] [PubMed] [Google Scholar]
- 29.Revicki DA, Camilleri M, Kuo B, Szarka LA, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the gastroparesis cardinal symptom index-daily diary (GCSI-DD). Neurogastroenterol Motil 2012;24:456–463, e215–e216 [DOI] [PubMed] [Google Scholar]
- 30.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil 2006;18:263–283 [DOI] [PubMed] [Google Scholar]
- 31.Wo JM, Ejskjaer N, Hellström PM, et al. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting—randomised clinical study subset data. Aliment Pharmacol Ther 2011;33:679–688 [DOI] [PubMed] [Google Scholar]
- 32.Ejskjaer N, Dimcevski G, Wo J, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil 2010;22:1069–e281 [DOI] [PubMed] [Google Scholar]
- 33.Fassino S, Daga GA, Mondelli V, et al. Hormonal and metabolic responses to acute ghrelin administration in patients with bulimia nervosa. Psychoneuroendocrinology 2005;30:534–540 [DOI] [PubMed] [Google Scholar]
- 34.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 2000;85:4908–4911 [DOI] [PubMed] [Google Scholar]
- 35.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004;25:426–457 [DOI] [PubMed] [Google Scholar]
- 36.Sharma D, Morrison G, Joseph F, Purewal TS, Weston PJ. The role of continuous subcutaneous insulin infusion therapy in patients with diabetic gastroparesis. Diabetologia 2011;54:2768–2770 [DOI] [PubMed] [Google Scholar]
- 37.Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol 2011;9:567-576, e1-4 [DOI] [PMC free article] [PubMed]
- 38.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol 2011;9:5–12; quiz e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musial F, Klosterhalfen S, Enck P. Placebo responses in patients with gastrointestinal disorders. World J Gastroenterol 2007;13:3425–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]