Abstract
OBJECTIVE
The incidence of the metabolic syndrome and type 2 diabetes mellitus (T2DM) is rising worldwide. Liver-derived fibroblast growth factor (FGF)-21 affects glucose and lipid metabolism. The aim of this study was to analyze the predictive value of FGF-21 on the incidence of T2DM and the metabolic syndrome.
RESEARCH DESIGN AND METHODS
The Metabolic Syndrome Berlin Potsdam (MeSyBePo) recall study includes 440 individuals. Glucose metabolism was analyzed using an oral glucose tolerance test, including insulin measurements. FGF-21 was measured using enzyme-linked immunosorbent assay. Primary study outcome was diabetes and the metabolic syndrome incidence and change of glucose subtraits.
RESULTS
During a mean follow-up of 5.30 ± 0.1 years, 54 individuals developed the metabolic syndrome, 35 developed T2DM, and 69 with normal glucose tolerance at baseline progressed to impaired glucose metabolism, defined as impaired fasting glucose, impaired glucose tolerance, or T2DM. FGF-21 predicted incident metabolic syndrome (lnFGF-21 odds ratio [OR] 2.6 [95% CI 1.5 – 4.5]; P = 0.001), T2DM (2.4 [1.2–4.7]; P = 0.01), and progression to impaired glucose metabolism (2.2 [1.3 – 3.6]; P = 0.002) after adjustment for age, sex, BMI, and follow-up time. Additional adjustment for waist-to-hip ratio, systolic blood pressure, HDL cholesterol, triglycerides, and fasting glucose did not substantially modify the predictive value of FGF-21.
CONCLUSIONS
FGF-21 is an independent predictor of the metabolic syndrome and T2DM in apparently healthy Caucasians. These results may indicate FGF-21 resistance precedes the onset of the metabolic syndrome and T2DM.
Numerous adipose tissue–derived hormones, the so-called adipokines, have been shown to predict and to be involved in the pathogenesis of type 2 diabetes mellitus (T2DM) (1,2). Recent data revealed increasing evidence that liver-derived hormones might affect glucose and lipid metabolism. Among these “hepatokines,” fibroblast growth factor (FGF)-21 has recently received increasing attention. FGF-21 expression and secretion is induced in the liver during periods of fasting (3,4). Recent studies suggested that fatty acids induce the expression and secretion of FGF-21 in a peroxisome proliferator–activated receptor-α (PPAR-α)–dependent fashion (5,6). FGF-21 signaling requires FGF receptor and the adapter molecule, β-Klotho (7,8), which targets FGF-21 primarily to the liver itself, but also to pancreas and adipose tissue. FGF-21 signaling has been suggested to affect glucose, lipid, cholesterol, and bile acid metabolism (3,9), which has turned FGF-21 into a reasonable candidate directly affecting the pathophysiology of the metabolic syndrome and T2DM. Notably, studies in animal models have found FGF-21 has antidiabetic properties (10), whereas a number of human studies observed increased circulating FGF-21 levels in subjects with existing insulin resistance, impaired glucose tolerance (IGT), and hypertriglyceridemia (11–13). Zhang and coworkers (13) demonstrated that FGF-21 is independently associated with the metabolic syndrome in Asian individuals, and another study in an Asian cohort recently demonstrated that genetic polymorphisms within a 3′-untranslated region of FGF-21 are also associated with the metabolic syndrome (14). Most interestingly an association between FGF-21 and incident diabetes was observed in an Asian cohort (15). However, whether FGF-21 predicts metabolic syndrome and whether the findings in Asian individuals are comparably found in Caucasian individuals is unclear. We therefore investigated whether FGF-21 predicts incident metabolic syndrome and T2DM, both defined by World Health Organization (WHO) criteria, and the progression of healthy controls to impaired glucose metabolism (IGM), defined as incident impaired fasting glucose (IFG), IGT, or incident diabetes, in a cohort of apparently healthy individuals.
RESEARCH DESIGN AND METHODS
A total of 582 individuals of the Metabolic Syndrome Berlin Potsdam (MeSyBePo) study were characterized in a follow-up study with a mean follow-up of 5.30 ± 0.04 years. Details of baseline phenotyping were described previously (16,17), and all individuals with at least 3 years of follow-up time were recruited to repeat phenotyping, which was virtually identical to the baseline characterization. All participants underwent a physical examination. Anthropometry was performed under standardized conditions by trained staff. All study samples were taken at 8:00 a.m. after an overnight fast. An oral glucose tolerance test (OGTT) was performed in all individuals, and fasting blood samples were taken before OGTT.
We took EDTA and Citrat plasma as well as serum from all participants. Plasma was placed on ice and spun down within 30 min at 4°C. After centrifugation, the plasma was immediately transferred to a −20°C freezer. Within the next 24 h, all samples were transferred on dry ice to a −80°C freezer, where aliquots were stored until assay. Serum was left at room temperature for 30 min to allow clotting, thereafter, the sample was centrifuged. The serum was then transferred to a −20°C freezer and within 24 h to a −80°C freezer, comparable to all plasma samples. FGF-21 was determined in aliquots without prior freeze and thaw cycles.
Considering the effect of intended weight reduction, we excluded 142 individuals who reported participation in weight-reduction courses during the follow-up, leaving 440 individuals for further analysis. The diagnostic criteria of the metabolic syndrome and T2DM were based on the criteria recommended by the WHO, including the 75-g OGTT. We used the criteria by the WHO (1999), which require presence of one of DM, IGT, or IFG, and two of the following: blood pressure ≥140/90 mmHg, dyslipidemia (triglycerides [TG] ≥1.695 mmol/L and HDL cholesterol ≤0.9 mmol/L [male] or ≤1.0 mmol/L [female]), central obesity (waist-to-hip ratio [WHR] >0.90 [male], >0.85 [female], or BMI >30 kg/m2), or microalbuminuria.
FGF-21 was measured in EDTA plasma aliquots, which had not been thawed and refrozen, with a commercially available ELISA (R&D Systems Inc., Minneapolis, MN). R&D Systems reports an intra-assay coefficient of variation (CV) of between 2.9 and 3.9%, as determined by three samples of known concentration tested 20 times on one plate. Interassay CV was reported at between 5.2 and 10.9%, as determined by three samples of known concentration tested in 40 separate assays. We also analyzed interassay CV by an internal standard that was determined on every plate and was 8.53% in our hands. FGF-21 values were log-transformed given that crude data were not normally distributed.
This study was carried out in accordance with the recommendations of the Declaration of Helsinki, and the experimental protocol of the study was approved by the institutional ethical committee. All subjects gave written informed consent.
Statistical calculations were performed using SPSS 20.0 software (IBM Statistics, Germany). All values are presented as mean and SEM, if not otherwise mentioned. The Student t test was applied if parameters were normally distributed; otherwise, the Mann-Whitney–Wilcoxon test was used. Logistic regression models were calculated to identify independent relations between FGF-21 and incident metabolic syndrome, T2DM, or development of impaired glycemic control. Two models were calculated: one crude model was adjusted for age, sex, BMI, and follow-up time, and a second model was additionally adjusted for WHR, HDL cholesterol, TG, systolic blood pressure, and fasting glucose (fully adjusted model). Odds ratios (ORs) and 95% CIs are reported.
The area under the receiver operating characteristic (ROC) curve (AUC) was used to define variables being additionally informative in the prediction of the metabolic syndrome, IGM, or T2DM, respectively. ROC curve analyses were performed according to Möhlig et al. (18).
RESULTS
A total of 440 individuals (151 men, 289 women) were available for analysis, and during the observation period, 54 (26 men, 28 women) developed novel metabolic syndrome according to WHO criteria, 35 (14 men, 21 women) developed incident T2DM, and 69 (29 men, 40 women) experienced a progression to IGM, defined as novel IFG, IGT, or T2DM. Baseline characteristics of individuals are presented in Tables 1–3. Briefly, individuals who developed the metabolic syndrome had a higher BMI, WHR, HbA1c, and diastolic blood pressure at baseline (Table 1). No significant differences were found for total cholesterol, LDL, HDL, TG, systolic blood pressure, and age. FGF-21 at baseline correlated significantly with systolic (r = 0.185; P < 0.001) and diastolic blood pressure (r = 0.169; P < 0.001), TG (r = 0.273; P < 0.001), WHR (r = 0.103; P = 0.032), BMI (r = 0.243; P < 0.001), age (r = 0.280; P < 0.001), HbA1c (r = 0.225; P < 0.001), fasting glucose (r = 0.156; P = 0.001), 2-h glucose (r = 0.241; P < 0.001), and homeostasis model assessment insulin-resistance (r = 0.189; P < 0.001). No significant correlations were found between FGF-21 and LDL and HDL cholesterol, free fatty acids, and intima media thickness.
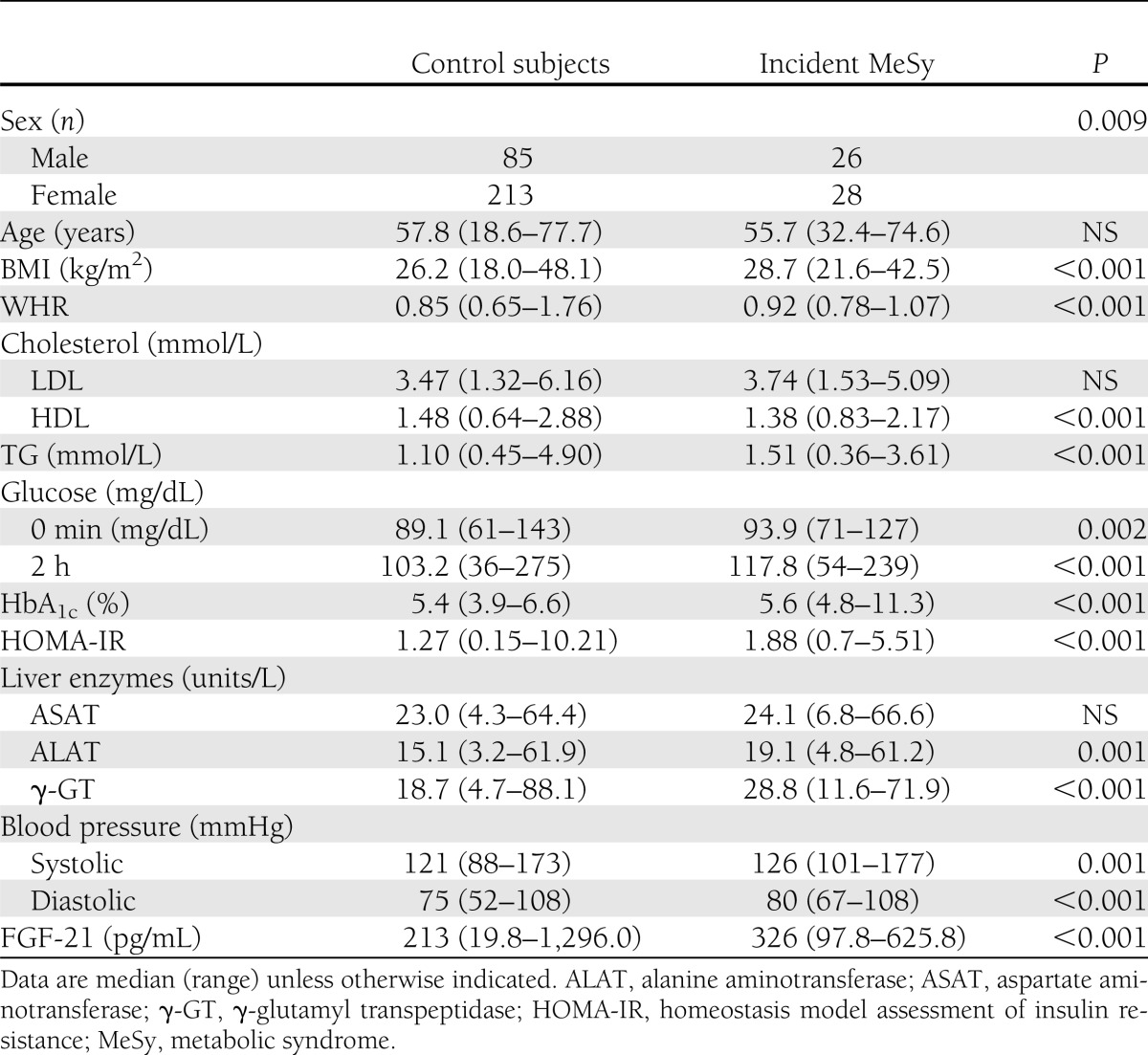
Table 1.
Comparison of control subjects vs. individuals with incident metabolic syndrome (n = 352)
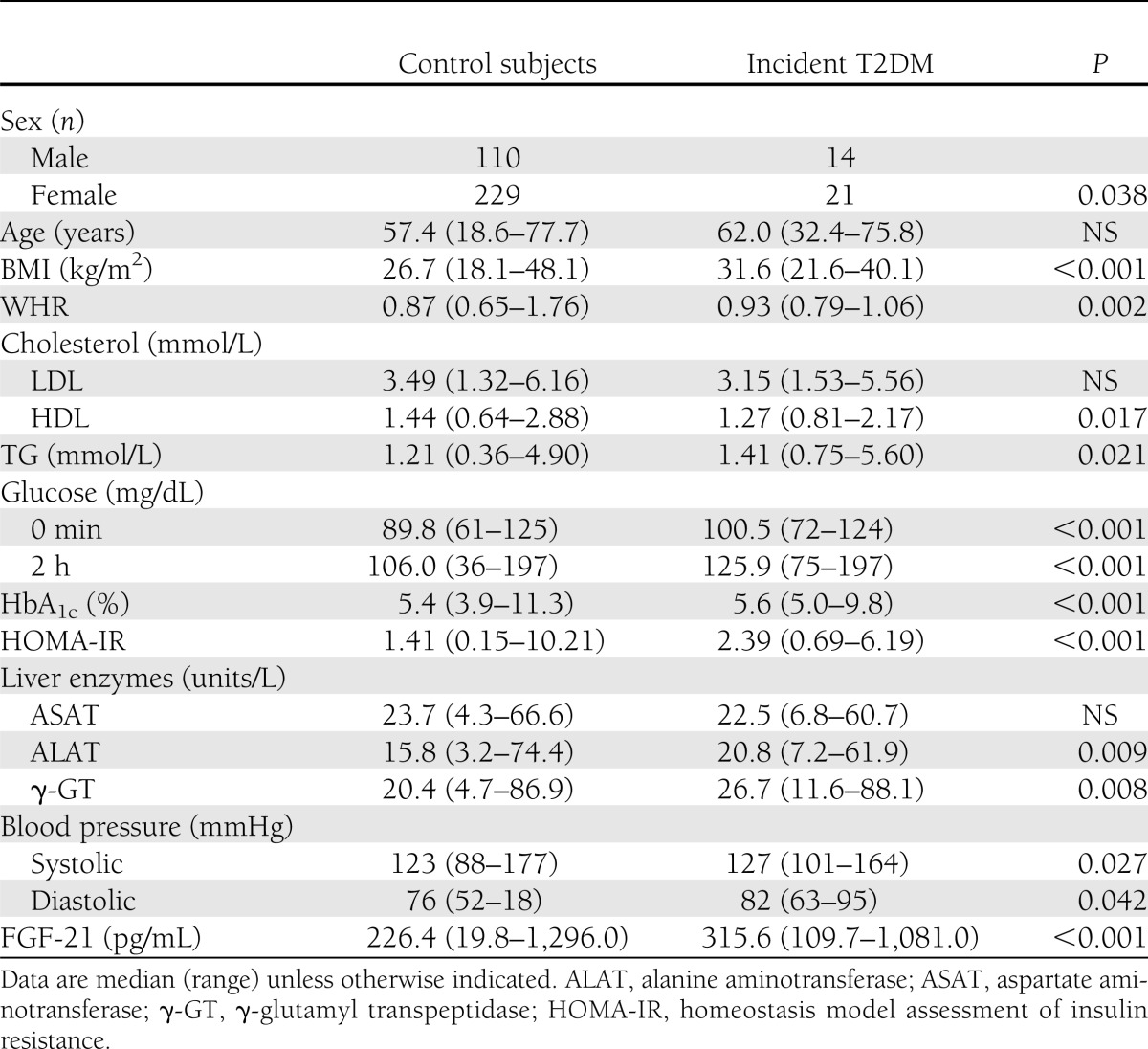
Table 3.
Comparison of control subjects vs. individuals with incident T2DM (n = 374)
Patients with incident metabolic syndrome had higher FGF-21 levels at baseline (326.1 ± 21.3 vs. 278.1 ± 10.1 pg/mL; P = 0.006). Logistic regression analysis revealed that FGF-21 independently predicted the risk to develop the metabolic syndrome (lnFGF-21 OR 2.6 [95% CI 1.5–4.5]; P = 0.001) after adjustment for age, sex, BMI, and time of follow-up (crude model). We additionally adjusted for WHR, systolic blood pressure, HDL cholesterol, TG, and fasting glucose, which slightly attenuated results, but FGF-21 still remained an independent predictor of future metabolic syndrome (2.2 [1.2–4.1]; P = 0.009).
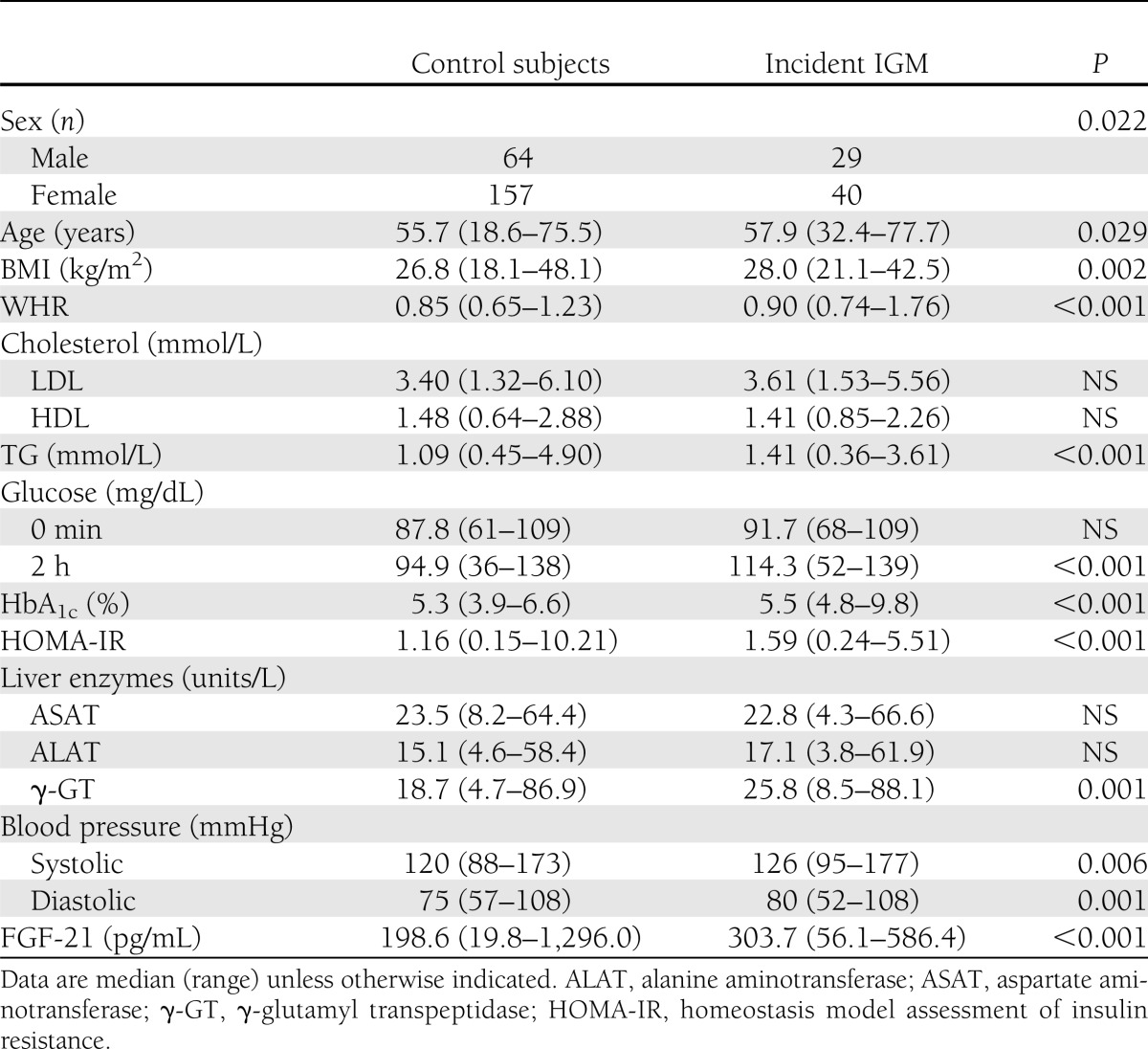
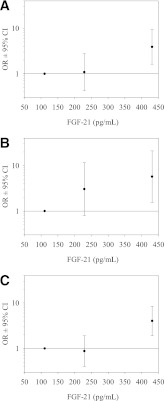
We next analyzed the relation between FGF-21 and incident IGM (Table 2) or incident T2DM (Table 3). Analyses were restricted to those individuals who did not have prevalent diabetes at baseline (incident T2DM) or normal glucose metabolism at baseline (incident IGM). Within the crude model (adjusted for age, sex, BMI, and time of follow-up) FGF-21 was associated with future T2DM (lnFGF-21 OR 2.4 [95% CI 1.2–4.7]; P = 0.01) and progression of healthy individuals to IGM (2.2 [1.3–3.6]; P = 0.002). Additional adjustment for WHR, systolic blood pressure, HDL cholesterol, TG, and baseline fasting glucose again modified results, but point estimates remained comparable to the crude analysis for incident T2DM (2 [0.91–4.3]; P = 0.086) and incident IGM (1.9 [1.2–3.3]; P = 0.012; Fig. 1A–C for risk according to FGF-21 tertiles). Including fasting insulin values or liver enzymes (aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase) into logistic regression models did not change observed results substantially. Neither incident changes of adiposity (BMI, WHR), nor systolic or diastolic blood pressure, nor changes of lipid metabolism (total cholesterol, LDL or HDL cholesterol, or TG) were associated with baseline FGF-21.
Table 2.
Comparison of control subjects vs. individuals with incident IGM (n = 290)
Figure 1.
Risk of subsequent metabolic syndrome (A), T2DM (B), and progression to IGM (IFG, IGT, or T2DM) (C) according to the respective median of ascending tertiles of FGF-21 concentrations. Adjustment for age, sex, BMI, and follow-up time. Error bars represent 95% CIs. Horizontal line at 1·0 represents the reference line. Participants were divided into tertiles according to FGF-21 concentrations. Tertile cut points were determined from the combined group of control subjects and case subjects.
We next aimed to analyze whether FGF-21 had any practical value in the prediction of T2DM or IGM and therefore calculated ROC curves for two models: one including age, sex, BMI, and time of follow-up, and a second model with the same variables and FGF-21. For IGM, the ROC AUC curve was 0.668 (95% CI 0.611–0.722) without FGF-21 and 7.08 (0.652–0.760) with FGF-21. This difference was not significant (P = 0.169). Comparable results were found for T2DM, with an AUC of 0.76 without FGF-21 and 0.79 with FGF-21 (P = 0.327).
CONCLUSIONS
We demonstrate within this study that FGF-21 predicts the metabolic syndrome and future glycemic control, as defined by T2DM and the compound outcome IGM. Those data suggest that the FGF-21 system is involved in the development of the metabolic syndrome and the pathophysiology of T2DM.
Interestingly, there are some data in cross-sectional human studies showing a positive correlation between FGF-21 values and obesity, T2DM, or the metabolic syndrome (13,19). Nevertheless the pathophysiologic effects of FGF-21 on glucose lipid metabolism are not well understood, especially because initial animal and in vitro studies proposed glucose-lowering effects and improved fat metabolism. For example in murine studies, FGF21 expression in the liver could be controlled by the transcription factor PPAR-α, which is activated during fasting (3,4). Interestingly FGF-21 expression can also be induced by PPAR-γ activation in 3T3-L1 cells, and FGF-21 synergizes with rosiglitazone to stimulate glucose transport by an increased GLUT1 expression (10,20). FGF-21 mRNA expression in liver and in adipose tissue, is increased by a high-fat diet and in mice with genetic obesity (13,21,22). In 3T3-L1 cells, stimulation with FGF-21 leads to elevated glycerol levels as a marker of increased lipolysis (3), whereas in primary cultures of human adipocytes, FGF-21 seems to promote inhibitory effects on lipolysis (23).
Positive metabolic effects on glucose and fat metabolism were also seen in diabetic rhesus monkeys that were treated for 6 weeks with FGF-21. Not only glucose and TG levels could be controlled but also LDL cholesterol was lowered and HDL cholesterol increased (24).
These differences in human in vitro data and human studies compared with data obtained in animal models are leading to a more complex view of FGF-21 metabolism. The increase of FGF-21 levels in obesity-related disorders might be caused by a resistance to FGF-21, leading to its compensatory upregulation. This scenario is comparable to hyperinsulinemia and hyperleptinemia in obesity-related disorders like T2DM.
The epidemiologic data presented here appear to be in some opposition to existing experimental data demonstrating that FGF-21 improves glycemic control (10). However, circulating levels of FGF-21 do not necessarily indicate the activity of the FGF-21 system. Comparable to insulin resistance, a significant FGF-21 resistance may exist in obesity (25). Next, one might argue that the effects of FGF-21 may be different in rodents and humans, although we believe that our observations are not suitable to challenge any experimental data. Finally, FGF-21 might be elevated to counterbalance very early pathophysiologic phenotypes, which were not analyzed in our study. Thus, FGF-21 is known to be increased in fatty liver disease (26), which in turn is known to precede the metabolic syndrome and T2DM. FGF-21 might therefore be a marker of very early metabolic disturbances preceding T2DM and the metabolic syndrome.
In general, there is a substantial need to identify individuals at increased risk for T2DM. Although logistic regression analysis identified FGF-21 as an independent risk factor of metabolic syndrome and glycemic control, we also performed ROC curve analyses to estimate the practical value of FGF-21 in addition to existing risk factors. Within a model including relevant risk factors (e.g., age, sex, BMI, and time of follow-up), FGF-21 did not significantly increase the AUC, thereby demonstrating that the additive value in daily clinical practice may be limited, although the pathophysiologic implications of our findings are tempting.
In summary, we demonstrated that FGF-21 is an independent predictor of the metabolic syndrome, T2DM, and IGM. In combination with existing experimental data, our data may suggest a state of FGF-21 resistance preceding the metabolic syndrome and impaired glycemic control.
Acknowledgments
This study was supported by the Deutsches Zentrum für Herz-Kreislauf-Forschung (German Centre for Cardiovascular Research) and by the Bundesministerium für Bildung und Forschung (German Ministry of Education and Research).
No potential conflicts of interest relevant to this article were reported.
T.B., F.S., and J.S. contributed to the conception and design of the project, contributed to discussion, collected and analyzed the data, and drafted, reviewed, and edited the manuscript. A.F.-R., M.M., A.F.H.P., and K.M. contributed to the conception and design of the project, researched data, contributed to discussion, and reviewed and edited the manuscript. T.B. and J.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 2.Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226–228 [DOI] [PubMed] [Google Scholar]
- 3.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–425 [DOI] [PubMed] [Google Scholar]
- 4.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–437 [DOI] [PubMed] [Google Scholar]
- 5.Mai K, Andres J, Biedasek K, et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 2009;58:1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mai K, Bobbert T, Groth C, et al. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. Am J Physiol Endocrinol Metab 2010;299:E126–E130 [DOI] [PubMed] [Google Scholar]
- 7.Kharitonenkov A, Dunbar JD, Bina HA, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 2008;215:1–7 [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y, Kurosu H, Yamamoto M, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 2007;104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest 2005;115:2202–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009;32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–375 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–1253 [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Zeng L, Wang YJ, An ZM, Ying BW. Associations of fibroblast growth factor 21 gene 3′ untranslated region single-nucleotide polymorphisms with metabolic syndrome, obesity, and diabetes in a Han Chinese population. DNA Cell Biol 2012;31:547–552 [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Cheung BM, Tso AW, et al. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care 2011;34:2113–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis J, Langenberg C, Prokopenko I, et al. DIAGRAM Consortium. GIANT Consortium. Global BPgen Consortium. Anders Hamsten on behalf of Procardis Consortium. MAGIC investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer-Rosinsky A, Fisher E, Kovacs P, et al. Lack of association between the tagging SNP A+930→G of SOCS3 and type 2 diabetes mellitus: meta-analysis of four independent study populations. PLoS ONE 2008;3:e3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Möhlig M, Spranger J, Ristow M, et al. Predictors of abnormal glucose metabolism in women with polycystic ovary syndrome. Eur J Endocrinol 2006;154:295–301 [DOI] [PubMed] [Google Scholar]
- 19.Li L, Yang G, Ning H, Yang M, Liu H, Chen W. Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract 2008;82:209–213 [DOI] [PubMed] [Google Scholar]
- 20.Moyers JS, Shiyanova TL, Mehrbod F, et al. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol 2007;210:1–6 [DOI] [PubMed] [Google Scholar]
- 21.Lundåsen T, Hunt MC, Nilsson LM, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 2007;360:437–440 [DOI] [PubMed] [Google Scholar]
- 22.Muise ES, Azzolina B, Kuo DW, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol 2008;74:403–412 [DOI] [PubMed] [Google Scholar]
- 23.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes - a possible link to improved insulin sensitivity. FEBS Lett 2008;582:1725–1730 [DOI] [PubMed] [Google Scholar]
- 24.Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007;148:774–781 [DOI] [PubMed] [Google Scholar]
- 25.Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010;59:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 2010;53:934–940 [DOI] [PubMed] [Google Scholar]