Abstract
OBJECTIVE
We sought to establish β-cell mass, β-cell apoptosis, and β-cell replication in humans in response to obesity and advanced age.
RESEARCH DESIGN AND METHODS
We examined human autopsy pancreas from 167 nondiabetic individuals 20–102 years of age. The effect of obesity on β-cell mass was examined in 53 lean and 61 obese subjects, and the effect of aging was examined in 106 lean subjects.
RESULTS
β-Cell mass is increased by ∼50% with obesity (from 0.8 to 1.2 g). With advanced aging, the exocrine pancreas undergoes atrophy but β-cell mass is remarkably preserved. There is minimal β-cell replication or apoptosis in lean humans throughout life with no detectable changes with obesity or advanced age.
CONCLUSIONS
β-Cell mass in human obesity increases by ∼50% by an increase in β-cell number, the source of which is unknown. β-Cell mass is well preserved in humans with advanced aging.
The incidence of type 2 diabetes increases with obesity and aging (1). There is a deficit in β-cell mass with increased β-cell apoptosis in type 2 diabetes (2). Although there are numerous studies of changes in β-cell mass and turnover in rodents, inevitably the data is much more limited in humans. As there is an increasing appreciation that regulation of β-cell mass in humans and rodents can be quite different, additional studies in humans, where possible, is important. In the current study, we addressed the following questions.
First, is β-cell mass adaptively increased in obese humans, and if so, is this through increased β-cell replication as widely reported in rodents? It has been reported that β-cell mass increases with obesity in age-matched individuals but β-cell replication was not reported (3). Second, is β-cell apoptosis increased with obesity? The increased β-cell apoptosis in type 2 diabetes (2) has been ascribed to lipotoxicity, based on increased β-cell apoptosis in rodents with obesity due to deficient leptin signaling (4). Since the relative fat content (fat-to-acinar ratio) accumulates in the pancreas in humans with obesity (5), if this is sufficient to induce increased β-cell apoptosis, then it would be anticipated that humans with marked obesity would have increased β-cell apoptosis.
Third, we questioned if β-cell mass adaptively decreases with aging, and if so, is this due to increased β-cell apoptosis? β-Cell function declines in humans with aging (6). The exocrine pancreas undergoes marked atrophy after 60 years of age, but there is limited data available about the changes in β-cell mass with aging, with one study reporting a marginal decline with age (3) but providing no measure of β-cell turnover.
RESEARCH DESIGN AND METHODS
Study design
Pancreas was obtained from Mayo Clinic autopsy archives. At autopsy, the pancreas is not routinely dissected free from retroperitoneal tissues to permit measurement of pancreas weight. To address this, we established population data for pancreas volume from abdominal computed tomography (CT) scans in 1,887 adults as previously reported (5) and validated (7). In that previously reported CT scan study, the relationship between obesity and pancreas volume and fat content was examined in 460 lean individuals (mean BMI 22 kg/m2), 430 overweight individuals (mean BMI 27 kg/m2), and 230 obese individuals (mean BMI 34 kg/m2), a range deliberately selected to encompass the range of cases in the current study. Also, the age range of individuals in the CT scan study, from 20 to 100 years of age, was also selected deliberately to permit the development of population data relevant to the present aging study. β-Cell mass was then computed as a product of fractional β-cell area and pancreas weight (assuming 1 cm3 pancreas = 1 g).
Subjects
University of California, Los Angeles (UCLA), and Mayo Clinic Institutional Review Board permission was obtained for these studies. Potential cases were first identified by retrospective analysis of the Mayo Clinic autopsy database. To be included, cases were required to have had 1) a full autopsy within 24 h of death; 2) pancreatic tissue stored that was of adequate size and quality; 3) no history of diabetes, pancreatitis, or pancreatic surgery; and 4) no use of glucocorticoids. Cases were excluded if pancreatic tissue had undergone autolysis. Preference was given to cases where the final illness was relatively short term (for example, trauma or sudden vascular event), so as to minimize the confounding effects of a prolonged final illness on the nutritional status of the individual and effects this may have had on islet morphology. Case subjects were identified based on these preferences at the Mayo Clinic, and the sections of selected case subjects were obtained and made available to UCLA investigators in a manner coded to conceal the personal identity of the subjects. The blood glucose value was obtained from the most recent ambulatory overnight-fasted value in the Mayo Medical Center clinical record, not from the final in-patient glucose values, which are subject to confounding factors such as premortem stress and intravenous glucose. The characteristics of the cases are summarized in Supplementary Table 1, with causes of death in Supplementary Table 2.
Lean (n = 53, BMI <25 kg/m2) and obese (n = 61, BMI ≥27 kg/m2) case subjects, 20–59 years of age, were included to evaluate the impact of obesity on β-cell mass (Supplementary Table 1A). Although this BMI cutoff for obesity is lower than current definitions of obesity, the two groups were selected with the intention of examining the impact of insulin resistance on β-cell mass, appreciating from available data that the selected ranges for BMI would have resulted in groups with contrasting insulin sensitivity. A limitation of human autopsy studies such as these is that insulin sensitivity cannot be measured in the two groups. Fasting plasma glucose (FPG) was slightly higher in the obese subjects than the lean subjects (P = 0.05) (Supplementary Table 1A), consistent with obesity-related insulin resistance (8).
For the aging study, 106 case subjects, 20–102 years of age, all with a BMI <25 kg/m2, were divided by decile age-groups (Supplementary Table 1B). Each decile group included 9–20 subjects, the BMI being matched between groups. Consistent with prior reports, FPG tended to increase with age (Supplementary Table 1B) (9,10).
Pancreatic tissue processing
At autopsy, the pancreas was resected from the tail and, with a sample of spleen, fixed in formaldehyde and embedded in paraffin for subsequent analysis. Sections (5 μm) were stained for 1) insulin (peroxidase staining) and hematoxylin for light microscopy; 2) insulin, Ki67, and DAPI; and 3) insulin, Tdt-mediated dUTP nick-end labeling (TUNEL), and DAPI (immunofluorescence), as previously described (2,11,12). For immunohistochemical staining, the following primary antibodies were used: guinea pig anti-insulin (1:100; Zymed Laboratories, San Francisco, CA) and mouse Ki67 (1:50, MIB-1; DAKO, Carpinteria, CA). Secondary antibodies labeled with Cy3 and fluorescein isothiocyanate (Jackson ImmunoResearch Laboratories, West Grove, PA) were used at a dilution of 1:100. For TUNEL staining, the in situ cell death detection kit TMR red (Roche Diagnostics, Mannheim, Germany) was used.
Morphometric analysis
All morphometric analyses were performed by two independent investigators (Y.S. and A.E.B.), and if results varied by >10% in any individual, the analyses were performed again by both investigators. The mean of the results by the two investigators was used. To quantify fractional β-cell area, the entire pancreatic section was imaged at 40× magnification (4× objective). The ratio of the β-cell area/total pancreas parenchymal area was digitally measured as previously described (2) using Image Pro Plus software (Media Cybernetics, Silver Springs, MD). After pancreas fixation, pancreas sections retain exocrine and endocrine tissue but not fat, which is removed during fixation. Therefore, the measured fractional β-cell area is a fraction of β-cell area to total pancreas parenchymal area.
To measure individual β-cell size and nuclear diameter, five islets per case (i.e., 100 islets lean vs. 100 islets obese) were selected at random and imaged at 400× (40× objective). β-Cell size was determined as mean individual β-cell cross-sectional area. For the mean individual β-cell cross-sectional area, the insulin-positive area of each islet was divided by the number of nuclei within the insulin-positive area. For the mean nuclear diameter, these islets were then examined to identify five representative β-cell nuclei each, as previously described (13,14). Once the identified nucleus was encircled, the measurement of 180 nuclear diameters per nucleus was performed using Image Pro Plus, which quantified these 180 diameters at 2° angles throughout the circumference of the nucleus. The individual β-cell size and nuclear diameter were also evaluated in BMI-matched cases, five each, from each decile (i.e., total of 200 islets from 40 cases) for the aging study.
β-Cell replication and apoptosis were quantified in 13 obese vs. 14 lean individuals. β-Cell replication and apoptosis were also quantified in 13 elderly individuals for the aging study. Since β-cell replication and apoptosis are rare in the human pancreas, every islet in each pancreas section (189 ± 8 islets per section) double stained by the insulin and Ki67 or TUNEL technique was imaged at 200× magnification using a Leica DM6000 microscope (Leica Microsystems, Wetzlar, Germany) and Openlab software (Improvision, Waltham, MA), and β-cell replication and apoptosis were documented. Then, the frequency of β-cell replication and apoptosis were expressed as percentage of β-cells. A total of 578,452 β-cells (6,288 ± 346 β-cells per section) were assessed for these analyses.
Pancreas parenchymal volume
To determine β-cell mass, the pancreas parenchymal volume was estimated by use of equations based on the population data described in detail in elsewhere (5). In brief, the pancreas parenchymal volume increases in childhood to reach a plateau at 20 years of age. From 20 to 60 years of age, pancreas parenchymal volume is stable and is described as a function of obesity. After 60 years of age, pancreatic parenchymal volume declines linearly, being described as a function of age.
Assessment of β-cell mass
β-Cell mass was calculated as a product of the fractional β-cell area determined by immunohistochemical staining in each individual, and the estimated pancreas parenchymal weight as above (assuming 1 g of weight per 1 cm3 pancreas volume).
Statistical analysis
Data are presented as mean ± SEM. Statistical comparisons were carried out using the Student t test or one-way ANOVA, with a P value of <0.05 taken as significant. A simple regression was carried out for the correlation analysis. The Wilcoxon rank sum test was performed to compare β-cell replication and apoptosis between groups due to the skewed distributions of the observations. Confidence intervals for group differences of mean β-cell turnover were constructed to obtain ranges of likely differences.
RESULTS
β-Cell mass in obesity
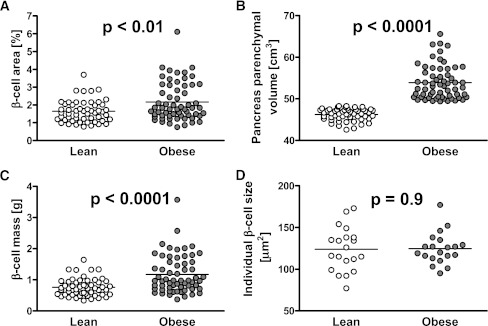
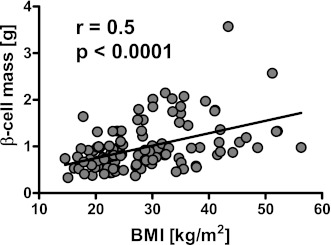
The fractional β-cell/pancreas parenchymal area is ∼30% higher in the obese compared with the lean group (2.2 ± 0.1 vs. 1.6 ± 0.1%, P < 0.01) (Figs. 1 and 2A). β-Cell mass is ∼50% higher in the obese compared with the lean group (1.2 ± 0.1 vs. 0.8 ± 0.04 g, P < 0.0001) (Fig. 2). Both the fractional β-cell area (r = 0.3, P = 0.001) and the calculated β-cell mass (r = 0.5, P < 0.0001) (Fig. 3) are increased as a function of BMI, although there is considerable variance in β-cell mass not explained by BMI. There is no correlation between β-cell mass and FPG, although the range of the FPG is, by design (all nondiabetic), narrow.
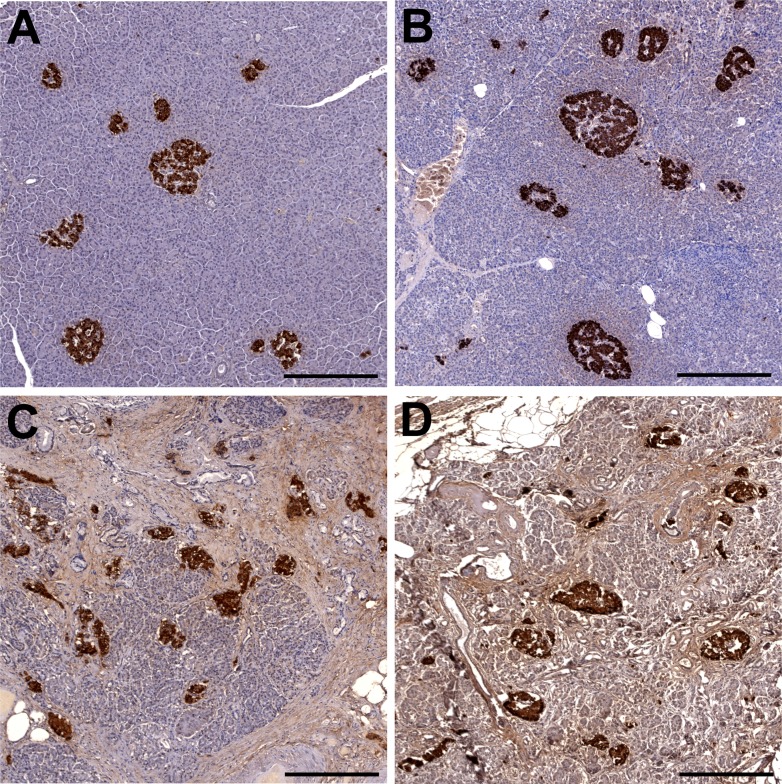
Figure 1.
Representative insulin (brown)-hematoxylin immunohistochemistry of pancreas (original magnification ×100). Examples of lean (male, 28 years of age, BMI 23.4) (A), obese (male, 44 years of age, BMI 33.6) (B), and elderly (male, 91 years of age, BMI 16.2; female, 100 years of age, BMI 24.9) (C and D) subjects. The fractional pancreatic insulin-positive area/pancreas parenchymal area, average islet size, and islet density are all modestly increased in obesity (B) compared with lean subjects (A). Pancreatic fat was also increased in obesity (B). Whereas atrophy, fibrosis, and fat accumulation are typical in the exocrine pancreas of elderly individuals (C and D), compared with a younger population (A), islet structure was remarkably maintained (C and D). As a result, fractional β-cell area/pancreas parenchymal area was increased in elderly vs. younger individuals (C and D vs. A). Scale bars, 200 μm. (A high-quality digital representation of this figure is available in the online issue.)
Figure 2.
Fractional β-cell area (A), estimated pancreas parenchymal volume (B), and computed β-cell mass (C) in lean and obese nondiabetic subjects. The pancreatic fractional β-cell area was ∼30% greater in the obese vs. the lean group (A). Estimated pancreas parenchymal volume (see research design and methods) was ∼15% greater in the obese vs. the lean subjects (B). In consequence, the computed mean β-cell mass was ∼50% higher in obese subjects (0.8 g in lean and 1.2 g in obese) (C). However, there was no increase in mean individual β-cell size in obese subjects (D).
Figure 3.
Correlation between β-cell mass and BMI in nondiabetic humans. There was a significant positive correlation between β-cell mass and BMI.
The mean individual β-cell cross-sectional area is comparable in the obese and lean groups (125 ± 4 vs. 124 ± 6 μm2, P = 0.9) (Fig. 2), implying that the increase in β-cell mass in obesity is accomplished by increased β-cell number rather than hypertrophy. The mean β-cell nuclear diameter, an indirect marker of secretory activity, is increased in obesity (6.9 ± 0.2 vs. 6.0 ± 0.2 μm, P < 0.001), consistent with prior findings (13).
β-Cell mass with aging
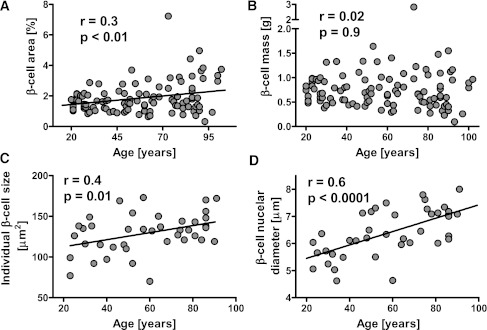
The most striking change in pancreas morphology with advanced age is atrophy of the exocrine pancreas with relative increasing fibrosis and fat accumulation (increased fat-to-acinar ratio) (Fig. 1). However, in contrast to the exocrine pancreas, islet structure is relatively maintained with advanced age in human pancreas. As a result, the fractional β-cell area increases with advanced age (r = 0.3, P < 0.01) (Fig. 4). There is no sex difference in the fractional β-cell area with aging (2.0 ± 0.2 vs. 2.2 ± 0.3% in males and females 60 years of age and over, P = 0.5). Despite the exocrine atrophy with advanced age, the calculated β-cell mass remained constant from 20 to 100 years of age (Fig. 4). Although there was no significant change in β-cell size with aging, the mean β-cell nuclear diameter increased with age (r = 0.6, P < 0.0001) (Fig. 4).
Figure 4.
Pancreatic fractional β-cell area (A) and computed β-cell mass (B) in lean nondiabetic subjects from 20 to 100 years of age. Pancreatic fractional β-cell area increased with age (A), but when β-cell mass was calculated from pancreatic parenchyma (Supplementary Fig. 1), β-cell mass remained constant to advanced age (B). The mean individual β-cell cross-sectional area (C) and β-cell nuclear diameter (D) both increased with age.
β-Cell replication and apoptosis with obesity and aging
The subsets of individuals in which β-cell turnover was evaluated were similar with respect to age and BMI to the larger cohort. For example, the lean young subjects studied for β-cell turnover had similar age and BMI to the overall lean young group. β-Cell replication was very infrequent and unchanged by either obesity (0.021 ± 0.007% in obese young group vs. 0.063 ± 0.026% in lean young group, P = 0.34, 95% CI for difference in means [−0.014 to 0.099]) or advanced age (0.020 ± 0.005% in lean old group, P = 0.24 vs. lean young group, 95% CI [−0.012 to 0.099]). Likewise, β-cell apoptosis was also infrequently detected, with nine events (observed in cells from five subjects) observed out of 236,771 cells examined. The proportion of apoptotic β-cells was unchanged by either obesity (0.0085 ± 0.0059% in obese young group vs. 0.0013 ± 0.0013% in lean young group, P = 0.48) or advanced age (0.0032 ± 0.0025% in lean old group, P = 0.53 vs. lean young group).
CONCLUSIONS
A deficit in β-cell mass with increased β-cell apoptosis is characteristic of both type 1 and 2 diabetes (2,11). Obesity and advancing age are both risk factors for the development of type 2 diabetes (1). However, most individuals with obesity or advanced age do not develop type 2 diabetes. In the current study, we sought to evaluate the changes in β-cell mass and β-cell turnover with obesity and aging in nondiabetic humans.
We report an ∼50% increase in β-cell mass induced by obesity but with a wide variance not explained by obesity or age (Supplementary Fig. 2). These findings are in broad agreement with the few studies available in humans. Ogilvie (15) reported that islets of Langerhans were enlarged in humans with obesity as early as 1933. Klöppel et al. (16) reported no increase in fractional β-cell area but a twofold increase in pancreas parenchymal volume in four obese humans compared with seven lean humans. Yoon et al. (17) reported a positive linear correlation between fractional β-cell area and BMI in nine nondiabetic humans with a BMI of 22–27 kg/m2 in patients undergoing partial pancreatectomy for pancreatic tumors. We previously reported an ∼50% increase in fractional β-cell area in 31 obese nondiabetic humans compared with 17 lean nondiabetic humans, but in that study, the lean and obese groups were not age matched as they were selected as control groups for lean and obese individuals with type 2 diabetes.
In an autopsy study, Rahier et al. (3) reported an increment in β-cell mass of 20% in individuals with a BMI of 26–40 kg/m2 (n = 25) compared with those with a BMI of <25 kg/m2 (n = 26). Despite a variety of methodological differences, the range of measured β-cell mass in the Rahier article and the current study are in broad agreement. For example, in the Rahier article, the β-cell fractional area of the pancreas was measured by manual point counting a quartile of sections obtained from the body and tail of the pancreas, whereas in the current study, the fractional β-cell area was measured in the entirety of sections from the tail by an automated image analysis system. Rahier et al. (3) observed marked variance between the body and tail within individuals not seen in a prior study (17) or in our own studies of pancreas procured from brain-dead organ donors. In the Rahier article, pancreas weight was measured at autopsy, whereas in the current study, population pancreas volumes were used as the pancreas weight was not available in the individuals from whom the pancreas samples were available. The large number of cases in both the Rahier and the present study likely compensate for the limitations of measurement of β-cell mass in human autopsy studies.
Also in agreement with Rahier et al. (3), we affirm that the increment in β-cell mass with obesity is due to increased numbers of cells rather than cell size. The increase in β-cells in response to obesity with no detectable increase in β-cell replication in humans noted here is consistent with the recent observation that β-cell mass is adaptively increased in human pregnancy but without a detectable increase in β-cell replication (18). Presumably these findings either reflect an increase in β-cell formation in response to obesity and pregnancy from sources other than the duplication of existing β-cells (so-called neogenesis) or an increase in β-cell replication that is too small to be detected. Alternatively, the increase in the number of β-cells may occur early in response to increasing obesity, with no further increase once obesity is established. We selected a wide range of BMIs for the obese group to include individuals classified as overweight rather than obese (BMI ≥27 kg/m2), but there was still no detectable increase of β-cell replication in individuals from the lower BMI tertile of the obese group. The majority of the β-cells observed to be replicating (64%) in the lean young group were observed in only 2 of the 14 subjects. Without those two cases, the average replication (0.030%) in the lean young group was numerically similar to the other two groups. Based on the few individuals in which β-cell replication was detected, even a very large number of additional study subjects would be unlikely to result in significant group differences. Given the absence of lineage tracing in humans, it is not possible at present to resolve the origins of newly forming β-cells in humans in pregnancy and obesity.
β-Cell apoptosis is increased in humans with type 2 diabetes and this has been ascribed to increased pancreatic fat with obesity leading to “lipotoxicity” (4). This hypothesis arose largely as a result of the increased β-cell apoptosis present in the absence of leptin signaling in a rodent model of diabetes (19). However, although fat accumulates in the pancreas in humans with obesity, the impact of this increased fat on induction of diabetes in humans is not well established (5). It has been reported that pancreatic fat is negatively associated with β-cell function in nondiabetic individuals but not in type 2 diabetes (20). However, there is no increase in β-cell apoptosis even in the presence of marked obesity when pancreatic fat is increased (5). Moreover, to our knowledge, there are no reports of increased pancreatic fat in humans with type 2 diabetes compared with nondiabetic individuals when matched for obesity. On the contrary, one large study reported no increase in pancreatic fat in humans with type 2 diabetes (5). As discussed by Unger and Zhou (4), lipotoxicity is based on the harmful partitioning of fat from the relatively safe storage in adipocytes compared with the relatively toxic accumulation of lipids within the tissue in question. It is plausible that fat accumulates in and around islets in humans with type 2 diabetes to a greater extent than in comparably obese nondiabetic humans, but this is yet to be established.
The remarkable preservation of β-cell mass in humans with advanced age despite marked loss of pancreatic acinar tissue noted in the present studies is consistent with one prior study in humans that reported a marginal decrease in β-cell mass with aging (no significant difference between the youngest and oldest age-group studied) (3). Here we extend those observations by including individuals to 100 years of age and evaluating β-cell turnover. There was no detectable change in β-cell apoptosis despite advanced age. Since it has also been shown that fat relative to acinar tissue accumulates in the pancreas with advanced age (5), apparent histologically in the present studies (Fig. 1), it is again notable that there was no increase in β-cell apoptosis despite advanced age.
With both obesity and advanced age, we report an increased β-cell nuclear diameter. Studies of the parathyroid gland have indicated a relationship between parathyroid hormone secretion and the mean nuclear diameter of parathyroid cells (21). The increase in mean β-cell nuclear diameter observed here with obesity is consistent with a prior report and an appreciation that daily insulin secretion is increased in obesity (13). Glucose-stimulated insulin secretion and insulin sensitivity decline with aging, with the result that fasting and postprandial glucose concentrations are increased (6). The net effect of these changes with aging in nondiabetic individuals is that insulin secretion is adaptively increased at the higher blood glucose levels (but not raised to levels considered diabetic) in both the fasting and fed state, with the result that β-cell workload is increased through the 24-h cycle consistent with the increased β-cell nuclear diameter with aging reported here. The extremely low frequency of β-cell apoptosis in humans across all age-groups is consistent with reports that in health β-cells are long lived (22). Here we extend this to include advanced old age.
As with all prior studies approaching the question of β-cell mass and turnover in humans, the current study has limitations. In the present studies, we have relied on population-based values obtained by CT scan for pancreas volumes rather than a direct measurement of pancreas weight mass in the individuals from whom the pancreas samples were obtained. We also assumed that 1 cm3 of pancreas weighs 1 g. A large number of subjects were studied to develop the population pancreas data, being specifically selected to encompass the age and BMI range of the current study subjects. Although the use of population pancreas volumes is clearly a limitation, given the retroperitoneal and locally adherent nature of the human pancreas, measuring the weight of pancreas resected at autopsy is not without error. Autopsy studies here are confined to the tail of the pancreas, and the changes in pancreas volume are calculated from population values ascertained in a large radiological study, an approach that was validated in nonhuman primates (7).
Another limitation of autopsy studies is that changes in β-cell mass or turnover may be present as a result of the final illness or postmortem. To minimize this, we sought to include cases where the course of the final illness was relatively short and the time between death and autopsy was no longer than 12 h. The very low frequency of TUNEL-positive β-cells provides reassurance that the quality of the pancreas included was not only well preserved in appearance but also had undergone minimal postmortem autolysis. In pancreas procured 24 h after death, we have noted a high frequency of TUNEL in all cell types, consistent with postmortem necrosis. The present studies did not include an obese elderly group. This was partly by design since the planned hypotheses required comparison between obese and lean individuals (age matched), and young and elderly individuals (BMI matched). The lack of an obese elderly group was also a practical issue as there are relatively few elderly obese individuals in the Mayo autopsy registry, presumably reflecting the impact of obesity on life expectancy.
By definition, autopsy studies are cross-sectional, introducing the possibility of confounding variables. For example, individuals who live to advanced old age are by definition a selected group compared with those who die young. Ideally, a cohort of individuals would be studied throughout life to establish changes in β-cell mass with aging. This is currently not possible since it is still not technically feasible to measure β-cell mass in vivo, and if it was possible, the longitudinal study to address the effects of age on pancreas shown here would take 100 years to complete. Moreover that approach would still not permit evaluation of β-cell turnover.
In conclusion, the mass and number of β-cells is increased in humans with obesity. Neither the timing of that increase nor its origins are known. The number and mass of β-cells are relatively well preserved compared with the exocrine pancreas in humans despite advanced age. Neither obesity nor advanced age in humans is characterized by increased β-cell apoptosis.
Acknowledgments
This study was supported by funding from the National Institutes of Health (DK-059579 and DK-077967), the Larry L. Hillblom Foundation, and the Manpei Suzuki Diabetes Foundation.
No potential conflicts of interest relevant to this article were reported.
Y.S. undertook the study, performed morphometric analysis, and helped write the manuscript. A.E.B. performed and supervised the morphometric analysis. E.M. participated in data analysis. D.E. performed statistical analysis of the data. R.A.R. obtained the pancreas specimen and supervised the abstracting of clinical data from the Mayo medical records. P.C.B. designed the study, participated in data analysis, and wrote the manuscript. P.C.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to colleagues in the Larry L. Hillblom Islet Research Center for their excellent suggestions. The authors acknowledge Inderroop Singh and David Kirakossian (UCLA David Geffen School of Medicine) for their assistance and Bonnie Lui (UCLA David Geffen School of Medicine) for her administrative assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0421/-/DC1.
See accompanying commentary, p. 4.
References
- 1.Wareham NJ. Epidemiology of type 2 diabetes. Endocrinol Nutr 2009;56(Suppl. 4):60–62 [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 3.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 4.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001;50(Suppl. 1):S118–S121 [DOI] [PubMed] [Google Scholar]
- 5.Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 2007;20:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003;52:1738–1748 [DOI] [PubMed] [Google Scholar]
- 7.Saisho Y, Manesso E, Butler AE, et al. Ongoing beta-cell turnover in adult nonhuman primates is not adaptively increased in streptozotocin-induced diabetes. Diabetes 2011;60:848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 9.Scheen AJ. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab 2005;31:Spec No 2:5S27–5S34 [DOI] [PubMed]
- 10.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab 2003;284:E7–E12 [DOI] [PubMed] [Google Scholar]
- 11.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 2007;50:2323–2331 [DOI] [PubMed] [Google Scholar]
- 12.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005;48:2221–2228 [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 2006;29:1554–1559 [DOI] [PubMed] [Google Scholar]
- 14.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogilvie RF. The islands of Langerhans in 19 cases of obesity. J Path Bact 1933;37:473–481 [Google Scholar]
- 16.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985;4:110–125 [DOI] [PubMed] [Google Scholar]
- 17.Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003;88:2300–2308 [DOI] [PubMed] [Google Scholar]
- 18.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010;53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pick A, Clark J, Kubstrup C, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998;47:358–364 [DOI] [PubMed] [Google Scholar]
- 20.Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007;30:2916–2921 [DOI] [PubMed] [Google Scholar]
- 21.Lloyd HM, Jacobi JM, Willgoss DA. DNA synthesis by pituitary tumours, with reference to plasma hormone levels and to effects of bromocriptine. Clin Endocrinol (Oxf) 1995;43:79–85 [DOI] [PubMed] [Google Scholar]
- 22.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 2010;53:321–330 [DOI] [PubMed] [Google Scholar]