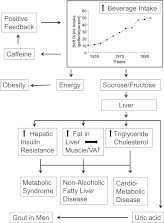
Fructose is a sweet tasting sugar that is found naturally in fruits and some vegetables and has been part of the human diet—in modest amounts—for eons. The increasing consumption of sugar has dramatically increased our exposure to fructose (1). Sugar consumption has risen more than 40-fold since the Declaration of Independence was signed 250 years ago, and more than 40% of the added sugars in our diet are in sugar-sweetened beverages and fruit drinks (2,3). Thus, the principal sources of fructose in our diet are now sugar and high-fructose corn syrup, each of which has about 50% fructose. The intake of soft drinks has risen fivefold since 1950 (4,5) (Fig.1) and with it the intake of fructose. The rise in the consumption of high-fructose corn syrup in beverages has paralleled the rise in the prevalence of obesity and the metabolic syndrome and is associated with the appearance of nonalcoholic fatty liver disease (6–8). Although association does not prove causation, it has stimulated research to understand whether current levels of fructose intake in beverages pose a health risk.
Figure 1.
Model showing some potential consequences of increasing fructose and energy intake from sugar or high-fructose corn syrup in beverages. VAT, visceral adipose tissue.
Background
Over the past decade fructose from either sucrose or high-fructose corn syrup has received growing attention as it has been associated with a widening group of health-related problems. Several meta-analyses have shown a relationship between the consumption of sugar-sweetened soft drinks and obesity (9–11). The relation of these beverages to obesity can be attributed to the increased caloric intake and to the fact that beverages do not suppress the intake of other foods to an appropriate degree—thus beverage calories serve as “add-on” calories enhancing the risk of obesity (12) (Fig. 1). Meta-analyses have also suggested that the consumption of sugar-sweetened beverages is related to the risk of diabetes, the metabolic syndrome, and cardiovascular disease (13).
Several short-term clinical trials have provided insights into the metabolic consequences of ingesting sugar-sweetened beverages. In one study there was an increase in body weight, blood pressure, and inflammatory markers (14,15), and in a second study there was an increase in triglycerides levels (particularly at night), a stimulation of de novo lipogenesis, and an increase in visceral fat (16,17). In the third study, which compared milk, diet cola, a sugar-sweetened cola, and water, the sugar-sweetened beverage increased liver fat, visceral fat, and triglycerides over the 6 months of beverage intake (18). The latter study suggests that consuming two 16-ounce sugar-containing beverages per day for 6 months can mimic many of the features of the metabolic syndrome and nonalcoholic fatty liver disease.
Brief overview
The article by Aeberli et al. (19) in this issue of Diabetes Care and their previous study (20) have added important data on the responses to fructose. They conducted a 4-week randomized crossover study with a 4-week wash-out between each diet in 9 healthy young men comparing 4 different soft drinks with levels of fructose, glucose, and sucrose that are closer to “normal” intake than some other studies. The low-fructose beverage had 40 g per day of fructose, which was the same amount of fructose as in the 80 g per day sucrose beverage (40 g). This is less fructose than is contained in two 16-ounce sugar-sweetened soft drinks with 10% sugar. There was also a high-glucose beverage (80 g per day), which is twice what was in the sucrose beverage, and an 80 g per day fructose beverage, which is also twice the amount in the sucrose and low-fructose beverages. With the hyperinsulinemic-euglycemic clamp, the authors examined insulin sensitivity of the liver and the whole body. Compared with the high-glucose beverage, the low-fructose beverage impaired hepatic insulin sensitivity, but not whole-body insulin sensitivity, pointing again to the pathophysiological effects that fructose can have on the liver. In addition, they found that total and LDL cholesterol were increased by fructose relative to glucose and that free fatty acids were increased or showed a trend toward an increase in the fructose beverage groups.
This article has several strengths, one of which is that it is a randomized crossover comparison of four beverages with two levels of fructose, glucose, and sucrose (50% fructose). Another strength is that the study used modest amounts of fructose and had a glucose control. One limitation is that it had only a small number of subjects and that they were all male, so we cannot be absolutely sure that these results extrapolate to females.
The authors did not find any effect on fasting triglycerides. However, they did not design the study to look at postprandial or nocturnal levels of triglycerides where they might have detected differences. In the comparison of the effect of glucose, fructose, and sucrose on plasma triglycerides, Cohen and Schall (21) found that both fructose in the amount found in sucrose AND sucrose increased triglycerides following a meal, but that glucose did not—leading them to conclude that the effects on lipids were due to the fructose either alone or as part of sucrose (table sugar), and not glucose.
This study adds to the information about the role of fructose either from sucrose (ordinary table sugar) or from high-fructose corn syrup in initiating liver dysfunction and possibly leading to nonalcoholic fatty liver disease and the metabolic syndrome, which have become increasingly prevalent. Figure 1 relates the findings from this study to those of other studies (13,16–18,22). The increasing intake of soft drinks (4,5) is viewed as the driver for the increase in energy and fructose, which may play a part in the development of obesity and the metabolic consequences depicted here (22). The caffeine present in these beverages is viewed as a positive feedback signal because of its ability to stimulate the central nervous system.
Two other meta-analyses of crystalline fructose added to the diet appeared to reach different conclusions. Livesey and Taylor (23) and Sievenpiper et al. (24) examined the effects of replacing carbohydrates in the diet with crystalline fructose. Both excluded high-fructose corn syrup and thus the beverage form of fructose, which seems to play the central role in the response to the fructose in beverages. Crystalline fructose added to the food supply represents only a few percent of the total “added sugars” and behaves differently from the fructose that is in beverages. The largest amount of dietary fructose comes from the fructose in sucrose or high-fructose corn syrup, both of which are the major components of calorie-sweetened beverages but were excluded from these meta-analyses.
One key question which Aeberli et al. begin to address is whether the detrimental effects of fructose are simply the result of a linear dose-response to our increasing dietary intake of fructose or whether there is a threshold below which fructose is without harm. The current data suggest that it is a “linear” response, and the reason we are now detecting the pathophysiological consequences of fructose is that its dietary load has continued to increase, largely as a consequence of increased soft drink and fruit drink consumption.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
See Aeberli et al., p. 150
References
- 1.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 2005;63:133–157 [DOI] [PubMed] [Google Scholar]
- 2.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 3.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003-2006. Crit Rev Food Sci Nutr 2010;50:228–258 [DOI] [PubMed] [Google Scholar]
- 4.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007;15:2739–2747 [DOI] [PubMed] [Google Scholar]
- 5.Putnam JJ, Allshouse JE. Food Consumption, Prices and Expenditure, 1970-1997 U.S. Department of Agriculture Food and Rural Economics Division, Economic Research Service Bulletin, No. 965, p. 34
- 6.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–543 [DOI] [PubMed] [Google Scholar]
- 7.Bray GA. How bad is fructose? Am J Clin Nutr 2007;86:895–896 [DOI] [PubMed] [Google Scholar]
- 8.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 2010;299:E685–E694 [DOI] [PubMed] [Google Scholar]
- 9.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 2007;97:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev 2009;10:68–75 [DOI] [PubMed] [Google Scholar]
- 11.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr 2009;89:438–439; author reply 439–440 [DOI] [PubMed] [Google Scholar]
- 12.Mourao DM, Bressan J, Campbell WW, Mattes RD. Effects of food form on appetite and energy intake in lean and obese young adults. Int J Obes (Lond) 2007;31:1688–1695 [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raben A, Vasilaras TH, Møller AC, Astrup AA. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–729 [DOI] [PubMed] [Google Scholar]
- 15.Sørensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr 2005;82:421–427 [DOI] [PubMed] [Google Scholar]
- 16.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanhope KL, Bremer AA, Medici V, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011;96:E1596–E1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–289 [DOI] [PubMed] [Google Scholar]
- 19.Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–485 [DOI] [PubMed] [Google Scholar]
- 21.Cohen JC, Schall R. Reassessing the effects of simple carbohydrates on the serum triglyceride responses to fat meals. Am J Clin Nutr 1988;48:1031–1034 [DOI] [PubMed] [Google Scholar]
- 22.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010;303:1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 2008;88:1419–1437 [DOI] [PubMed] [Google Scholar]
- 24.Sievenpiper JL, de Souza RJ, Mirrahimi A, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012;156:291–304 [DOI] [PubMed] [Google Scholar]