Abstract
OBJECTIVE
To investigate associations between serum cathepsin S, impaired insulin sensitivity, defective insulin secretion, and diabetes risk in a community-based sample of elderly men without diabetes.
RESEARCH DESIGN AND METHODS
Serum cathepsin S, insulin sensitivity (euglycemic-hyperinsulinemic clamp), and insulin secretion (early insulin response during an oral glucose tolerance test) were measured in 905 participants of the Uppsala Longitudinal Study of Adult Men (mean age, 71 years). Thirty participants developed diabetes during 6 years of follow-up.
RESULTS
After adjustment for age, anthropometric variables, and inflammatory markers, higher cathepsin S was associated with decreased insulin sensitivity (regression coefficient per SD increase −0.09 [95% CI −0.14 to −0.04], P = 0.001), but no association with early insulin response was found. Moreover, higher cathepsin S was associated with a higher risk for developing diabetes (odds ratio per SD increase 1.48 [1.08–2.01], P = 0.01).
CONCLUSIONS
Cathepsin S activity appears to be involved in the early dysregulation of glucose and insulin metabolism.
Adipokines, inflammatory cytokines secreted from adiopose tissue, have been suggested to play a key role in the development of insulin resistance and diabetes (1). Cathepsin S is a potent cysteine protease that is highly expressed and secreted in adipose tissue of obese individuals (2) and has been suggested to be an important regulator of inflammatory activity (3). We thus hypothesized that cathepsin S levels would be involved in the early dysregulation of glucose and insulin metabolism before development of diabetes. Accordingly, we investigated the association between serum cathepsin S and the two major underlying causes of diabetes—impaired insulin sensitivity and impaired insulin secretion—in a community-based sample of elderly men without diabetes. In secondary analyses, we also investigated the longitudinal association between serum cathepsin S and the incidence of diabetes.
RESEARCH DESIGN AND METHODS
The design and selection criteria of the Uppsala Longitudinal Study of Adult Men (ULSAM) have been described previously (4), and further details can be found on the Internet (www.pubcare.uu.se/ULSAM/). The present analyses are based on the third examination cycle (baseline 1991–1995; n = 1,221, mean age 71 years) where 1,161 men were free from diabetes. Of these, 905 men had valid measurements of cathepsin S and covariates. Follow-up data on diabetes status at the fourth examination cycle (1998–2002) were available for 597 participants.
Venous blood samples were drawn at baseline and stored at –70°C until analysis. Serum levels of cathepin S was measured by enzyme-linked immunosorbant assay (human cathepsin S [Total], DY1183, R&D Systems) in frozen samples (mean freezer time 14.6 years [range 12.9–16.7]) (5). Serum levels of high-sensitivity C-reactive protein, interleukin (IL)-6, adiponectin, cystatin C, and triglycerides were performed as previously described (5). Diabetes was diagnosed as fasting plasma glucose ≥7.0 mmol/L (≥126 mg/dL) or use of oral hypoglycemic agents or insulin.
The euglycemic-hyperinsulinemic clamp technique according to DeFronzo (6) was used, with a slight modification to suppress hepatic glucose production (7), for estimation of in vivo sensitivity to insulin. The glucose infusion rate during the last hour (M value) was used as the measure of insulin sensitivity. An oral glucose tolerance test (OGTT) was performed, and β-cell function was estimated by the early insulin response: [(insulin30min − insulin0min)/(glucose30min − glucose0min)].
Statistical analysis
Linear regression analyses were used in separate multivariable models to assess cross-sectional associations between cathepsin S (independent variable) and insulin sensitivity (dependent variable) or insulin secretion (dependent variable) (Table 1). Logistic regression was used to investigate the longitudinal association between cathepsin S and the development of diabetes.
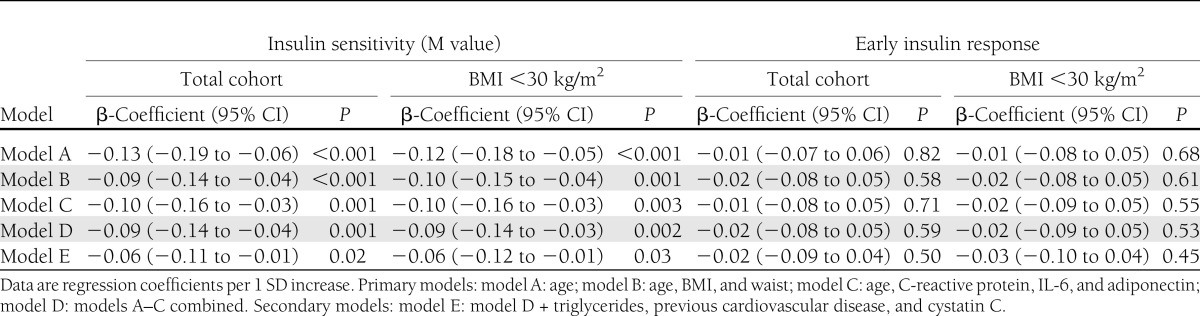
Table 1.
Cross-sectional associations between cathepsin S, insulin sensitivity, and early insulin response (n = 905)
RESULTS
Baseline characteristics of the study population are presented in Supplementary Table 1. Higher serum cathepsin S was significantly associated with decreased insulin sensitivity (glucose disposal rate, M) in all multivariable (models A–E, Table 1), but no association was found between cathepsin S and early insulin response. The results were similar in participants with BMI <30 kg/m2 (Table 1).
At the follow-up after 6 years (median follow-up 6.5 years [range 4.5–9.2]), 41 participants had developed diabetes. One SD increase in cathepsin S at baseline was associated with a 41–48% risk of developing diabetes in all multivariable models (model D: odds ratio per SD increase 1.48 [95% CI 1.08–2.01], P = 0.01, Supplementary Table 2).
CONCLUSIONS
Our study suggests that increased cathepsin S levels are involved in the early dysregulation of glucose and insulin metabolism, before the development of diabetes.
Comparison with the literature
Previous data on the association between circulating cathepsin S and the underlying causes of diabetes are scarce. A small study in women observed no associations between serum cathepsin S and insulin sensitivity, as evaluated by the quantitative insulin-sensitivity check index (QUICKI) (8). However, QUICKI results have limitations as an indicator of insulin sensitivity (9) which may explain the discrepancy with the current study. One study reported increased cathepsin S levels in patients with type 2 diabetes (10). However, the longitudinal association of cathepsin S and diabetes incidence has not been reported previously.
Potential mechanisms
Our understanding of the importance of adipose tissue-induced inflammation in the development of insulin resistance and diabetes is increasing (1). Cathepsin S may play a part in this process. Cathepsin S is released by macrophages (11) and participates in the pathophysiologic remodeling of extracellular matrix (12), which leads to adipogenesis and/or adipose cell hyperthrophy (13). This adipose tissue expansion may trigger hypoxia, which in turn results in local low-grade inflammation that has been suggested to be a causal link to insulin resistance (14). Also, cystatin C, the endogenous inhibitor of cathepsin S, has been found to be elevated in obese subjects, both in the circulation and in adipose tissue expression, independently of reduced estimated glomerular filtration rate, which could be a reflection of adipose tissue growth control through cathepsin inhibitions (13). The associations between cathepsin S and insulin sensitivity were independent of adiposity measures, inflammatory markers, and cystatin C in the current study, which would argue against adipose tissue–derived inflammation as the sole mechanistic explanation of our results. Still, we cannot rule out that there may be substantial residual confounding because the adiposity measurements and circulating inflammatory markers used in the current study may both be poor proxies for specific inflammation in adipose tissue. Cathepsin S has also been shown to be associated with triglyceridemia (8) and an increased cardiovascular risk (5), but these mechanisms did not appear to mediate the present associations (model E, Table 1).
Clinical implications
The development of selective inhibitors of cathepsin S is currently pursued by several pharmaceutical companies (15), but whether cathepsin S inhibitors improve insulin sensitivity or prevent diabetes remains to be established.
Limitations
Limitations of the study include the unknown generalizability to women and other age and ethnic groups, the large number of participants lost to follow-up, and the modest number of incident diabetes events during follow-up. Also, it is not possible to establish causality with a cross-sectional study design, and there is a risk of reverse causation. Further studies are needed for validation, for exploration of the underlying pathophysiology, and for evaluation of the clinical utility of measuring cathepsin S.
Acknowledgments
This study was supported by the Swedish Research Council (2006-6555), Swedish Heart-Lung Foundation, Thuréus Foundation, Dalarna University, and Uppsala University. The funding sources did not play any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
E.J. wrote the manuscript, researched data, and contributed to discussion. U.R., E.I., and J.S. reviewed the manuscript and contributed to discussion. M.J., E.N., D.I., S.B., and L.L. reviewed the manuscript. A.L. contributed data and reviewed the manuscript. J.Ä. researched data, edited the manuscript, contributed to discussion, and provided funding. J.Ä. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0494/-/DC1.
References
- 1.Li ZY, Wang P, Miao CY. Adipokines in inflammation, insulin resistance and cardiovascular disease. Clin Exp Pharmacol Physiol 2011;38:888–896 [DOI] [PubMed] [Google Scholar]
- 2.Taleb S, Lacasa D, Bastard JP, et al. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J 2005;19:1540–1542 [DOI] [PubMed] [Google Scholar]
- 3.Jobs E, Risérus U, Ingelsson E, et al. Serum cathepsin S is associated with serum C-reactive protein and interleukin-6 independently of obesity in elderly men. J Clin Endocrinol Metab 2010;95:4460–4464 [DOI] [PubMed] [Google Scholar]
- 4.Hedstrand H. A study of middle-aged men with particular reference to risk factors for cardiovascular disease. Ups J Med Sci Suppl 1975;19:1–61 [PubMed] [Google Scholar]
- 5.Jobs E, Ingelsson E, Risérus U, et al. Association between serum cathepsin S and mortality in older adults. JAMA 2011;306:1113–1121 [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 7.Pollare T, Vessby B, Lithell H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler Thrombol 1991;11:1192–1203 [DOI] [PubMed] [Google Scholar]
- 8.Naour N, Rouault C, Fellahi S, et al. Cathepsins in human obesity: changes in energy balance predominantly affect cathepsin S in adipose tissue and in circulation. J Clin Endocrinol Metab 2010;95:1861–1868 [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes 2010;1:36-47 [DOI] [PMC free article] [PubMed]
- 10.Liu J, Ma L, Yang J, et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis 2006;186:411–419 [DOI] [PubMed] [Google Scholar]
- 11.Shi GP, Munger JS, Meara JP, Rich DH, Chapman HA. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem 1992;267:7258–7262 [PubMed] [Google Scholar]
- 12.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A 1995;92:3849–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafarge JC, Naour N, Clément K, Guerre-Millo M. Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie 2010;92:1580–1586 [DOI] [PubMed] [Google Scholar]
- 14.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347–355 [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Singh RK, Dastidar S, Ray A. Cysteine cathepsin S as an immunomodulatory target: present and future trends. Expert Opin Ther Targets 2008;12:291–299 [DOI] [PubMed] [Google Scholar]