Abstract
OBJECTIVE
Ketosis-prone atypical diabetes (KPD) is a subtype of diabetes in which the pathophysiology is yet to be unraveled. The aim of this study was to characterize β- and α-cell functions in Africans with KPD during remission.
RESEARCH DESIGN AND METHODS
We characterized β- and α-cell functions in Africans with KPD during remission. The cohort comprised 15 sub-Saharan Africans who had been insulin-free for a median of 6 months. Patients in remission were in good glycemic control (near-normoglycemic) and compared with 15 nondiabetic control subjects matched for age, sex, ethnicity, and BMI. Plasma insulin, C-peptide, and glucagon concentrations were measured in response to oral and intravenous glucose and to combined intravenous arginine and glucose. Early insulin secretion was measured during a 75-g oral glucose tolerance test. Insulin secretion rate and glucagon were assessed in response to intravenous glucose ramping.
RESULTS
Early insulin secretion and maximal insulin secretion rate were lower in patients compared with control participants. In response to combined arginine and glucose stimulation, maximal insulin response was reduced. Glucagon suppression was also decreased in response to oral and intravenous glucose but not in response to arginine and insulin.
CONCLUSIONS
Patients with KPD in protracted near-normoglycemic remission have impaired insulin response to oral and intravenous glucose and to arginine, as well as impaired glucagon suppression. Our results suggest that β- and α-cell dysfunctions both contribute to the pathophysiology of KPD.
Ketosis-prone atypical diabetes (KPD) is a frequent specific subtype of diabetes in African Americans and sub-Saharan Africans (1–3). Patients with KPD present at onset with acute hyperglycemia and ketosis or ketoacidosis owing to an insulin secretory deficiency, but autoimmune markers against islet β-cells are absent (4–6). A prolonged insulin-free near-normoglycemic remission phase frequently follows the acute phase after insulin treatment and is associated with a significant recovery of the insulin secretory function (4,7,8). These observations have suggested that the blunting in insulin secretion at disease onset may be due to a functional disorder of β-cells rather than to cell destruction. We previously hypothesized that KPD is a subtype of type 2 diabetes with acute onset at diagnosis as the result of an environmental triggering factor, such as a viral infection, that severely impairs glucose-stimulated insulin secretion and favors ketogenesis (9). KPD patients display insulin resistance at the level of muscles, liver, and adipose tissue during remission (10). However, maximal insulin secretory capacity and surrogates of α-cell mass have not been evaluated, and whether α-cell dysfunction also contributes to the pathophysiology of KPD, as described in type 2 diabetes (11–14) is not known.
In the current study, we therefore measured early insulin secretion in response to oral glucose, dose-response insulin secretion to intravenous glucose, and maximum secretory response to arginine combined with glucose (glucose potentiation of arginine-induced insulin secretion) in Africans with KPD during near-normoglycemic remission compared with control subjects of the same ethnic background. Function of α-cells was assessed by measuring glucagon in response to glucose, insulin, and arginine.
RESEARCH DESIGN AND METHODS
Subjects
The study was undertaken at the clinical investigation center of Saint-Louis University Hospital, Paris, France. We studied 15 subjects of sub-Saharan African origin with KPD, who were in insulin-free remission, and 15 healthy control subjects from the same geographic origin with normal glucose tolerance. All participants were born in Africa, all four grandparents were from West or Central Africa, and participants had migrated to France as adults. KPD was defined as new-onset diabetes without precipitating illness (infection, stress), with the presence of strong ketosis (urine ketones >80 mg/dL) or diabetic ketoacidosis, and in the absence of islet cell and GAD 65 antibodies, as previously described (7). All patients had been diagnosed, had received insulin treatment at diagnosis, and were followed up in the Department of Diabetes and Endocrinology of our hospital. Insulin-free remission was defined as maintenance of an HbA1c level ≤7.0% at least 3 months apart after withdrawal of exogenous insulin treatment. Healthy control subjects were recruited by advertisement, were matched to patients for age, sex, and BMI, and were free of known family history of type 2 diabetes in first-degree relatives.
Participants were eligible if they had normal results on the physical examination and routine laboratory tests. Diabetes Control and Complications Trial standardized HbA1c level was confirmed in patients during the screening visit. Percentage of fat, fat mass, and fat-free mass were measured by dual-energy X-ray absorptiometry (Hologic QDR-1000/W, Wilmington, MA). Patients taking oral antidiabetic drugs were asked to stop them at least 5 days before the procedures. All patients had fasting blood glucose levels below 8.2 mmol/L.
The study was approved by the Paris Saint-Louis ethics committee, and each participant gave a written informed consent to participate.
Metabolic assessments
Insulin and glucagon secretions were evaluated in response to oral glucose tolerance test (OGTT), intravenous glucose (glucose ramp), and combined intravenous glucose and arginine. In addition, glucagon secretion was assessed in response to insulin during a euglycemic hyperinsulinemic clamp.
OGTT.
A 75-g OGTT was performed over 120 min during the screening visit after a 12-h overnight fast. Blood samples were collected from an antecubital vein before and 30 and 120 min after a 75-g oral glucose load for glucose, insulin, and glucagon determination. Normal glucose tolerance was confirmed in control subjects using the current American Diabetes Association criteria (15).
Euglycemic clamp.
A two-step euglycemic hyperinsulinemic clamp was performed within the week after the screening visit, as previously described (10). In brief, it consisted of a first step at 10 mU of insulin infusion per meter square body surface per minute for 100 min (low-dose insulin infusion), followed by a primed 100-min step at 80 mU ⋅ m−2 ⋅ min−1 insulin infusion (high-dose insulin infusion). Endogenous glucose production was also measured during the whole procedure using deuterated glucose. During the test, blood glucose was clamped at 5.5 mmol, thus variation in glucagon level was independent of glucose levels and reflected the effect of plasma insulin.
Glucose ramp.
The day after the clamp study, after a 12-h in-hospital overnight fast, the graded glucose infusion (glucose ramp) was performed. The test consisted of five consecutive 40-min intravenous infusion periods of 20% glucose at 2, 4, 6, 8, and 10 mg/kg of body weight per min, as previously described (16,17). Arterialized blood samples were obtained −10 and 0 min before, and every 10 min during the whole procedure (200 min).
Arginine test.
At the end of the glucose ramp, glucose infusion rate was increased, if needed, to obtain a plasma glucose level of 22 ± 2 mmol/L. Glucose infusion was then maintained at the same rate, and an intravenous bolus of arginine chlorhydrate (5 g) was administered in 45 s, with venous blood sample collection at baseline and 2, 3, 4, 5, 10, and 15 min after. This test was primarily aimed at evaluating the maximum β-cell insulin secretory capacity, but glucagon levels were also measured.
Analytic measures
All assays were run in duplicate. Plasma insulin was measured using immunoradiometric assays (BI-INSULIN IRMA, Cis Bio-International, Gif-Sur-Yvette, France) with a detection limit of 0.2 mU/L and an intra-assay and interassay coefficient of variation (CV) of less than 9.5%. C-peptide was measured by immunoradiometric assay (IRMA-C-PEP, CIS International) with intra-assay CV of 3.7–6.6% and interassay CV of 4.4–8.0%. Samples for glucagon assay were collected in aprotinin-EDTA tubes and were immediately centrifuged and stored at −80°C until biochemical analysis. Plasma glucagon was measured by radioimmunoassay (Adaltis, Casalecchio Di Reno, Italy) with a detection limit of 14.5 pg/mL, an intra-assay CV of 9.5%, and an interassay CV of 9%. Plasma glucose was measured by the hexokinase method (Roche Diagnostics GmbH, Mannheim, Germany). All other biochemical tests were done using routine laboratory methods.
Calculations
OGTT-derived indices.
The insulinogenic index (Δinsulin0–30/Δglucose0–30) was used to estimate early insulin secretion during OGTT. We proposed the product glucagon × glucose (10−3 pg ⋅ mmol ⋅ mL−2) to estimate the resistance to suppression of glucagon secretion by glucose (α-cell resistance index) at baseline and at 120 min after glucose load during OGTT.
Glucose ramp.
Insulin secretion in response to glucose ramping was assessed from the changes in C-peptide concentrations and the prehepatic insulin secretion rate (ISR). We derived the ISR by deconvolution, assuming a two-compartmental model of C-peptide clearance kinetic using Insulin SECretion (ISEC) 3.4a software. Individual kinetic variables of C-peptide clearance were calculated from standard kinetic parameters, taking into account age, sex, body surface area, and glucose tolerance status (18). Mean insulin secretion rate for every time period was plotted against the corresponding mean glucose concentration, thereby establishing a dose–response relationship between plasma glucose and the secretion rate for each participant. The dose–response effect of glucagon during the glucose ramp was estimated by comparing the slope of the glucagon levels as a function of the glucose levels.
Arginine test.
The incremental area under the curve (AUCI) of insulin and glucagon levels during the first 5 min (AUCI-5) or the whole 15 min (AUCI-15) of the arginine test was calculated using the trapezoidal rule (19). We also determined the acute insulin response (AIR) and glucagon responses to arginine as the mean of the 2-, 3-, 4-, and 5-min plasma level of each minus the basal (prearginine) plasma concentration (20).
Glucose clamp.
Glucose utilization (M values, as previously published [10]) were calculated according to DeFronzo’s method (21) and served for the adjustment of insulin secretion values in the current study. Three blood samples were taken 10-min apart during the last 20 min of the low-dose and high-dose insulin infusion steps of the glucose clamp, and the steady-state plasma insulin (SSPI1 and SSPI2) and glucagon (SSPG1 and SSPG2) concentrations were calculated as the average of the three values for each step. To assess insulin-mediated glucagon suppression, the percentage suppression of glucagon at low- and high-dose insulin infusions steps was calculated as [100 × (basal glucagon − SSPG1)/basal glucagon], and [100 × (SSPG1 – SSPG2)/SSPG1], respectively. Glucagon suppression was also divided by the SSPIs to adjust for plasma insulin levels.
Statistical analysis
Results are presented as percentage and mean ± SEM and other appropriate measures of central tendency and dispersion. Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, IL) and R 2.6.2 (The R Foundation for Statistical Computing, Vienna, Austria) software. We used the Fisher exact test to compare categoric variables and the nonparametric Mann-Whitney U test or Wilcoxon rank sum test for quantitative variables. The product glucagon × glucose was compared between KPD patients and control subjects at baseline using ANOVA and at 120 min using ANCOVA adjusted for baseline values of the product and plasma insulin at 120 min. The relationship between ISR or glucagon and glucose levels during glucose ramp was analyzed using mixed-model ANCOVA. Fixed effects in the model included BMI, a linear and a quadratic effect for glucose levels, a group effect (KPD vs. control), and their interactions. Random subject effects (intercept and slopes) were added to the models. In response to arginine, adjustment for prestimulus insulin and glucose levels was done. All tests were two-sided, and the level of significance was set at P < 0.05.
RESULTS
Participant characteristics
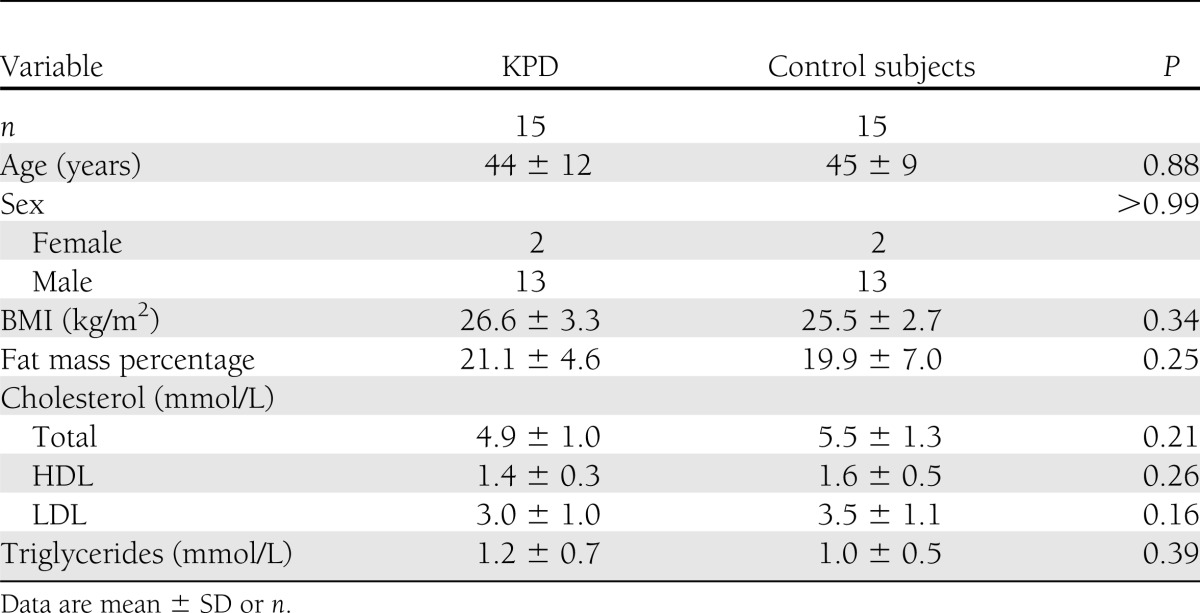
Diabetes (KPD) duration was a median 24 months (range 6–72 months). At inclusion, patients had been in insulin-free near-normoglycemic remission for a median of 6 months (range 3–45 months). The mean ± SD HbA1c level was 6.2% ± 0.7%. Antidiabetic treatment before inclusion in the study consisted of metformin in most patients, alone (n = 8) or combined with a sulfonylurea (n = 2). Two patients were being treated with diet alone, one was taking a sulfonylurea alone, one was taking a glinide, and one was taking acarbose. No patient had been taking a glitazone. There was no significant between-group difference regarding other clinical and biochemical parameters, including BMI, percentage of fat mass, and lipid profile (Table 1). During OGTT, three patients had the profile of impaired glucose tolerance, and the others fulfilled the criteria of diabetes, despite fasting plasma glucose level ≤7.0 mmol/L in 10 patients.
Table 1.
Clinical and biochemical characteristics of participants
Insulin and glucagon secretion during OGTT
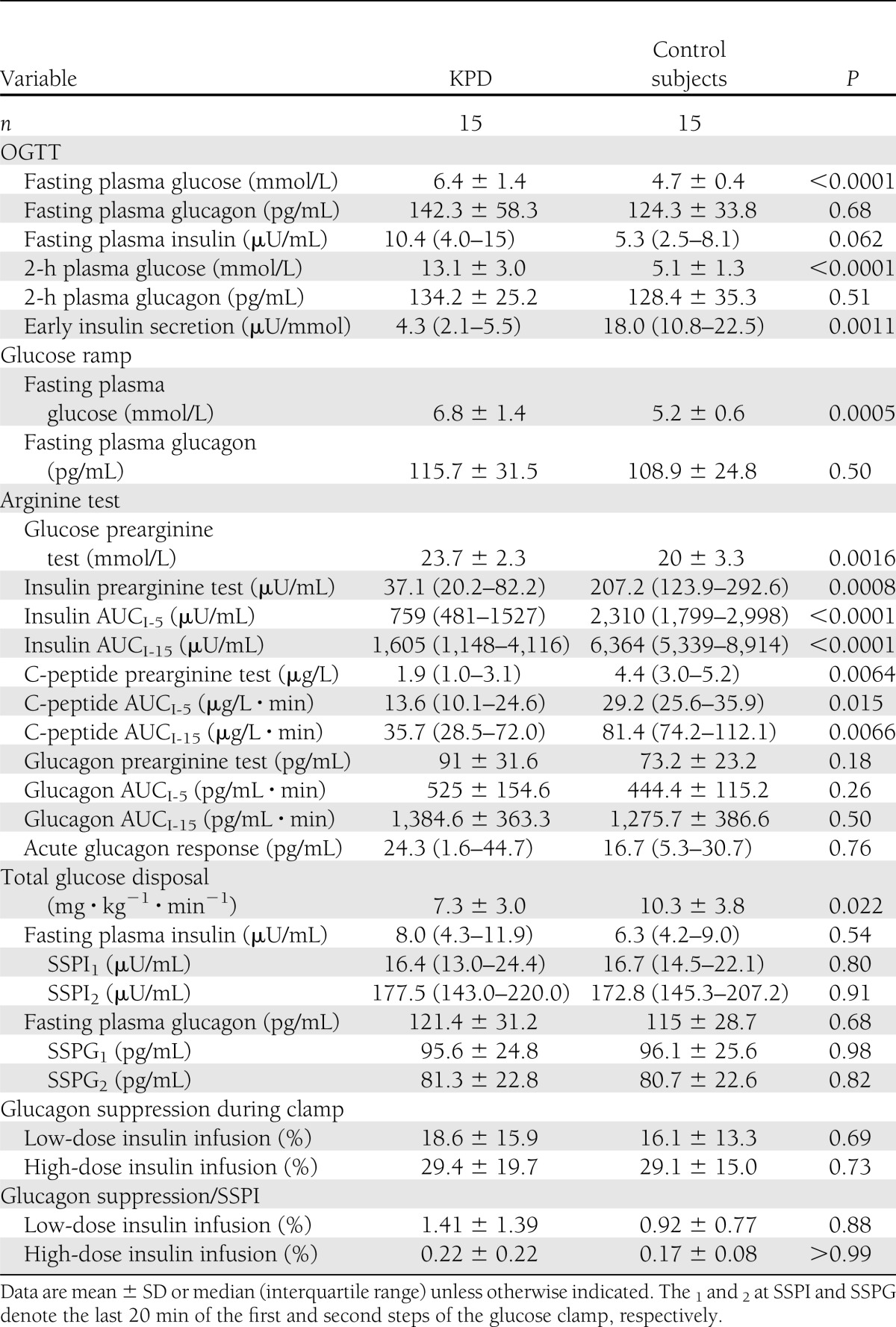
Early insulin secretion, as measured by insulinogenic index, was significantly lower in patients (P < 0.001). Mean fasting and 2-h plasma glucose concentrations were both significantly higher in patients compared with control subjects (Table 2).
Table 2.
Metabolic characteristics and parameters of β- and α-cell function
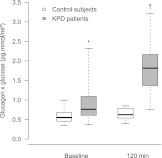
Plasma glucagon levels were similar in patients and controls at fasting or 2 h after the glucose load (Table 2), despite higher glucose levels in patients. As a consequence, the product glucagon × glucose (Fig. 1) was higher in patients at baseline (P = 0.03). The product was also significantly higher in patients at 120 min and remained so after adjustment for the baseline product and plasma 2-h insulin (P < 0.0001).
Figure 1.
Distribution of the product glucagon × glucose during OGTT at baseline and at 120 min in KPD patients (gray) and control subjects (white). The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box represent the first and third quartiles of the distribution; and the whiskers represent 1.5 times the interquartile range. *P = 0.03; †P < 0.0001 vs. control subjects.
ISR and glucagon suppression during intravenous glucose ramp
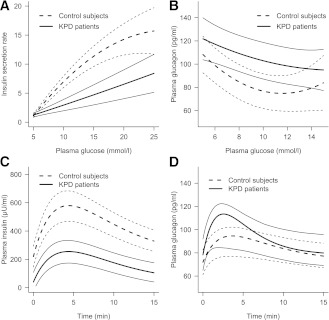
In response to the glucose ramp, the relationship between insulin and glucose was not linear (Fig. 2A). ISR rose sharply with increasing blood glucose levels in both groups. However, the slope was sharper in control subjects than in patients (P < 0.001), showing an impaired dose-response effect of glucose on β-cell in patients compared with control subjects. The maximum ISR reached with comparable blood glucose was as twice as low in patients than in control subjects.
Figure 2.
ISR (A) and glucagon secretion (B) during glucose ramp in KPD patients (continuous lines) and control subjects (dashed lines). Variations of insulin (C) and glucagon (D) levels in response to arginine in KPD patients (continuous lines) and control subjects (dashed lines). Thick dark lines are average model prediction; thin gray lines are 95% CI.
As glucose levels increased progressively during glucose ramp, plasma glucagon concentrations decreased in the two groups. The relationship between glucagon and glucose was not linear (Fig. 2B). The global trend of the curves was significantly different between the two groups, even when taking into account the achieved plasma insulin concentrations (likelihood ratio test P = 0.04), showing a hyposuppressibility of glucagon by the increasing glucose levels in patients compared with control subjects.
Insulin and glucagon secretion in response to arginine
During arginine stimulation, insulin concentrations increased in both groups during the first three assessments to reach a maximum at 3 min (patients) or 4 min (control subjects) and declined thereafter (Fig. 2C). Patients had significantly lower insulin secretion, at least a 50% reduction compared with control subjects, whatever the parameter used to measure it: insulin AUCs, C-peptide AUCs, or AIR (Table 2).
Glucagon profile followed a similar pattern (Fig. 2D). Although the peak of plasma glucagon seemed higher in KPD patients than in control subjects, the difference between the two curves was not significant (P = 0.074). Also, no significant difference was found in the incremental AUCs and the acute glucagon response between the two groups, even after adjustment for prestimulus insulin and glucose concentrations (Table 2).
Glucagon suppression during euglycemic hyperinsulinemic clamp
The mean fasting (FPI) and steady state plasma insulin (SSPI1 and SSPI2) concentrations were similar between the two groups (Table 2). Plasma glucagon declined significantly and similarly in the two groups from baseline (FPG clamp) to the end of the low-dose (SSPG1) and high-dose (SSPG2) steps. Glucagon levels, percent glucagon suppression, and percent glucagon suppression-to-SSPI ratio did not differ between patients and control subjects (Table 2).
CONCLUSIONS
The major findings of our study are that during protracted insulin-free near-normoglycemic remission, patients with KPD have a reduced insulin secretory response to oral and intravenous glucose and a reduced glucose potentiation of arginine-induced insulin secretion compared with matched nondiabetic control subjects. In addition, we show that the glucagon level is higher at baseline in patients when taking into account blood glucose concentrations and is less suppressed by oral and intravenous glucose compared with control subjects.
Previous studies have shown severe insulin secretory deficiency during the acute ketotic phase (4,7). This deficiency was characterized by a loss of acute-phase insulin secretion in response to intravenous glucose (4) or a decrease in C-peptide response to glucagon (4,7). They also showed that the clinical remission phase paralleled a restoration in insulin secretion in response to the same stimuli, after achievement of good metabolic control, but remained lower than that in healthy controls (4,7,8). Almost all of those studies used C-peptide response to intravenous glucagon or insulin response to an intravenous glucose tolerance test as indicators. Here, we confirm those observations using the early insulin secretion in response to oral glucose. Furthermore, using an intravenous glucose ramp, we show that at similar glucose levels, insulin secretion in KPD patients is lower (approximately half that of nondiabetic subjects) and progresses more slowly, indicating that KPD subjects display a defect in β-cell sensitivity to glucose.
In our study, we used a combined arginine and glucose stimulus as an indicator of the maximal insulin secretion capacity, as proposed earlier (22). We show that KPD is associated with a large decrease in the maximal ISR based on the AIR to arginine and also on the incremental AUC. Whether this defect is related to a decrease in β-cell mass, as suggested in dogs (23) or in humans after islet autotransplantation (24) or after hemipancreatectomy (25), is still questioned. Recently, Umpierrez and colleagues (26) investigated African Americans with newly diagnosed KPD soon after the occurrence of insulin-induced near-normoglycemia. They found a similar glucose potentiation of arginine-induced insulin secretion compared with nonketotic type 2 diabetes and nondiabetic obese control subjects. Their findings support a functional insulin secretory defect rather than a reduced β-cell mass at diabetes onset. However, the maximum glucose level at which they performed arginine stimulation was only modest (∼9.6 mmol/L), whereas arginine stimulation in our study was at glucose levels of up to 24 mmol/L. Such levels have been reported to elicit maximal responsiveness to arginine (22) and might then give a better idea of β-cell mass. Consequently, our results suggest that KPD is associated with reduced β-cell mass in addition to decreased β-cell sensitivity to glucose as in classical type 2 diabetes.
We report that KPD is associated with basal hyperglucagonemia when taking into account glucose levels. To illustrate the inappropriate glucagon concentration with respect to glucose levels, we proposed an index (glucagon × glucose) similar to the homeostasis model assessment concept, which is higher in KPD, suggesting exaggerated glucagon secretions. The altered glucagon suppression in response to glucose observed in our KPD patients has previously been shown in patients with type 1 or type 2 diabetes (27,28). Hyperglucagonemia is well recognized as a metabolic feature of type 2 diabetes, even in well-controlled subjects, (27) suggesting that it participates in the pathophysiology of the disease (review in 29,30). Hyperglucagonemia may result from the β-cell dysfunction because insulin exerts a paracrine suppressive effect on α-cells (review in 31). In 2002, Banerji et al. (32) also demonstrated decreased glucagon suppression in response to meal testing in type 2 diabetic children and adolescents, of whom 40% had had diabetic ketoacidosis at onset. Here, we observed no significant difference between KPD patients and control subjects regarding glucagon secretion in response to arginine. This contrasts with previous reports in type 2 diabetes where significantly exaggerated glucagon secretion in response to arginine was described, whatever the glucose level at which the arginine test was performed (20,22,28). The very good metabolic control of our patients (mean HbA1c at 6.2% and fasting plasma glucose levels at 6.2 mmol/L) may explain this discrepancy.
Similarly to observations by Abdul-Ghani et al. (33) in well-controlled type 2 diabetic patients, we do not find a difference in glucagon suppression by insulin in KPD patients compared with control subjects. This suggests that islet α-cell response to insulin is preserved.
The small number of subjects and the lack of a non-KPD type 2 diabetes control group should be acknowledged as potential limitations. Although the patients were in good glycemic control, most remained with abnormal glucose tolerance. Therefore, we are not able to discern whether the insulin secretory defect is a consequence of the glucose intolerance state at the time of the study or contributes to the onset of the disease.
In conclusion, patients with KPD in protracted near-normoglycemic remission display a defect in β- and α-cell sensitivity to glucose and a decrease in glucose potentiation of arginine-induced insulin secretion. Thus, our findings support the use of therapeutic agents targeting both β- and α-cell dysfunctions in this form of diabetes.
Acknowledgments
This work was funded by an institutional grant (PHRC02 088) from Assistance Publique–Hôpitaux de Paris, and by grants from the French Diabetes Association and the Francophone Diabetes Society, all nonprofit organizations.
No potential conflicts of interest relevant to this article were reported.
S.-P.C., E.S., and J.-F.G. designed the study, recruited the volunteers, undertook the metabolic assessment, contributed to the analysis of data, and wrote the first draft of the manuscript. P.B. and F.I. contributed to the design of the study, carried out all the hormonal assays, and reviewed the manuscript. L.-S.F., P.V., and F.C. contributed to the design of the study and the clinical assessment and reviewed the manuscript. R.P. carried out the statistical analyses and reviewed the manuscript. B.B. and F.M.-J. contributed to the interpretation of data, writing of reports, and reviewed the manuscript. J.-F.G. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the participants, the nurse staff of the Clinical Investigation Center, and the technical staff of the Hormones Laboratory at Saint-Louis Hospital for their dedication. They also thank Professor Pascal Ferré from Cordeliers Research Center (INSERM UMRS 872), Paris, France, for his comments on the manuscript. ISEC software was provided free of charge to the authors by Roman Hovorka (Diabetes Modelling Group, Department of Paediatrics, University of Cambridge, Cambridge, U.K.).
Footnotes
See accompanying commentary, p. 8.
References
- 1.Sobngwi E, Mauvais-Jarvis F, Vexiau P, Mbanya JC, Gautier JF. Diabetes in Africans. Part 2: Ketosis-prone atypical diabetes mellitus. Diabetes Metab 2002;28:5–12 [PubMed] [Google Scholar]
- 2.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med 2006;144:350–357 [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 2008;29:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes 1995;44:790–795 [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs type 2 diabetes and the effect of ethnicity. Arch Intern Med 1999;159:2317–2322 [DOI] [PubMed] [Google Scholar]
- 6.Sobngwi E, Vexiau P, Levy V, et al. Metabolic and immunogenetic prediction of long-term insulin remission in African patients with atypical diabetes. Diabet Med 2002;19:832–835 [DOI] [PubMed] [Google Scholar]
- 7.Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes 2004;53:645–653 [DOI] [PubMed] [Google Scholar]
- 8.Maldonado MR, Otiniano ME, Cheema F, Rodriguez L, Balasubramanyam A. Factors associated with insulin discontinuation in subjects with ketosis-prone diabetes but preserved beta-cell function. Diabet Med 2005;22:1744–1750 [DOI] [PubMed] [Google Scholar]
- 9.Sobngwi E, Choukem SP, Agbalika F, et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-Saharan Africans. JAMA 2008;299:2770–2776 [DOI] [PubMed] [Google Scholar]
- 10.Choukem SP, Sobngwi E, Fetita LS, et al. Multitissue insulin resistance despite near-normoglycemic remission in Africans with ketosis-prone diabetes. Diabetes Care 2008;31:2332–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–4058 [DOI] [PubMed] [Google Scholar]
- 12.Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med 1998;15:290–296 [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1987;64:106–110 [DOI] [PubMed] [Google Scholar]
- 14.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2000;85:4053–4059 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes—2007. Diabetes Care 2007;30(Suppl. 1):S4–S41 [DOI] [PubMed] [Google Scholar]
- 16.Gautier JF, Wilson C, Weyer C, et al. Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 2001;50:1828–1833 [DOI] [PubMed] [Google Scholar]
- 17.Sobngwi E, Boudou P, Mauvais-Jarvis F, et al. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 2003;361:1861–1865 [DOI] [PubMed] [Google Scholar]
- 18.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed 1996;50:253–264 [DOI] [PubMed] [Google Scholar]
- 19.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003;28:916–931 [DOI] [PubMed] [Google Scholar]
- 20.Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772–777 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 22.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward WK, Wallum BJ, Beard JC, Taborsky GJ, Jr, Porte D., Jr Reduction of glycemic potentiation. Sensitive indicator of beta-cell loss in partially pancreatectomized dogs. Diabetes 1988;37:723–729 [DOI] [PubMed] [Google Scholar]
- 24.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes 1998;47:324–330 [DOI] [PubMed] [Google Scholar]
- 25.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest 1992;89:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosmanov AR, Smiley D, Robalino G, et al. Effects of intravenous glucose load on insulin secretion in patients with ketosis-prone diabetes during near-normoglycemia remission. Diabetes Care 2010;33:854–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsson A, Sundkvist G, Groop L, Tuomi T. Insulin and glucagon secretion in patients with slowly progressing autoimmune diabetes (LADA). J Clin Endocrinol Metab 2000;85:76–80 [DOI] [PubMed] [Google Scholar]
- 28.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 1970;49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 30.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A 2010;107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerji MA. Impaired beta-cell and alpha-cell function in African-American children with type 2 diabetes mellitus—“Flatbush diabetes”. J Pediatr Endocrinol Metab 2002;15(Suppl. 1):493–501 [PubMed] [Google Scholar]
- 33.Abdul-Ghani M, DeFronzo RA. Fasting hyperglycemia impairs glucose- but not insulin-mediated suppression of glucagon secretion. J Clin Endocrinol Metab 2007;92:1778–1784 [DOI] [PubMed] [Google Scholar]