Abstract
OBJECTIVE
There has been growing evidence that inflammatory markers play a role in the development of type 2 diabetes. We aimed to systematically review prospective studies on the associations of elevated levels of interleukin-6 (IL-6) and C-reactive protein (CRP) with increased risk of type 2 diabetes by conducting a meta-analysis.
RESEARCH DESIGN AND METHODS
A systematic search of the PubMed, EMBASE, ISI Web of Knowledge, and Cochrane Library databases up until 10 February 2012 was conducted to retrieve prospective studies matched to search terms. We used generalized least-squares trend estimation to assess dose-response relationships. The summary risk estimates were pooled using either fixed-effects or random-effects models to incorporate between-study variation.
RESULTS
The meta-analysis, including 10 prospective studies, with a total of 19,709 participants and 4,480 cases, detected a significant dose-response association of IL-6 levels with type 2 diabetes risk (relative risk [RR] 1.31 [95% CI 1.17–1.46]). For CRP, the meta-analysis involving 22 cohorts, with a total of 40,735 participants and 5,753 cases, showed that elevated CRP levels were significantly associated with increased risk of type 2 diabetes (1.26 [1.16–1.37]), with the absence of publication bias. Sensitivity and subgroup analyses further supported the associations.
CONCLUSIONS
This meta-analysis provides further evidence that elevated levels of IL-6 and CRP are significantly associated with increased risk of type 2 diabetes.
The rapid worldwide increase in the prevalence of type 2 diabetes has become a serious public health problem (1). Type 2 diabetes may be accompanied by long-term microvascular and macrovascular complications, which lead to both morbidity and mortality (2). In addition, as many as one-third of individuals with type 2 diabetes are undiagnosed. However, accumulating evidence shows that inflammation may play a crucial intermediary role in the pathogenesis of type 2 diabetes, thus relating diabetes to a number of commonly coexisting conditions thought to originate via inflammatory mechanisms (3). In this regard, more recent data suggest that interleukin-6 (IL-6) and C-reactive protein (CRP) are associated with type 2 diabetes (4–10). IL-6, a pleiotropic proinflammatory cytokine, is produced by a variety of cells, including activated leukocytes, endothelial cells, and adipocytes (11). CRP is an acute-phase plasma protein synthesized by the liver and has been shown to be a sensitive, systemic biomarker of inflammation (3). The stability of this protein during long-term frozen blood storage and the availability of inexpensive, precise, and standardized assays have assisted studies of CRP (12).
One potential implication of the many studies suggesting a relation between inflammation and diabetes is that inflammatory markers may be used to refine diabetes risk prediction and thus better target individuals for lifestyle interventions. However, the results reported on the association between IL-6 and diabetes risk have varied across studies (13–16). To date, no systematic review has been performed to evaluate the available evidence on the association of IL-6 levels with the risk of type 2 diabetes. Two previous meta-analyses evaluating the association of CRP and diabetes risk have yielded contradictory results. One previous meta-analysis (17) suggested that a positive association exists between CRP and diabetes risk. In contrast, another meta-analysis (18) concluded that CRP may not be an independent risk factor for the development of diabetes.
The objective of the current study was to estimate the magnitude of the relationships between IL-6 and CRP levels and the risk of type 2 diabetes in prospective studies and to quantify these relationships in a meta-analysis.
RESEARCH DESIGN AND METHODS
Search strategy
We conducted the present meta-analysis in accordance with the guidelines of the Meta-analysis of Observation Studies in Epidemiology Group (19). A systematic literature search was performed to identify all studies published before 10 February 2012 that investigated the association between inflammatory markers and the risk of type 2 diabetes. Electronic databases, including PubMed, EMBASE, ISI Web of Knowledge, and the Cochrane Library, were explored using a combination of the following terms: inflammation, inflammatory markers, inflammatory biomarkers, inflammatory mediators, inflammatory cytokines, C-reactive protein, CRP, interleukin 6, IL-6, type 2 diabetes, T2D, type 2 diabetes mellitus, T2DM, diabetes mellitus, DM, and diabetes. Studies written in all languages without any special restriction were included. We found additional articles through a manual search of the reference lists of prior meta-analyses and reviews.
Study selection
We included only prospective studies that reported original data relevant to measuring the increased risk for type 2 diabetes associated with elevated levels of IL-6 and CRP. A study was considered eligible for inclusion if the study design was a prospective cohort study, a case-cohort study, or a nested case-control study. In these studies, participants were excluded on the basis of having previously had diabetes recorded and a follow-up duration of <1 year. We did not select cross-sectional studies, literature reviews, studies on cell lines or animals, and studies of gestational diabetes or type 1 diabetes.
Data extraction
A standardized data collection form was used to extract the following information from the published article for each included article: first author’s name, publication year, sample size, study design, mean (SD) for IL-6 and CRP levels, geographic location of participants, mean age, race/ethnicity, duration of follow-up, proportion of women, outcome assessment, reported relative risks (RRs) or hazard ratios (HRs) of type 2 diabetes and the corresponding 95% CIs, statistical adjustment for the major confounding factors, and statistical methods used for the analysis. The full text and any supplementary materials were examined for data extraction. The bibliographic search and data extraction were performed independently by two authors (W.B. and J.L.), and any disagreements between the two authors were resolved by consensus with a third investigator (L.-G.L.).
For most original studies, IL-6 and CRP levels were classified by tertiles, quartiles, and quintiles. For each category, we extracted numbers of cases/noncases, median values, RRs, and 95% CIs. We also extracted the effect estimate that was most fully adjusted for potential confounders if studies reported several multivariable-adjusted RRs.
Study quality assessment
We assessed study quality and established a quality assessment scale according to the Newcastle-Ottawa quality assessment scale (Supplementary Table 1). Study quality was evaluated on the basis of selection of participants and study design, measurement of IL-6 and CRP levels, reliability of studies on outcome assessment, and comparability of studies on confounders. We assigned low-, moderate-, and high-quality labels to scores of 0–2, 3, and 4–5, respectively.
Statistical analysis
The multivariable-adjusted HR or odds ratios reported in the eligible studies were extracted and considered directly as RR in our analysis. RR was used to measure the relationship between IL-6 and CRP levels and the risk of type 2 diabetes. To estimate dose-response associations, we used generalized least-squares trend estimation (GLST) analysis on the basis of the methods developed by Greenland and Longnecker (20,21) according to categories of IL-6 and CRP levels on median dose, number of participants and cases, and effect estimates with corresponding standard errors. If medians for categories of IL-6 and CRP levels were not reported, we estimated approximate medians by using the midpoint of the lower and upper bounds or by using the mean if the midpoint could not be estimated. Given the open-ended categories, the median values were estimated assuming a normal distribution density function (22). Furthermore, six studies reported results for log-transformed CRP (7,13,15,23–25), and one study did so for log-transformed IL-6 levels (15), which could be included in the analysis of IL-6 and CRP levels and type 2 diabetes risk. In addition, 13 studies that reported RRs for categories of IL-6 and CRP levels were eligible for GLST dose-response analysis. For these studies, we estimated RR per 1 log mg/L increase in CRP and per 1 log pg/mL increase in IL-6 levels by regressing the natural log RRs according to the categories IL-6 and CRP levels, which was performed using the GLST method.
Heterogeneity across studies was tested using the Cochrane Q test and the I2 test (26). Data from the studies were combined using a fixed-effects model or a random-effects model. In the presence of statistically significant heterogeneity, a random-effects model was adopted to calculate the overall OR value.
To explore the source of heterogeneity, we first performed stratified analyses by meta-regression, and then we conducted subgroup analyses according to location (U.S., Europe, Asia, and Aboriginal populations), sex (proportion of women and two categories), the study population (continuous and two categories), duration of follow-up (continuous and two categories), adjustment for glycemia (homeostasis model assessment [HOMA-IR], fasting blood glucose, fasting insulin, impaired glucose tolerance, or HbA1c) (two categories), adjustment for waist circumference/waist-to-hip ratio (WC/WHR) (two categories), study design (two categories), and study quality (three categories).
To verify the robustness of our findings and explore possible sources of statistical heterogeneity, we also performed sensitivity analysis. The absence or presence of publication bias was assessed using the Begg and Egger test. Publication bias was further assessed by the application of contour-enhanced funnel plots (27).
All statistical analyses and contour-enhanced funnel plots were performed using Stata 11.2 (Stata-Corp, College Station, TX).
RESULTS
Identifying studies
We initially retrieved 3,071 citations from the database. Of these, the majority were excluded. After full-text review of 33 articles, 15 studies were excluded because they were missing sufficient data, did not report RR or HR estimates, and assessed cardiovascular disease with type 2 diabetes or genetic variants of CRP. Two studies (18,28) were subsequently excluded because they did not report sufficient data for using GLST dose-response analysis. An additional three studies were included from the bibliographies of prior meta-analyses. Finally, the 19 remaining studies (4–10,13–17,23–25,29–32) were included in our meta-analysis. A flowchart presenting the study selection is shown in Supplementary Fig. 1.
Study characteristics
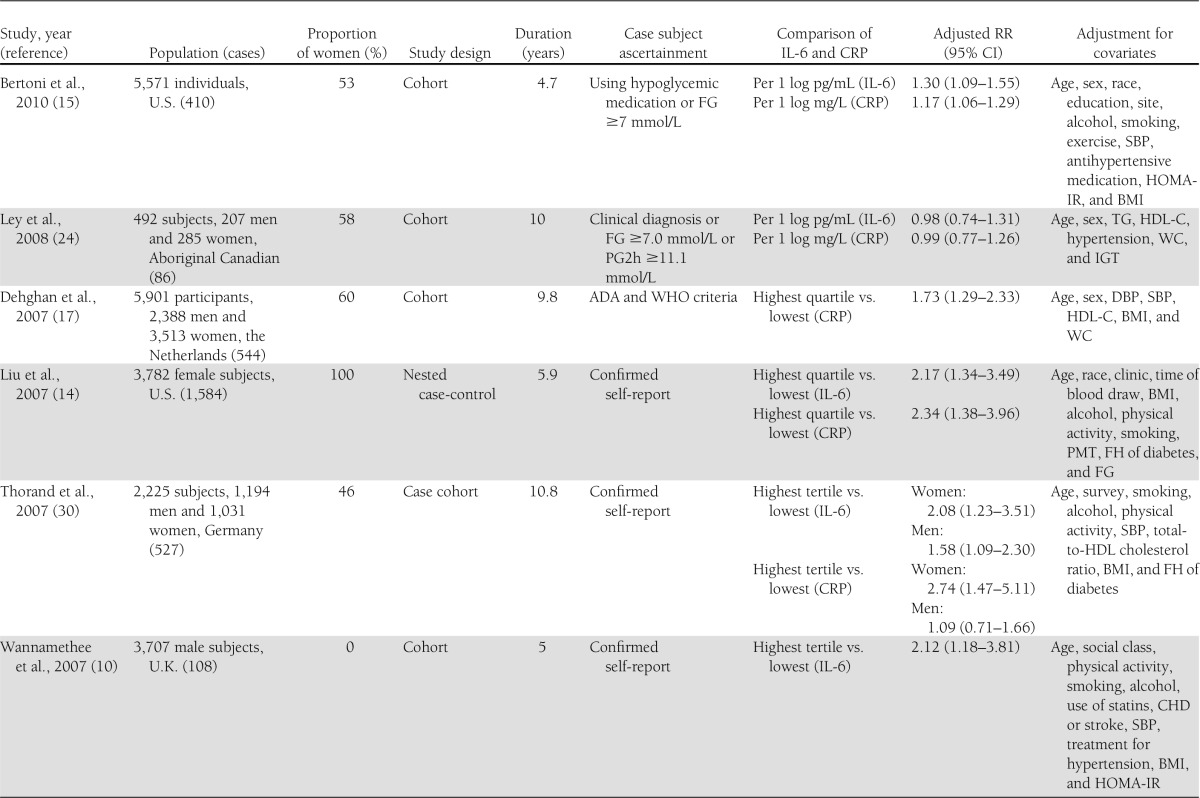
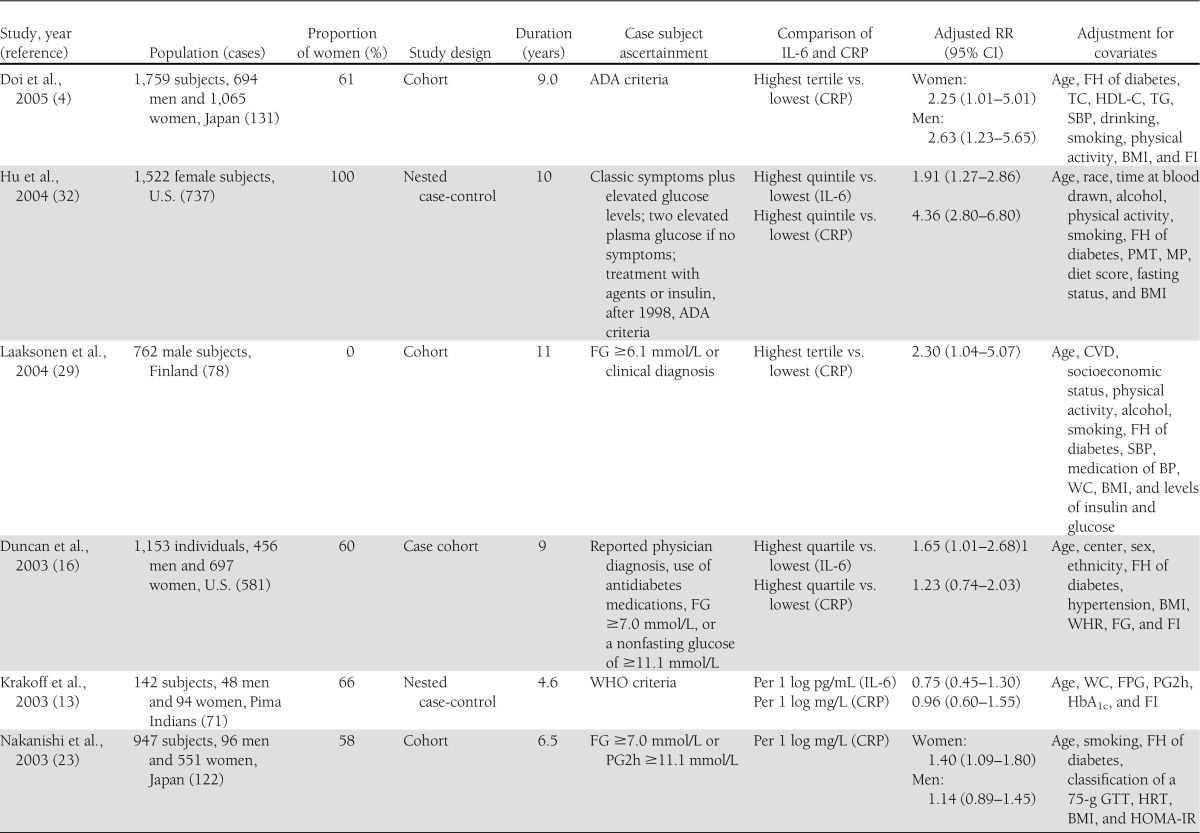
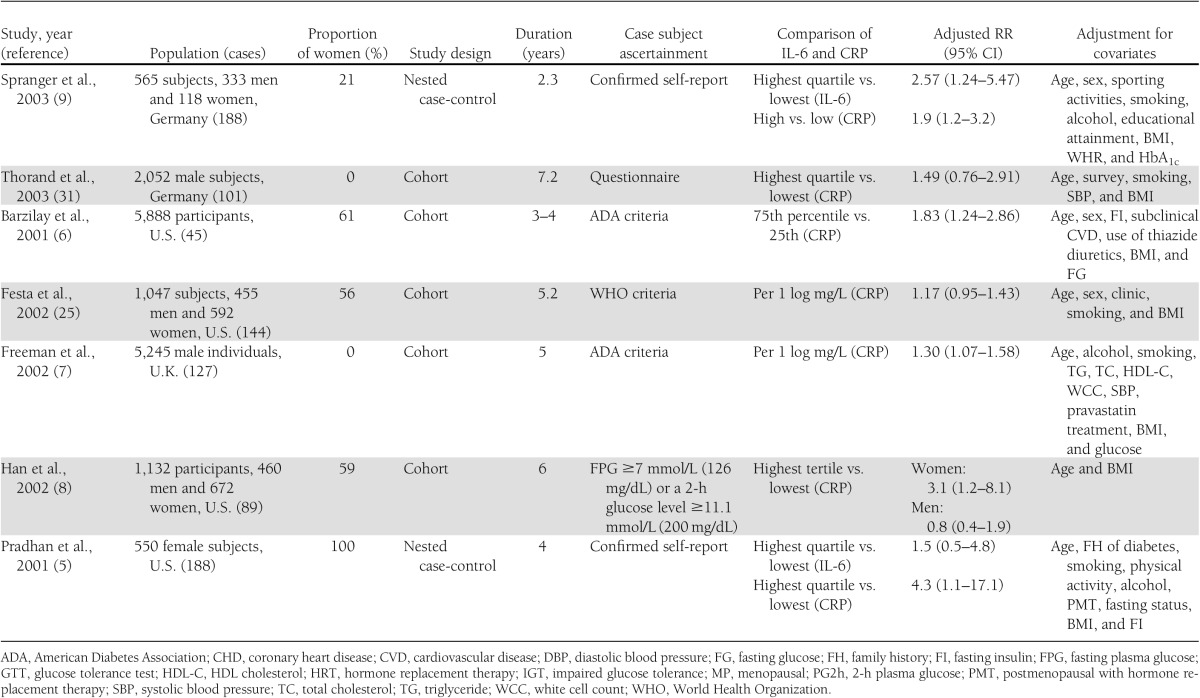
Table 1 shows the characteristics of the 19 included studies of IL-6 and/or CRP levels and the risk of type 2 diabetes. Of these, 10 studies (5,9,10,13–16,24,30,32) reported data on the relation of IL-6 and type 2 diabetes, and 18 studies (4–9,13–17,23–25,29–32) on CRP and type 2 diabetes. The eligible studies included 12 cohort studies, 5 nested case-control studies, and 2 case-cohort studies. Two studies (13,24) involved small samples (<500 subjects). Four studies (4,8,23,30) reported outcomes separately for men and women.
Table 1.
Study characteristics and RRs of type 2 diabetes risk associated with CRP and/or IL-6
Analysis of IL-6
Ten prospective studies (5,9,10,13–16,24,30,32) were included in the meta-analysis for the association of IL-6 levels with the risk of type 2 diabetes. Of these, five included both men and women, four consisted entirely of women, and one consisted of men only. Five of these studies were performed in the U.S., three in Europe, and two in North American Aboriginal populations.
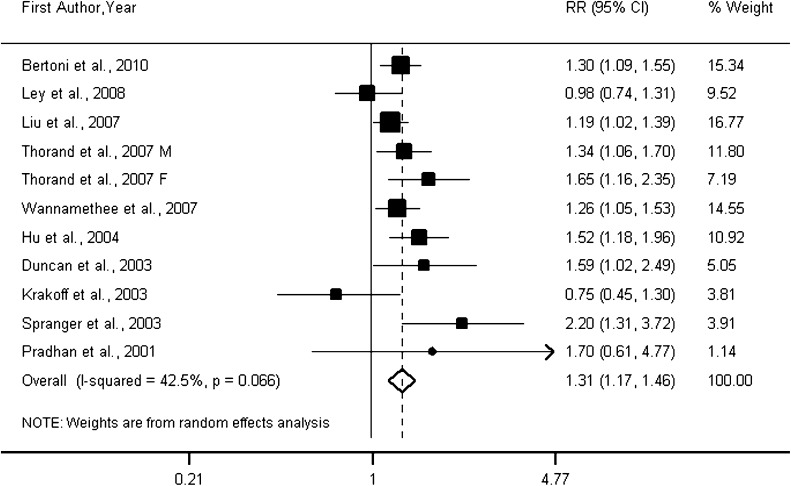
The overall RR of type 2 diabetes was 1.31 (95% CI 1.17–1.46; P = 0.000) per 1 log pg/mL increment in IL-6 levels (Fig. 1), which roughly corresponds to the difference between the medians of the highest and the lowest tertiles of the eligible studies. Between-study heterogeneity was found among studies (Pheterogeneity = 0.066; I2 = 42.5%).
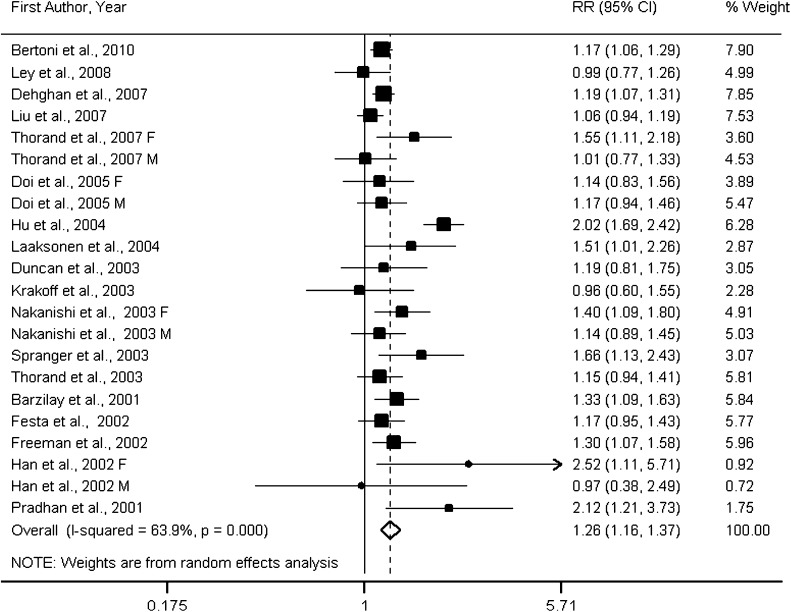
Figure 1.
Forest plot of prospective studies examining IL-6 level and risk of type 2 diabetes. F, female; M, male.
Sensitivity and subgroup analysis
A sensitivity analysis was performed to confirm the robustness of our findings. We recalculated the pooled risk estimates for the remainder of the studies by omitting one study at a time, which resulted in little change of the observed risk estimates from 1.28 (95% CI 1.14–1.41) to 1.34 (1.20–1.19). Furthermore, we also recalculated the pooled RR of type 2 diabetes using a fixed-effects model instead of a random-effects model, which yielded an RR of 1.29 (1.19–1.39), which was not significantly different from the original risk estimate. In addition, excluding two small studies (13,24) did not appreciably change the pooled RR (1.35 [1.23–1.47]) of type 2 diabetes, but between-study heterogeneity was significantly decreased (I2 from 42.5 to 13.7%) with the removal of the two studies.
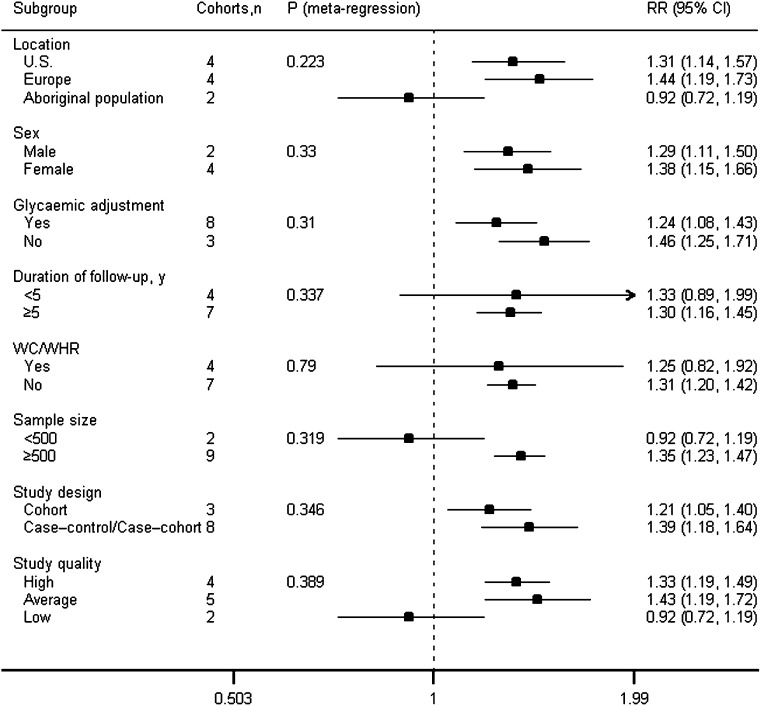
To test the robustness of our results, we conducted subgroup analyses. Figure 2 shows the results of the analyses of all subgroups at the IL-6 level. The association between elevated levels of IL-6 and diabetes risk was consistently observed in studies from the U.S. and Europe. However, data from two studies in Aboriginal populations suggested that IL-6 did not predict diabetes (RR 0.92 [95% CI 0.72–1.19]; P = 0.531). In subgroup analyses by sex, IL-6 was significantly associated with an increased risk of diabetes in both women and men.
Figure 2.
Analyses of subgroups relating IL-6 to type 2 diabetes. For sex (proportion of women), sample size, and duration of follow-up, the P value was obtained by modeling these variables as continuous variables in meta-regression analysis.
We also performed subgroup analyses by whether or not a study adjusted for WC/WHR or glycemia. In the studies that adjusted for glycemia, the relationship remained significant but slightly attenuated (RR 1.24 [95% CI 1.08–1.43]; P = 0.002). In studies that adjusted for WC/WHR, IL-6 levels were not associated with diabetes risk (1.25 [0.82–1.92]; P = 0.303). However, after excluding the two low-quality studies, the relationship remained significant (1.82 [1.29–2.56]; P = 0.001).
Regardless of two small studies, the association between IL-6 and risk of type 2 diabetes was not substantially modified by follow-up length, study quality, and study design.
Analysis of CRP
Eighteen prospective studies (4–9,13–17,23–25,29–32) were included in the meta-analysis for the associations of CRP levels with type 2 diabetes. Three studies consisted entirely of men, three of women only, and twelve of both men and women. Four studies (4,8,23,30) reported data separately for men and women, and our analyses of CRP therefore included a total of 22 cohorts. Of these, nine cohorts were conducted in the U.S., four in Asia, seven in Europe, and two in North American Aboriginal populations. On the basis of 22 cohorts, the overall RR of type 2 diabetes was 1.26 (95% CI 1.16–1.37; P = 0.000) per 1 log mg/L increment in CRP levels (Fig. 3). Substantial between-study heterogeneity (Pheterogeneity = 0.000; I2 = 63.9%) was found in the analysis.
Figure 3.
Forest plot of prospective studies examining CRP level and risk of type 2 diabetes. F, female; M, male.
Sensitivity and subgroup analysis
A sensitivity analysis was conducted, which yielded a range of RRs from 1.20 (95% CI 1.13–1.27) to 1.28 (1.17–1.39). The analysis indicated that one study (32) had a substantial influence on between-study heterogeneity. Although the magnitude of the association was not significantly changed (1.20 [1.13–1.27]), between-study heterogeneity was significantly decreased (I2 from 63.9 to 23.2%) with the removal of that study (32). Restricting analyses to high- and average-quality studies did not appreciably change the results (1.28 [1.18–1.40]).
To further confirm our results, we conducted stratified analyses. Supplementary Fig. 2 shows the results of the analyses of all subgroups at the CRP level. The association between CRP and diabetes was consistently observed in the U.S., Europe, and Asia, but not in Aboriginal populations. The associations remained significant but attenuated in studies adjusted for glycemia or WC/WHR.
Notably, stratification by sex revealed that elevated levels of CRP were associated with an increased risk of diabetes in women and men. However, for the subgroup, in women, significant heterogeneity existed between studies (Pheterogeneity = 0.000; I2 = 85.7%). In contrast, for the subgroup, in men, no heterogeneity was observed between studies (Pheterogeneity = 0.685; I2 = 0.0%). One study from the subgroup was adjusted only for age and BMI in women (8). Excluding that study did not appreciably change the results of the subgroup in women.
Except for two small studies, the association between CRP and the risk of type 2 diabetes was not substantially modified by follow-up length, study quality, and study design.
Associations of IL-6 and CRP with type 2 diabetes
To further determine whether IL-6 is more strongly associated with type 2 diabetes than CRP, we also conducted a subgroup analysis for the results of IL-6 and CRP arising from the same studies.
A total of 10 cohorts reported simultaneous IL-6 and CRP levels in the same studies. Pooled RRs of type 2 diabetes were 1.33 (95% CI 1.17–1.51) for IL-6 and 1.28 (1.09–1.51) for CRP.
Study quality and publication bias
According to study quality, we performed subgroup analyses (Fig. 2 and Supplementary Fig. 2). IL-6 and CRP were significantly associated with type 2 diabetes in high- and average-quality studies, but not in low-quality studies.
The Begg (P = 0.350) and Egger test (P = 0.422) suggested the absence of publication bias in the analysis relating IL-6 to type 2 diabetes. Visual inspection of contour-enhanced funnel plots (Supplementary Fig. 3) did not result in the identification of substantial asymmetry. For the relation of CRP and diabetes, contour-enhanced funnel plots (Supplementary Fig. 4) were symmetrical, and neither the Begg (P = 0.063) nor the Egger test (P = 0.149) suggested publication bias.
CONCLUSIONS
The present meta-analysis of prospective studies suggests a significant association of elevated levels of IL-6 and CRP with type 2 diabetes risk. Our findings further support the hypothesis that chronic inflammation is a predictor of type 2 diabetes development.
In subgroup analyses, the associations of IL-6 and CRP with diabetes were not substantially changed by geographic region, with the exception of Aboriginal populations. In view of only two small studies involving these populations, further studies will be required to confirm these data in Pima Indians/Aboriginal Canadians.
Differences between sexes in the diabetes risk associated with inflammatory markers have been reported (8,28,30). Two studies in Japanese subjects showed that CRP was significantly associated with type 2 diabetes risk in both men and women (4,23). Consistent with this, our results of stratification by sex demonstrated that elevated IL-6 and CRP levels were predictors of the development of type 2 diabetes in both men and women. Of note, in the analysis of CRP, stratification by sex showed substantial between-study heterogeneity in the subgroup when only women were included, but no between-study heterogeneity in the subgroup when only men were included. We made an attempt to determine the reason by examining those studies in women. First, there was a wide range of RRs for diabetes among those studies from the subgroup in women. Second, excluding one study (8) that adjusted only for age and BMI did not appreciably change the results. Moreover, the lack of adjustment for hormone replacement therapy in some studies might have confounded results among women. Two of the studies in women (5,32) demonstrated that CRP was more strongly associated with type 2 diabetes, but those two studies adjusted for being postmenopausal with hormone replacement therapy. Therefore, it remains unclear whether it is a reflection of a different confounding in those studies in women or whether it is a change in CRP levels of the biological process in women.
Some studies observed no significant associations between IL-6 and CRP levels and type 2 diabetes after adjusting for BMI or WC/WHR (13,18). Although the RRs adjusted for BMI in the original studies were used in our analyses, our findings showed significant associations. The association of CRP with diabetes remained significant but attenuated in the studies that adjusted for WC/WHR. For IL-6, after excluding the two low-quality studies, the relationship also remained significant. This finding suggests that although there may be complex interrelationships between adiposity, inflammation, and diabetes, the associations between inflammatory markers and diabetes risk cannot be explained fully by adiposity.
Stratified analyses also showed that the magnitude of associations between IL-6 and CRP and diabetes remained statistically significant but were slightly attenuated in the studies that adjusted for glycemia. It is possible that inflammation directly causes elevations in blood glucose and/or insulin resistance and subsequent diabetes. In the past decade, the role of inflammatory markers in the development of type 2 diabetes has been increasingly recognized. However, the biological mechanisms through which the levels of IL-6 and CRP increase the risk of type 2 diabetes are not well understood. Potential environmental triggers such as infection, chemicals, and overnutrition can be detected through a series of sentinel cells, which are a major component of innate immunity (33). On and in sentinel cells, a variety of germ line–encoded pattern recognition receptors recognize and bind potentially harmful substances, which activates signaling pathways and releases proinflammatory cytokines mainly including IL-6 and tumor necrosis factor-α (34). IL-6 may contribute to the pathology and physiology of type 2 diabetes through its interaction with insulin-signaling pathways and β-cell function (35). In addition, IL-6 stimulates the production of CRP (34). In the current study, our findings showed that IL-6 was more strongly associated with type 2 diabetes than CRP. Subgroup analyses for the results of IL-6 and CRP arising from the same studies further provided a more direct comparison of the associations. This further supports the notion that CRP is more likely to be a downstream intermediate rather than a true causal intermediate (36). Furthermore, the recent Mendelian randomization studies by Brunner et al. (37) failed to demonstrate a causal role between CRP levels and diabetes.
In contrast to previous meta-analyses, first, the current study simultaneously explored the relationships between the two inflammatory cytokines IL-6 and CRP and type 2 diabetes risk. This may provide more complete evidence for the notion that IL-6 and CRP are etiologically involved in the pathogenesis of diabetes. Second, due to the addition of recent studies, our analysis included 22 cohorts, involving 40,735 participants and 5,753 cases, and used GLST analysis to evaluate the dose-response relationships. In contrast, one previous meta-analysis that included 16 studies was performed by the conversion of estimates into thirds of the CRP distribution (18). Another meta-analysis included 10 studies and synthesized estimates by different CRP distributions (17). Third, the current study, including 10 prospective studies with a total of 19,709 participants and 4,480 cases, provided for the first time, to our knowledge, pooled estimates of the association of elevated IL-6 levels with diabetes risk. Previous meta-analyses have focused solely on the relationship between CRP and type 2 diabetes. Finally, we used the same method to explore the associations of levels of IL-6 and CRP with type 2 diabetes risk, which allowed us to better compare the magnitude of the associations with the risk of type 2 diabetes between IL-6 and CRP levels.
Limitations of the current study should be addressed. The primary limitation is that the methods used to diagnose diabetes were different in each study. For example, the oral glucose tolerance test retains a higher sensitivity when compared with FPG only. FPG was used as the only method for the screening of diabetes, which does not seem to show high enough sensitivity in obese subjects (38). Misclassification of diabetes cases is inevitable. These misclassifications could weaken the association obtained in our findings. Furthermore, the adjustments for potential confounders differed in the original studies. Although we used adjusted estimates from multivariate models from each included study in our analyses, we cannot exclude the possibility that this aspect affected the results of the study. Finally, we could not completely exclude the possibility that inaccurately measured IL-6 and CRP are partly responsible for the observed associations.
These findings may have important implications for the prevention and treatment of type 2 diabetes. Measurements of IL-6 and CRP in apparently healthy subjects may help to identify high-risk populations for type 2 diabetes. Lifestyle interventions such as weight loss and exercise can reduce serum CRP levels and other inflammatory markers (39). Therefore, elevated IL-6 and CRP can also serve as a common target for lifestyle and therapeutic interventions for type 2 diabetes. However, randomized trial data also suggested that low-dose aspirin use did not prevent the development of clinical type 2 diabetes in women (40). Future research for understanding the potential causal association of inflammation with diabetes may yield novel avenues for the prevention and treatment of type 2 diabetes. However, it remains important to focus on modifying unhealthy diet and lifestyle factors for the prevention and management of diabetes.
In summary, this systematic review and meta-analysis indicates that elevated levels of IL-6 and CRP are significantly associated with an increased risk of type 2 diabetes. Our findings support the concept that the pathogenesis of type 2 diabetes can be considered an autoinflammatory disease.
Acknowledgments
This study was supported by the National High Technology Research and Development Program of China (2010AA023003) and the National Science and Technology Support Program of China (2012BAI02B02).
No potential conflicts of interest relevant to this article were reported.
X.W. designed the study, collected and analyzed data, contributed to discussion, and wrote, reviewed, and edited the manuscript. W.B., J.L., and Y.-Y.O.Y. designed the study, collected and analyzed data, and contributed to discussion. D.W., S.R., X.X., Z.-L.S., Y.Z., and P.Y. collected and analyzed data and contributed to discussion. L.-G.L. designed the study, collected and analyzed data, contributed to discussion, and reviewed and edited the manuscript.
The authors thank Frank B. Hu (Harvard School of Public Health and Harvard Medical School, Boston, MA) for his help in reviewing and editing the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0702/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2011;29:116–122 [Google Scholar]
- 3.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 4.Doi Y, Kiyohara Y, Kubo M, et al. Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: the Hisayama Study. Diabetes Care 2005;28:2497–2500 [DOI] [PubMed] [Google Scholar]
- 5.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 6.Barzilay JI, Abraham L, Heckbert SR, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes 2001;50:2384–2389 [DOI] [PubMed] [Google Scholar]
- 7.Freeman DJ, Norrie J, Caslake MJ, et al. West of Scotland Coronary Prevention Study C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002;51:1596–1600 [DOI] [PubMed] [Google Scholar]
- 8.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean MEJ, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 2002;25:2016–2021 [DOI] [PubMed] [Google Scholar]
- 9.Spranger J, Kroke A, Möhlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003;52:812–817 [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Lowe GDO, Rumley A, Cherry L, Whincup PH, Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care 2007;30:1200–1205 [DOI] [PubMed] [Google Scholar]
- 11.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8(Suppl 2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control and Prevention. American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511 [DOI] [PubMed] [Google Scholar]
- 13.Krakoff J, Funahashi T, Stehouwer CDA, et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care 2003;26:1745–1751 [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med 2007;167:1676–1685 [DOI] [PubMed] [Google Scholar]
- 15.Bertoni AG, Burke GL, Owusu JA, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2010;33:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan BB, Schmidt MI, Pankow JS, et al. Atherosclerosis Risk in Communities Study Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2003;52:1799–1805 [DOI] [PubMed] [Google Scholar]
- 17.Dehghan A, Kardys I, de Maat MPM, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 2007;56:872–878 [DOI] [PubMed] [Google Scholar]
- 18.Lee CC, Adler AI, Sandhu MS, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia 2009;52:1040–1047 [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 20.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology 1993;4:218–228 [DOI] [PubMed] [Google Scholar]
- 21.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–57 [Google Scholar]
- 22.Gravetter FJ, Wallnau LB. Essentials of Statistics for the Behavioral Sciences. 6th ed. Belmont, CA, Wadsworth, 2008 [Google Scholar]
- 23.Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N. Elevated C-reactive protein is a risk factor for the development of type 2 diabetes in Japanese Americans. Diabetes Care 2003;26:2754–2757 [DOI] [PubMed] [Google Scholar]
- 24.Ley SH, Harris SB, Connelly PW, et al. Adipokines and incident type 2 diabetes in an Aboriginal Canadian [corrected] population: the Sandy Lake Health and Diabetes Project. Diabetes Care 2008;31:1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM, Insulin Resistance Atherosclerosis Study Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2002;51:1131–1137 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991–996 [DOI] [PubMed] [Google Scholar]
- 28.Snijder MB, Dekker JM, Visser M, et al. Prospective relation of C-reactive protein with type 2 diabetes: response to Han et al. Diabetes Care 2003;26:1656–1657; author reply 1657–1658 [DOI] [PubMed] [Google Scholar]
- 29.Laaksonen DE, Niskanen L, Nyyssönen K, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia 2004;47:1403–1410 [DOI] [PubMed] [Google Scholar]
- 30.Thorand B, Baumert J, Kolb H, et al. Sex differences in the prediction of type 2 diabetes by inflammatory markers: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Diabetes Care 2007;30:854–860 [DOI] [PubMed] [Google Scholar]
- 31.Thorand B, Löwel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984-1998. Arch Intern Med 2003;163:93–99 [DOI] [PubMed] [Google Scholar]
- 32.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004;53:693–700 [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med 2000;343:338–344 [DOI] [PubMed] [Google Scholar]
- 34.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- 35.Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:305–311 [DOI] [PubMed] [Google Scholar]
- 36.Oh J, Teoh H, Leiter LA. Should C-reactive protein be a target of therapy? Diabetes Care 2011;34(Suppl. 2):S155–S160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunner EJ, Kivimäki M, Witte DR, et al. Inflammation, insulin resistance, and diabetes—Mendelian randomization using CRP haplotypes points upstream. PLoS Med 2008;5:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox ME, Edelman D. Tests for screening and diagnosis of type 2 diabetes. Clin Diabetes 2009;27:132–138 [Google Scholar]
- 39.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ 2005;172:1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradhan AD, Cook NR, Manson JE, Ridker PM, Buring JE. A randomized trial of low-dose aspirin in the prevention of clinical type 2 diabetes in women. Diabetes Care 2009;32:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]