We show that pneumococcal colonization rates and composition greatly change during human immunodeficiency virus infection. These changes are associated with fewer Th1 pneumococcal-specific responses but unaltered Th17 responses. Altered pneumococcal colonization and impaired pneumococcal-specific immunity are not rectified by highly active antiretroviral therapy.
Keywords: Human, T Cells, Mucosa, Streptococcus pneumoniae; HIV
Abstract
Background. African adults infected with human immunodeficiency virus (HIV) have high rates of pneumococcal colonization and invasive disease. Here we have investigated the possibility that HIV disrupts the normal balance of pneumococcal-specific helper T cell (Th) 1/Th17 immunity to colonization, resulting in a more permissive nasopharyngeal niche.
Methods. One hundred thirty-six HIV-infected and -uninfected Malawian adults were enrolled in the study. Changes in rates and composition of nasopharyngeal pneumococcal colonization were analyzed using microarray. The underlying pneumococcal-specific Th1/Th17 responses associated with altered pneumococcal colonization were investigated using flow cytometry.
Results. We find that pneumococcal carriage is only modestly increased in asymptomatic HIV-infected Malawian adults but that colonization rates rise dramatically during symptomatic disease (HIVneg 13%, HIVasy 19%, and HIVsym 38%). These rates remain high in subjects established on antiretroviral therapy (ART): 33% (at 6–12 months) and 52% (at 18 months), with HIV-infected individuals carrying a broader range of invasive and noninvasive serotypes compared with HIV-negative controls. The frequency of multiple serotype carriage (>1 serotype HIVneg 26%, HIVasy 30%, HIVsym 31%, HIVART 31%) is not affected. These changes in colonization are associated with generalized CD4 T-cell depletion, impaired antigen-specific proliferation, and a defect in pneumococcal-specific T-cell interferon-γ but not interleukin 17 production.
Conclusions. These data reveal the persistently poor control of pneumococcal colonization in HIV-infected adults following immune ART-mediated reconstitution, highlighting a potential reservoir for person-to-person spread and vaccine escape. Novel approaches to control colonization either through vaccination or through improvements in the quality of immune reconstitution are required.
Worldwide, invasive pneumococcal disease (IPD), in the form of pneumonia, bacteremia, and meningitis, is a leading cause of mortality [1, 2]. In particular, sub-Saharan Africa has a disproportionate burden of IPD, which in adults is largely associated with the human immunodeficiency virus (HIV) epidemic and persists despite antiretroviral therapy (ART) [3]. As with many opportunistic pathogens, pneumococcal colonization of the mucosal surface generally precedes a multistep process that leads to invasive infection [4] and provides a reservoir for person-to-person transmission within the population [5]. In humans, certain capsule serotypes are associated with carriage, while others with a different invasive potential are more commonly associated with IPD [6]. The nasopharyngeal niche supports the carriage of multiple pneumococcal serotypes, particularly in children [7], facilitating horizontal gene transfer between pneumococcal strains and across bacterial species [8, 9]. However, the onset and maintenance of carriage in the nasopharynx, and the emergence of variation by this commensal, is controlled by host immune surveillance [10–14].
Recent data suggest that 2 separate lineages of CD4 T cells facilitate the clearance of pneumococcal colonization within the nasopharynx (Th1/Th17) and control the multiplication of bacteria following dissemination (principally Th1) [15]. While this concept is largely based on studies performed in murine models [16, 17], there is increasing evidence that Th1 and Th17 are important in human naturally acquired immunity to pneumococcus [11–14, 18]. It has been shown that individuals with hyper-IgE syndrome are defective in Th17 differentiation and susceptible to recurrent pulmonary infections [18], and that pneumococcal-specific interleukin 17 (IL-17) CD4 T cells are present within the human mucosa [13, 14]. Furthermore, Th1 interferon gamma (IFN-γ) responses to the pneumococcus are found within the mucosal and systemic compartments along with T-cell-dependent isotype-switched immunoglobulin G and immunoglobulin A antibody to pneumococcal protein antigens [11, 12]. Any underlying change in Th1/Th17 responses during HIV infection may disrupt either mucosal and/or systemic pneumococcal immune defenses. Th17 supports mucosal defense by producing antimicrobial β-defensins [19] and promoting phagocytic recruitment to the nasopharynx for pneumococcal engulfment [13]. Th1 supports systemic and mucosal immune compartments by enhancing phagocytic function and helping generate pneumococcal-specific memory B cells, which produce opsonizing and complement fixing antibodies.
Although HIV infection is associated with higher rates of pneumococcal colonization [20, 21] and invasive disease [22, 23], changes in pneumococcal-specific T-cell immunity and consequential effects on colonizing pneumococci during HIV infection have not been extensively described. We have previously shown that early in disease progression, HIV-infected Malawian adults and children have evidence of immune activation and senescence associated with impaired pneumococcal-specific immune memory [24, 25]. We have now explored the hypothesis that Th1/Th17-mediated immune control is impaired in HIV, resulting in colonization by multiple serotypes not typically seen in healthy individuals. We show striking increases in pneumococcal colonization with a broad range of serotypes during the progression of HIV infection in adults. These are associated with dynamic changes in peripheral pneumococcal-specific Th1 IFN-γ responses but not IL-17 production. Immune reconstitution with ART does not lead to restoration of immune control, which may promote pathogen adaptation and transmission within this growing population.
MATERIALS AND METHODS
Ethics Statement
The study was approved by research ethics committees at The College of Medicine, Malawi (P.07/10/960) and The Liverpool School of Tropical Medicine (11.00). Individuals were recruited from the voluntary counseling and testing clinic at Queen Elizabeth Central Hospital, Blantyre, Malawi, following written informed consent.
Subjects
Of 136 subjects, 32 were healthy controls confirmed by 2 HIV rapid antibody tests, Uni-gold (Trinity Biotech) and Determine (Abbott Laboratories) Of 104 subjects infected with HIV, 32 subjects were asymptomatic (World Health Organization [WHO] stage I), and 24 were symptomatic (WHO stage IV). Of the 48 subjects who were established on ART (27 were on ART for 6–12 months and 21 for 18–24 months). Peripheral blood CD4 T-cell counts were determined by flow cytometry using FACSCount (BD Biosciences).
Table 1.
Patient Demographics, Age, CD4 Count, Rate of Pneumococcal Carriage, and World Health Organization Clinical Staging of HIV Infection
| HIVneg (n = 32) | HIVasymp (n = 32) | HIVsym (n = 24) | ART 6–12 mo (n = 27) | ART ≥18 mo (n = 21) | |
|---|---|---|---|---|---|
| Age, y, median (range) | 30 (21–51) | 33 (22–51) | 34 (21–53) | 34 (21–52) | 38 (26–51) |
| CD4 counta, median (range) | 679 (471–1330) | 307 (36–761) | 218 (7–593) | 324 (85–929) | 328 (130–1172) |
| Carriage WHO stage | 13% | 19% Stage 1 | 38% Stage 3/4 | 33% | 52% |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; WHO, World Health Organization.
a Cells per microliter of peripheral blood.
Assessment of Pneumococcal Colonization
Nasopharyngeal swabs were placed into a vial of skimmed milk-tryptone-glucose-glycerol (STGG) transport medium [26]. Streptococcus pneumoniae was identified by morphologic characteristics and optochin susceptibility following growth on gentamicin blood agar.
Pneumococcal DNA Extraction and Serotyping
Pneumococci in STGG were diluted 1:10 and 1:100 using brain heart infusion broth; 50 µL was plated onto a colistin-oxolinic acid-blood agar and incubated overnight at 37°C in 5% CO2. Colonies were harvested using the plate sweep technique [7] into 1.5-mL tubes, and bacteria were centrifuged at 7500 rpm for 10 minutes. DNA was extracted from the pellet using QIAamp DNA Mini Kit (Qiagen). Molecular serotyping was performed on DNA extracts using the BµG@S SP-CPS v1.4.0 microarray, which determines multiple serotypes and their relative abundance [7]. The output was analyzed using empirical Bayesian model [27].
Antigens
Pneumococcal cell culture supernatant (CCS) was prepared from a standard encapsulated type 2 (D39) S. pneumoniae strain and an isogenic pneumolysin-deficient mutant (Ply−) [28]. The concentration of CCS was measured by Bradford protein assay (Sigma-Aldrich), and CCS was heat-inactivated at 56°C for 30 minutes to reduce toxicity of pneumococcal proteins. Mycobacterium tuberculosis purified protein derivative (PPD RT49) was obtained from Statens Serum Institut, Denmark. Influenza antigens were derived from dialyzed inactive trivalent split virion influenza vaccine (Enzira 2006/2007) obtained from Aventis-Pasteur. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were obtained from Sigma-Aldrich.
Cells and Reagents
Peripheral blood mononuclear cells (PBMCs) were isolated from blood by 25 minutes’ centrifugation at 400 g on Histopaque density-gradient medium (Sigma). PBMCs were harvested, washed in Hanks’ balanced salt solution (Invitrogen) and resuspended in complete RPMI (RPMI-1640 with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 4 mM l-glutamine and 10 mM HEPES buffer). PBMCs were counted using 0.4% (wt/vol) trypan blue (Sigma), reconstituted in complete RPMI at 1 × 106 cells/mL with 2% (vol/vol) heat-inactivated human AB-serum (National Blood Services, Malawi), and incubated at 37°C in 5% CO2.
Proliferation and Intracellular Cytokine Staining Assay
PBMCs were labeled with 2.5 µm carboxyfluorescein diacetate succinimidyl ester (CFSE) dye (Invitrogen) to identify dividing T cells. Labeled cells were cultured at 1 × 106 cells/mL in 48-well plates with wild-type (WT) CCS (8 µg/mL), Ply− CCS (8 µg/mL), PPD (10 µg/mL), influenza (0.9 µg/mL), phytohemagglutinin (5 µg/mL) or media only. On day 8 PBMCs were further stimulated with PMA (100 ng/mL) and ionomycin (500 ng/mL) for 6 hours and pneumococcal-specific CFSElow proliferating cells were evaluated for IFN-γ and IL-17 production using intracellular cytokine staining. Brefeldin A (10 µg/mL) (Sigma) was added 1 hour after polyclonal stimulation to block cytokine secretion. Cells were harvested and stained with anti-CD4 peridinin-chlorophyll-protein, CD8-ECD, CD56-allophycocyanin (APC) (Beckman Coulter), and CD3-allophycocyanin-H7 (APC-H7) (BD Biosciences) for 10 minutes. After washing with phosphate-buffered saline, cells were permeabilized and fixed using cytofix/cytoperm (BD Biosciences). Cells were then incubated for 30 minutes at 4°C with anti-IFN-γ–APC and IL-17–PE (BD Biosciences) antibodies. Cells were washed with 1 × Perm Wash (BD Biosciences), resuspended, and analyzed by flow cytometry. For analysis, 5000–10 000 T cells were analyzed across all conditions. All CFSElow cells that produced cytokines in media controls were subtracted from each antigenic stimuli and the proportion of T cells producing IFN-γ or IL-17 was recalculated. During preliminary experiments, CD56 was used to evaluate natural killer T-cell proliferation, which was consistently ≤1% of total proliferating CD3+CD8– T cells.
Laboratory Methods
Because of low PBMC yield in some participants, we were unable to perform all assays on every subject. Perinasal sampling was refused by 3 study subjects.
Statistical Analysis
Statistical analyses and graphical presentation were performed using GraphPad Prism 5. Nonparametric data were analyzed using the Mann-Whitney U test; results show medians and ranges. The presence or absence of nasopharyngeal colonization and carriage of a noninvasive or invasive serotypes in HIV-infected and -uninfected individuals was analyzed using Fisher exact test. Differences were considered significant if P ≤ .05.
RESULTS
Pneumococcal Colonization Increases During HIV Progression and Remains Elevated Despite ART
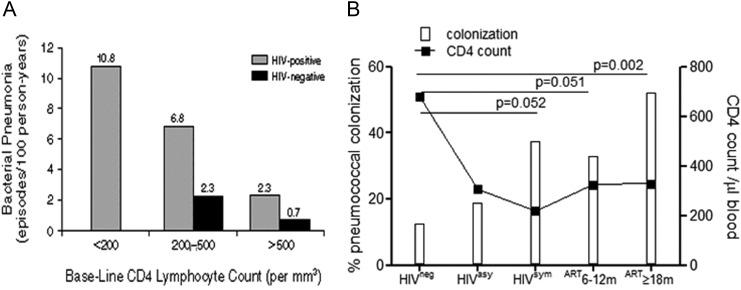
As peripheral blood CD4 T cells decline during HIV infection, the incidence of pneumococcal pneumonia increases (Figure 1A) [29]. As colonization generally precedes invasive disease, we investigated the relationship between the decline of CD4 T cells and the immune control of pneumococcal colonization. Individuals were classified as WHO stage I asymptomatic, WHO stage IV symptomatic, HIV-infected individuals established on ART (6–12 month or ≥18 months), or HIV-uninfected controls (Table 1). We found that although peripheral blood CD4 counts declined (median, 307 [range, 36–761] cells/µL) in asymptomatic HIV infection, the incidence of pneumococcal colonization only marginally increased compared to HIV-uninfected controls (HIVneg 13% vs HIVasy 19%), whereas colonization increased dramatically in symptomatic HIV-infected individuals (HIVsym 38%, P = .052; median CD4 count, 218 [range, 7–593] cells/µL). Although median CD4 counts increased in subjects established on ART (324 [range, 85–929] cells/µL at 6–12 months to 328 [range, 130–1172] cells/µL at ≥18 months) in line with HIV-asymptomatic individuals, the rates of pneumococcal colonization remained high in comparison (ART: 6–12 months, 33% [P = .051]; ≥18 months, 52% [P = .002]) (Figure 1B). Seasonality can influence rates of carriage with colonization peaking in Malawi between October and January (Ho et al, personal communication); all study participants were sampled between February and June outside of the peak in pneumococcal colonization.
Figure 1.
Pneumococcal colonization increases as peripheral blood CD4 T-cell counts decrease and human immunodeficiency virus (HIV) infection progresses. Incidence of bacterial pneumonia increases as CD4 T-cell counts decrease (published with permission from Dr Hirschtick and NEJM [29]) A, Microbiological identification of pneumococcal carriage in HIV-uninfected (n = 32), HIV-asymptomatic (n = 32), HIV-symptomatic (n = 24), and HIV-infected on antiretroviral therapy (ART) for 6–12 months (n = 27) or ≥18 months (n = 21; white bars). B, Median peripheral blood CD4 T-cell counts in HIV-uninfected, HIV-asymptomatic, HIV-symptomatic, and HIV ART (6–12 months and ≥18 months; black line).
HIV-Infected Individuals Carry a Wider Range of Invasive and Noninvasive Pneumococcal Serotypes
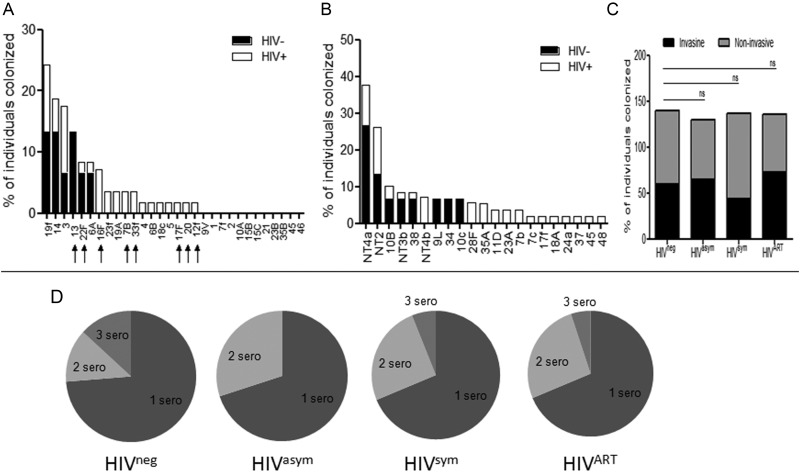
Having shown the incidence of pneumococcal colonization increased sequentially, as HIV infection progressed, and remained elevated in those established on ART, we investigated whether the serotype composition changed under altered immune pressure. Of known global pneumococci [6], HIV-infected individuals carried a wider range of invasive serotypes (HIVpos, 17 vs HIVneg, 6 serotypes) (Figure 2A) and noninvasive (HIVpos, 18 vs HIVneg, 8 serotypes) (Figure 2B) serotypes as compared with HIV-negative controls. However, the likelihood of carrying an invasive or noninvasive serotype (Figure 2C) or single/multiple serotypes (Figure 2D) was comparable with HIV-negative controls and remained unaltered by ART. As study participant group sizes differed, the data were normalized; each serotype was expressed as a percentage of the total individuals within the group.
Figure 2.
Carriage of pneumococcal invasive and noninvasive serotypes in adults infected with human immunodeficiency virus (HIV). Microarray of invasive pneumococcal serotypes (A) and noninvasive serotypes (B) in HIV-uninfected (n = 15; white bars) and HIV-infected Malawian adults (n = 55; black bars). Arrows represent invasive serotypes that are not covered by the current pneumococcal conjugate vaccines. C, Proportion of HIV-uninfected (n = 15), HIV-asymptomatic (n = 20), HIV-symptomatic (n = 16), and HIV-infected individuals established on antiretroviral therapy (ART) for 6–24 months (n = 19) carrying either invasive or noninvasive pneumococcal serotypes. Data were normalized for group number by expressing the percentage of total individuals who carried the serotype in each group. Data were analyzed using Fisher exact test and shown to be nonsignificant (P = >.05). D, Proportion of HIV-uninfected, HIV-asymptomatic, HIV-symptomatic, and HIV-infected individuals on ART with single or multiple carriage of 1, 2, or 3 serotypes.
Impaired Pneumococcal-Specific T-Cell Proliferation Precedes Loss of Control of Pneumococcal Colonization and Is Not Reversed by ART
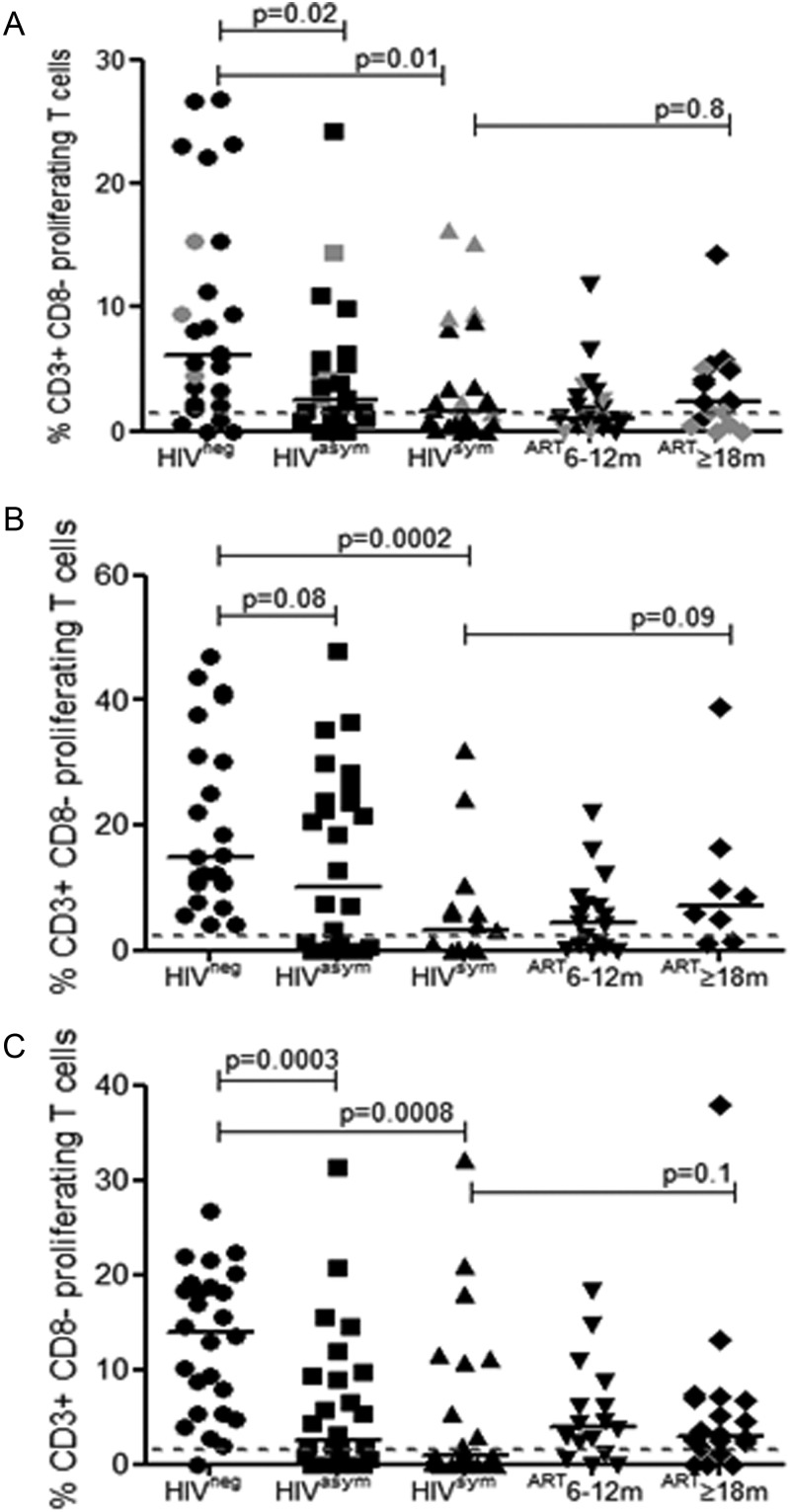
Recently, we reported that CD4 T-cell depletion and pneumococcal-specific T-cell dysfunction occur early in asymptomatic HIV infection [24]. Furthermore, we find that pneumococcal colonization increases as peripheral blood CD4 T cells decline (Figure 1B). Therefore, we investigated the relationship between prevalence of colonization and number and function of pneumococcal-specific proliferating T cells; specifically, whether a sequential decrease in pneumococcal-specific T-cell proliferation resulted in increased colonization or, conversely, whether high colonization boosted pneumococcal-specific T-cell responses following initiation of ART. As part of our experimental approach, we combined a CFSE proliferation assay with intracellular cytokine staining; the data shown in Figure 3 are proliferative responses of CD3+CD8– T cells. We find a sequential decrease in CD3+CD8– T-cell proliferation in response to WT CCS (Figure 3A) and in asymptomatic (median, 2.5% [0%–24.2%], P = .02) and symptomatic (median, 1.6% [0%–16.3%], P = .01) HIV-infected individuals compared with healthy controls (median, 6.2% [0%–26.8%]) as pneumococcal colonization increases from HIVneg 13%, HIVasy 19%, and HIVsym 38%, respectively. The high rates of pneumococcal colonization in those established on ART (HIVART 33% [6–12 months] and 52% [≥18 months]) did not significantly increase CD3+CD8– responses (HIVART median, 1.1% [0%–11.8%] for 6–12 months and median, 2.4% [0%–14.2%] for ≥18 months) to WT CCS antigens (Figure 3A). In comparison, T-cell proliferative responses to other respiratory antigens, M. tuberculosis PPD (Figure 3B) and influenza (Figure 3C), were also impaired as HIV infection progressed and were not improved by ART. In parallel, we evaluated the intrinsic capacity of CD4 T cells to respond to phytohemagglutinin during HIV progression and found no significant differences in T-cell proliferation in individuals with variable CD4 counts (data not shown).
Figure 3.
Proliferative capacity of antigen-specific T cells in human immunodeficiency virus (HIV) infection. Collated flow cytometric data demonstrating the proportion of CD3+CD8– T cells proliferating in response to wild-type (WT) cell culture supernatants (A), Mycobacterium tuberculosis (MTB) purified protein derivative (B), and influenza (C) in HIV-uninfected (circles: WT n = 26, MTB n = 23, Flu n = 26), HIV-asymptomatic (squares: n = 26), HIV-symptomatic (triangles: WT n = 24, MTB n = 15, Flu n = 23), and HIV-infected individuals established on antiretroviral therapy (ART) for 6–12 months (inverted triangles: WT n = 24, MTB n = 17, Flu n = 16) or ≥18 months (diamonds: WT n = 18, MTB n = 8, Flu n = 20). Gray data points represent individuals who were positive for pneumococcal nasopharyngeal carriage. Black horizontal bars represent median values of total antigen-specific responses above background. Statistical significance was analyzed by the Mann-Whitney U test. Differences were considered significant if P ≤ .05.
Defective Pneumococcal-Specific T-Cell IFN-γ Production Is Restored Following Increased Rates of Pneumococcal Colonization Whereas IL-17 Responses Remain Unchanged
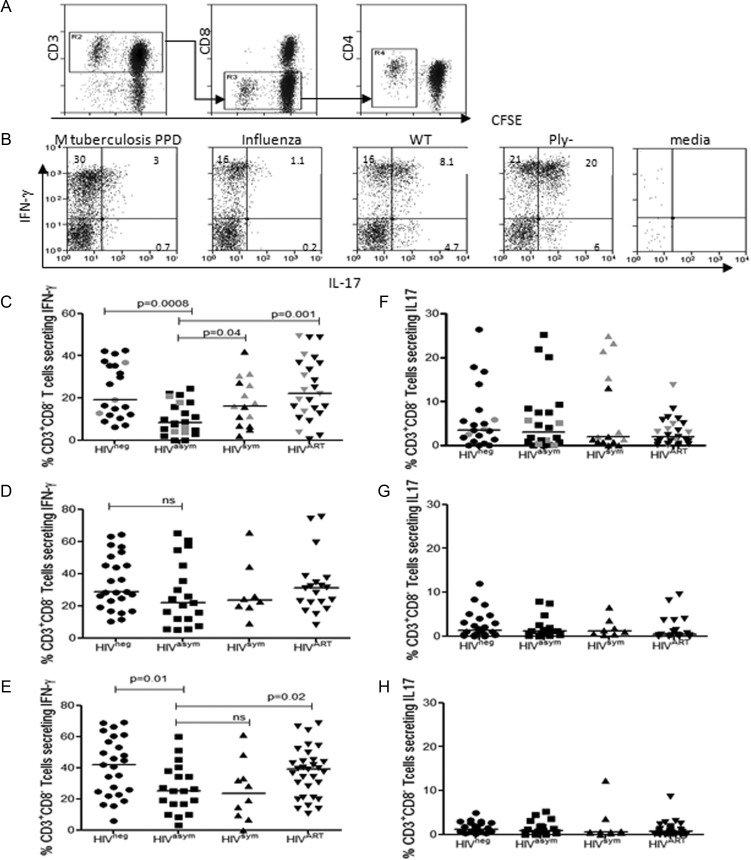
As Th1 and Th17 have been implicated in the clearance of nasopharyngeal pneumococci, we investigated the underlying balance of peripheral blood Th1/Th17 responses in relation to pneumococcal colonization. CFSE-labeled PBMCs were stimulated with WT CCS, Ply− CCS, M. tuberculosis PPD, and influenza antigens to drive antigen-specific T-cell expansion. After 7 days, cells were restimulated with PMA and ionomycin to generate cytokine production, and antigen-specific proliferating CFSElow T cells were gated for analysis of Th1/IFN-γ and Th17/IL-17. Surface expression of CD4 was downregulated following restimulation; therefore, analysis focused on cytokine production by proliferating CD3+CD8– T cells (Figure 4A). To ensure that only cytokine profiles from robust antigen-specific responses were evaluated, poor T-cell proliferative responses (≤1.5% proliferating T cells) were excluded from the analysis. In HIV-uninfected individuals, we found that CD3+CD8– proliferating T cells generated distinct antigen-specific cytokine profiles. IFN-γ was produced in response to pneumococcal, M. tuberculosis PPD, and influenza antigens. In contrast, stimulation with WT CCS and Ply− CCS produced a high proportion of T-cell IL-17 responses, whereas IL-17 production was lower in response to M. tuberculosis PPD and undetectable to influenza (Figure 4B).
Figure 4.
Pneumococcal-specific Th1 and Th17 responses in peripheral blood during human immunodeficiency virus (HIV) infection. Representative flow cytometric data from a HIV-uninfected control demonstrating CD3+CD8– T-cell proliferation (A) and interferon gamma (IFN-γ) and interleukin 17 (IL-17) production (B) in response to Mycobacterium tuberculosis (MTB) purified protein derivative (PPD), influenza, wild-type (WT) cell culture supernatant (CCS), Ply− CCS, and media only. The percentage indicated in the upper-left quadrant represents the cell population that produced cytokines following 7-day expansion and restimulation with phorbol 12-myristate 13-acetate and ionomycin. Collated flow cytometric data demonstrating the proportion of CD3+CD8– T cells producing IFN-γ in response to WT CCS (C), M. tuberculosis PPD (D), and influenza (E) in HIV-uninfected (circles: WT n = 21, MTB n = 22, Flu n = 24), HIV-asymptomatic (squares: n = 19), HIV-symptomatic (triangles: WT n = 15, MTB n = 8, Flu n = 10), and all HIV-infected individuals established on antiretroviral therapy (ART) (6–24 months) (inverted triangles: WT n = 25, MTB n = 19, Flu n = 31). Proportion of CD3+CD8– T cells producing IL-17 in response to to WT CCS (F), M. tuberculosis PPD (G), and influenza (H) in HIV-uninfected, HIV-asymptomatic, HIV-symptomatic, and all HIV-infected individuals established on ART (6–24 months). Gray data points represent individuals who were positive for pneumococcal nasopharyngeal carriage. Black horizontal bars represent median values of total antigen-specific responses above background. Statistical significance was analyzed by the Mann-Whitney U test. Differences were considered significant if P ≤ .05.
In asymptomatic HIV infection, significant decreases in T-cell IFN-γ responses to WT CCS (Figure 4C) were followed by stark increases in pneumococcal colonization; increased colonization then coincided with the early restoration of pneumococcal-specific IFN-γ responses in HIV-symptomatic individuals in line with healthy controls. In comparison, T-cell IFN-γ responses to M. tuberculosis PPD (Figure 4D) remained consistent throughout HIV progression and on ART, whereas responses to influenza (Figure 4E) decreased in both asymptomatic and symptomatic HIV-infected individuals and levels were only restored following ART. We found that Th17/IL-17 production was greatest in response to pneumococcal antigens (Figure 4F) as compared to M. tuberculosis PPD (Figure 4G) and influenza (Figure 4H); but, contrary to our hypothesis, IL-17 responses neither decreased during HIV progression nor increased in response to higher rates of colonization. The dynamic changes in pneumococcal-specific IFN-γ production were not seen in IL-17 responses even in the context of increasing rates of pneumococcal colonization.
DISCUSSION
How healthy individuals control pneumococcal colonization and largely avoid invasive disease remains unclear. The high frequency of carriage and disease in HIV-infected compared to HIV-uninfected individuals [30] suggests that T-cell immunity at the mucosal surface and in the blood plays a critical role [31]. Therefore, we investigated the relationship between pneumococcal colonization and underlying pneumococcal-specific T-cell immunity during HIV progression. We show that rates of pneumococcal colonization in this African adult population are relatively well controlled in asymptomatic HIV infection but that colonization dramatically increases in symptomatic individuals. These HIV-infected adults carry a broad range of invasive and noninvasive pneumococcal serotypes compared with HIV-uninfected controls, suggesting a loss of immune control that allows poor pneumococcal colonizers to occupy the nasopharyngeal niche. Surprisingly, carriage remains high in those established on ART. As we have reported previously [24], pneumococcal-specific T-cell proliferation is impaired in asymptomatic HIV infection. We now show that immune memory deteriorates as HIV infection progresses and that even when individuals are well-established on ART with good CD4 recovery (6–12 and ≥18 months), defects in pneumococcal-specific T-cell proliferation persist. These data highlight the complex nature of the immunological control of pneumococcal commensalism and prevention of invasion.
In healthy adults, pneumococcal colonization and disease decline with the acquisition of naturally acquired immune memory. This immunity was thought to be largely mediated by anticapsular antibodies [10]; however, recent carriage experiments comparing antibody-deficient with T-cell-deficient mice suggest that CD4 T cells are key to mucosal clearance of pneumococci [16, 32]. Antibodies to pneumococcal antigens appear to correlate with, but may not be required for, protection against colonization. Following recent data suggesting that Th1/Th17 are important in pneumococcal clearance [12, 16, 18] we investigated whether an underlying qualitative imbalance in Th1/Th17 pneumococcal-specific immunity contributed to our observed changes in pneumococcal colonization. We find that CD4 T-cell IFN-γ production is impaired in asymptomatic HIV-infected individuals, but restored following a dramatic increase in rates of pneumococcal colonization in HIV-symptomatic individuals. Importantly, we find no impairment of pneumococcal-specific IL-17 responses in HIV infection, no increase in IL-17 responses in the context of higher rates of colonization, and no change in IL-17 responses with ART-mediated immune reconstitution. While the importance of Th17-mediated immunity is not excluded by our data, we strongly implicate Th1 responses in the control of pneumococcal colonization in humans. Although Th1 IFN-γ production is restored in symptomatic HIV infection and in individuals on ART, overall there are fewer pneumococcal-specific T cells per se due to prolonged proliferative impairment, which is likely to hinder pneumococcal clearance.
We and others have shown that in populations with a relatively low carriage rates, T-cell responses to mucosal pathogens are somewhat compartmentalized [14, 33, 34]. However, in African populations with higher frequency of colonization, we find a closer relationship between pneumococcal-specific T-cell responses in the upper respiratory tract (URT) mucosa vs periphery (unpublished). We therefore speculate that there is more overspill of T-cell immunity from the mucosa to the blood driven by carriage. Indeed, in this study, the dynamic restoration of pneumococcal-specific IFN-γ responses in heavily colonized HIV-symptomatic individuals supports this possibility. However, potentially the effector:regulatory T cell (Treg) balance within URT mucosa is altered during HIV infection by generalized mucosal T-cell depletion [35], but preserved pneumococcal [14, 33] and HIV-specific Tregs [36] that reduce peripheral Th1 responses. As HIV infection progresses and Tregs decrease [37], higher rates of colonization may help restore pneumococcal-specific Th1 responses in our HIV-infected symptomatic cohort. The relative control of nasopharyngeal pneumococcal clearance in asymptomatic compared with symptomatic HIV-infected individuals raises a number of possibilities. Although mucosal T-cell depletion occurs early on in HIV infection [35] there may be sufficient remnant T-cell immunity together with β-defensins [38], pneumococcal-specific antibodies, complement, and epithelial interleukin 8 recruitment of phagocytes [39] to control colonization. The sudden increase in colonization in HIV-symptomatic individuals may be partly due to the exhaustion and/or overregulation of both innate and adaptive immune control, possibly amplified by opportunistic coinfections, which increase as HIV infection progresses. URT infections cause tissue damage that can favor bacterial attachment [40, 41] and modulate the immune response [42, 43].
The increased carriage of diverse invasive pneumococcal serotypes in HIV-infected individuals has implications for transmission within host populations and adaptation of the pathogen. Our data suggest that HIV-infected adults have impaired mucosal immunity and as a result, serotypes that were rarely carried are now able to colonize. The carriage of a broad range of serotypes provides a reservoir for the transmission of non-vaccine-preventable pneumococci to other vulnerable groups. Furthermore, multiple carriage of noninvasive and invasive pneumococci increases the chance of horizontal gene transfer (both intra- and interspecies) of antibiotic resistance and virulence genes, contributing to the adaptation of the pathogen [8, 9] and increasing the risk of disease.
In conclusion, our findings strongly support the previously murine-dominated data implicating CD4 T-cell-mediated immunity in the control of pneumococcal colonization, providing a mechanistic basis for this immune defect. However, our results are indicative of a more important role for Th1 as opposed to Th17 cells in mediating this protective effect. They also highlight the complex nature of the immune processes involved, which require further investigation at the mucosal epithelial level. Our data also raise the possibility that persistently high rates of pneumococcal colonization in HIV-infected individuals will perpetuate person-to-person spread within the general and HIV-affected population. Novel approaches that control colonization either through improved vaccine efficacy or better pneumococcal-specific T-cell immune reconstitution are required.
Notes
Acknowledgments. The authors thank the volunteers and staff of the Queen Elizabeth Central Hospital, Blantyre, Malawi, for their cooperation with this study, especially Rose Nkhata, our research nurse. We thank James Paton for kindly providing the pneumococcal strains.
Financial support. This work was supported by a grant to R. S. H and N. A. W and the MLW Core Programme grant (R. S. H.) from the Wellcome Trust (grant number 083603/A/07/Z) and by the Bill & Melinda Gates Foundation (grant number JXR10110 to D. E.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci. 1999;354:777–85. doi: 10.1098/rstb.1999.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Nunes MC, von Gottberg A, de Gouveia L, et al. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS. 2011;25:453–62. doi: 10.1097/QAD.0b013e328341b7f1. [DOI] [PubMed] [Google Scholar]
- 4.Gray BM, Converse GM, 3rd, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 5.Auranen K, Ranta J, Takala AK, Arjas E. A statistical model of transmission of Hib bacteria in a family. Stat Med. 1996;15:2235–52. doi: 10.1002/(SICI)1097-0258(19961030)15:20<2235::AID-SIM354>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;10:7. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner P, Hinds J, Turner C, et al. Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J Clin Microbiol. 2011;49:1784–9. doi: 10.1128/JCM.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nesin M, Ramirez M, Tomasz A. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis. 1998;177:707–13. doi: 10.1086/514242. [DOI] [PubMed] [Google Scholar]
- 9.Havarstein LS, Hakenbeck R, Gaustad P. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J Bacteriol. 1997;179:6589–94. doi: 10.1128/jb.179.21.6589-6594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipsitch M, Whitney CG, Zell E, et al. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med. 2005;2:e15. doi: 10.1371/journal.pmed.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Choo S, Finn A. Immune responses to novel pneumococcal proteins pneumolysin, PspA, PsaA, and CbpA in adenoidal B cells from children. Infect Immun. 2002;70:5363–9. doi: 10.1128/IAI.70.10.5363-5369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Bernatoniene J, Bagrade L, et al. Regulation of production of mucosal antibody to pneumococcal protein antigens by T-cell-derived gamma interferon and interleukin-10 in children. Infect Immun. 2006;74:4735–43. doi: 10.1128/IAI.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pido-Lopez J, Kwok WW, Mitchell TJ, Heyderman RS, Williams NA. Acquisition of pneumococci specific effector and regulatory Cd4 T cells localising within human upper respiratory-tract mucosal lymphoid tissue. PLoS Pathog. 2011;7:e1002396. doi: 10.1371/journal.ppat.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp K, Bruunsgaard H, Skinhoj P, Klarlund Pedersen B. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect Immun. 2002;70:5019–25. doi: 10.1128/IAI.70.9.5019-5025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malley R, Trzcinski K, Srivastava A, et al. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina MJ, Greene CM, Gertz RE, et al. Novel antibiotic-resistant pneumococcal strains recovered from the upper respiratory tracts of HIV-infected adults and their children in Kisumu, Kenya. Microb Drug Resist. 2005;11:9–17. doi: 10.1089/mdr.2005.11.9. [DOI] [PubMed] [Google Scholar]
- 21.Gwanzura L, Pasi C, Nathoo KJ, et al. Rapid emergence of resistance to penicillin and trimethoprim-sulphamethoxazole in invasive Streptococcus pneumoniae in Zimbabwe. Int J Antimicrob Agents. 2003;21:557–61. doi: 10.1016/s0924-8579(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 22.Buie KA, Klugman KP, von Gottberg A, et al. Gender as a risk factor for both antibiotic resistance and infection with pediatric serogroups/serotypes, in HIV-infected and -uninfected adults with pneumococcal bacteremia. J Infect Dis. 2004;189:1996–2000. doi: 10.1086/386548. [DOI] [PubMed] [Google Scholar]
- 23.Scott JA, Hall AJ, Muyodi C, et al. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet. 2000;355:1225–30. doi: 10.1016/s0140-6736(00)02089-4. [DOI] [PubMed] [Google Scholar]
- 24.Glennie SJ, Sepako E, Mzinza D, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS One. 2011;6:e25610. doi: 10.1371/journal.pone.0025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwajomo OH, Finn A, Moons P, et al. Deteriorating pneumococcal-specific B-cell memory in minimally symptomatic African children with HIV infection. J Infect Dis. 2011;204:534–43. doi: 10.1093/infdis/jir316. [DOI] [PubMed] [Google Scholar]
- 26.Mureithi MW, Finn A, Ota MO, et al. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis. 2009;200:783–93. doi: 10.1086/605023. [DOI] [PubMed] [Google Scholar]
- 27.Newton R, Hinds J, Wernisch L. Empirical Bayesian models for analysing molecular serotyping microarrays. BMC Bioinformatics. 2011;12:88. doi: 10.1186/1471-2105-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry AM, Ogunniyi AD, Miller DC, Paton JC. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect Immun. 1999;67:981–5. doi: 10.1128/iai.67.2.981-985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333:845–51. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 30.Jordano Q, Falco V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38:1623–8. doi: 10.1086/420933. [DOI] [PubMed] [Google Scholar]
- 31.Glennie SJ, Williams NA, Heyderman RS. Mucosal immunity in resource-limited setting: is the battle ground different? Trends Microbiol. 2011;18:487–93. doi: 10.1016/j.tim.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Trzcinski K, Thompson C, Malley R, Lipsitch M. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect Immun. 2005;73:7043–6. doi: 10.1128/IAI.73.10.7043-7046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Leong SC, McNamara PS, et al. Characterisation of regulatory T cells in nasal associated lymphoid tissue in children: relationships with pneumococcal colonization. PLoS Pathog. 2011;7:e1002175. doi: 10.1371/journal.ppat.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davenport V, Groves E, Hobbs CG, Williams NA, Heyderman RS. Regulation of Th-1 T cell-dominated immunity to Neisseria meningitidis within the human mucosa. Cell Microbiol. 2007;9:1050–61. doi: 10.1111/j.1462-5822.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- 35.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss L, Donkova-Petrini V, Caccavelli L, et al. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–56. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 37.Angin M, Kwon DS, Streeck H, et al. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J Infect Dis. 2012;205:1495–1500. doi: 10.1093/infdis/jis236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HY, Andalibi A, Webster P, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dogan S, Zhang Q, Pridmore AC, et al. Pneumolysin-induced CXCL8 production by nasopharyngeal epithelial cells is dependent on calcium flux and MAPK activation via Toll-like receptor 4. Microbes Infect. 2011;13:65–75. doi: 10.1016/j.micinf.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 42.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–21. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;121:3657–65. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]