Abstract
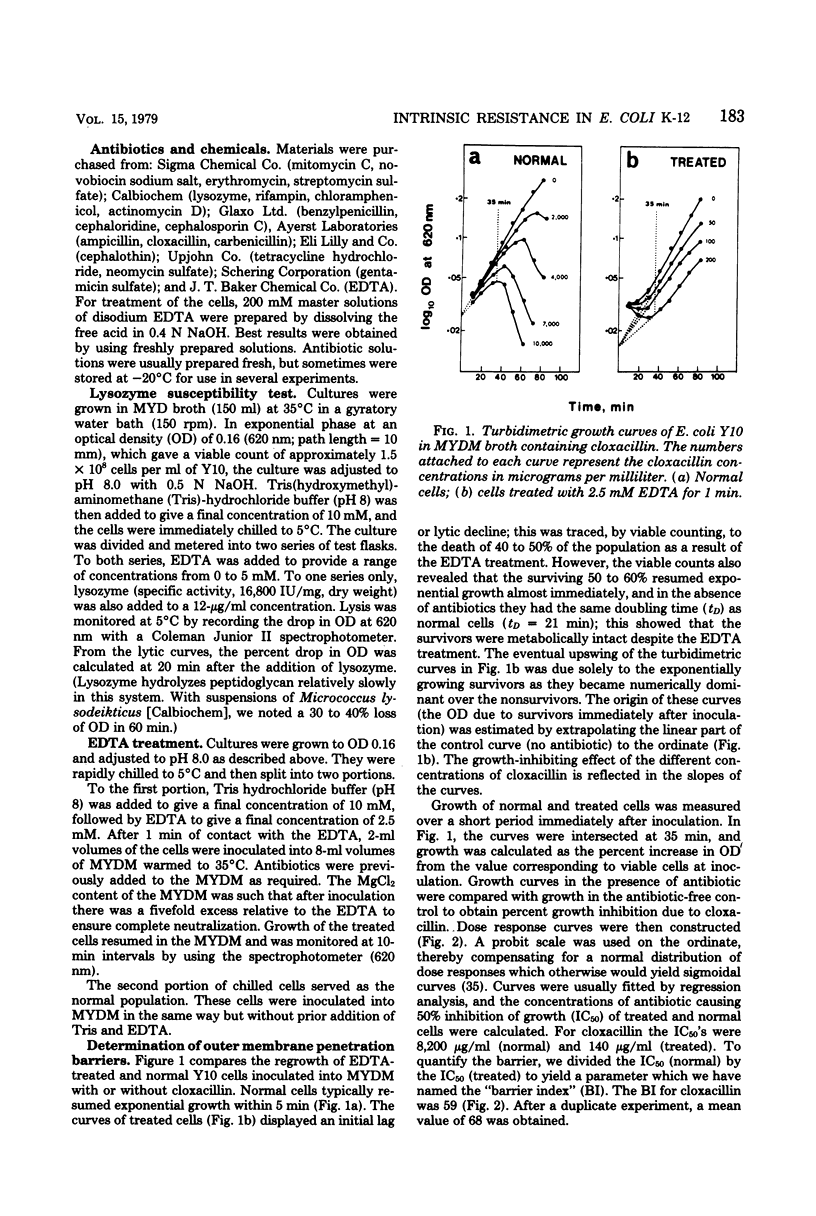
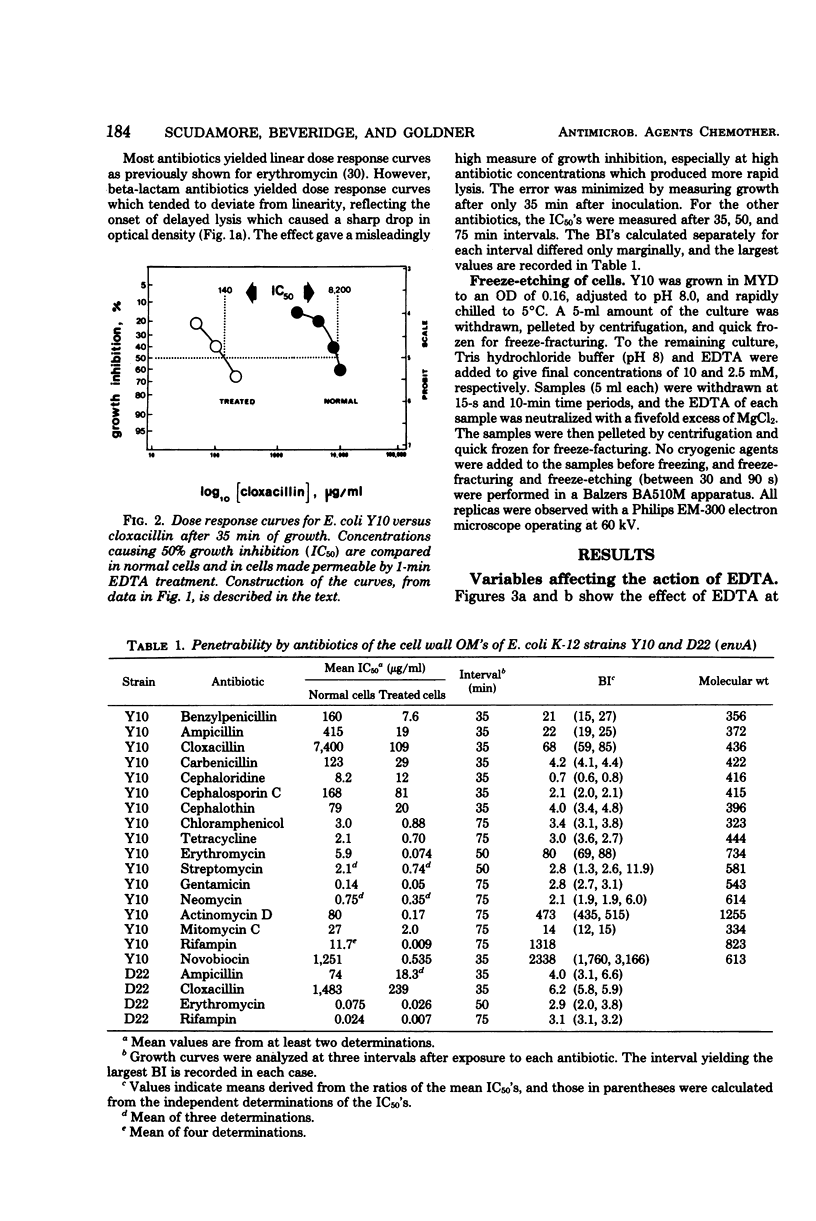
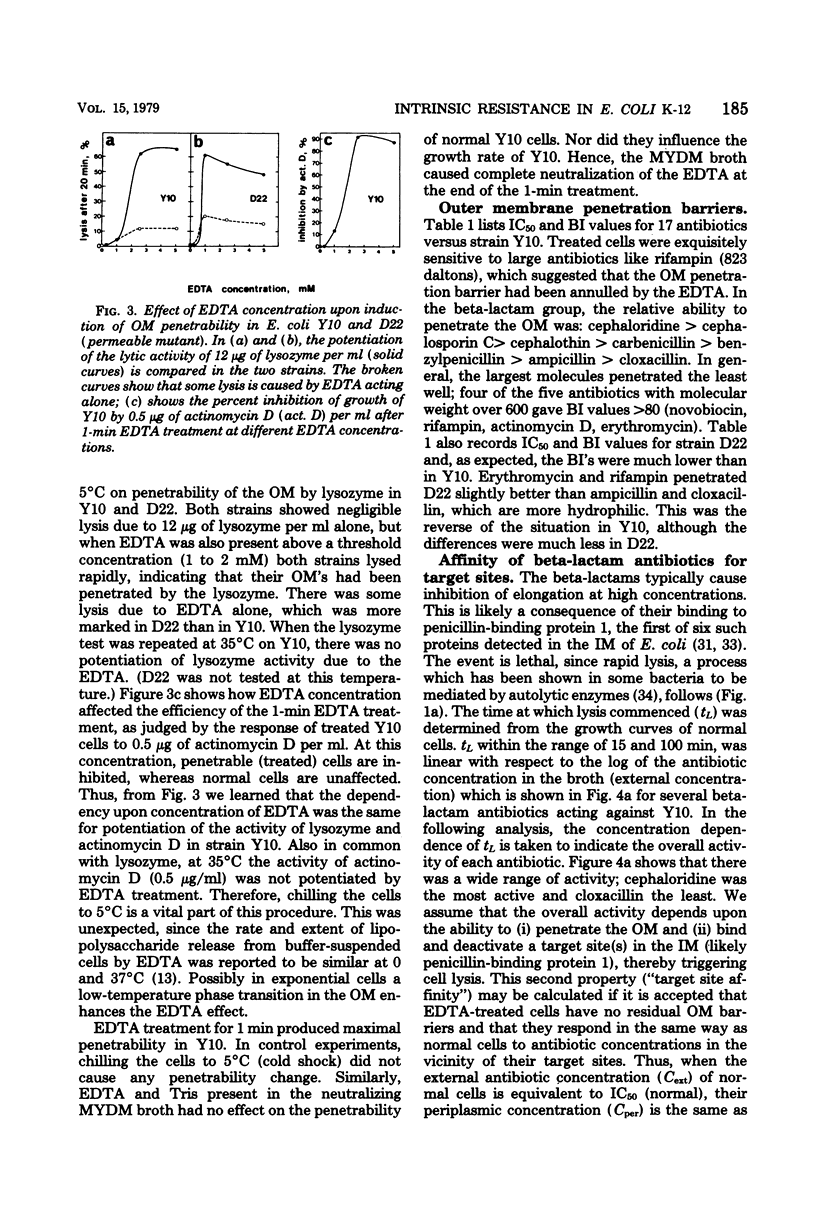
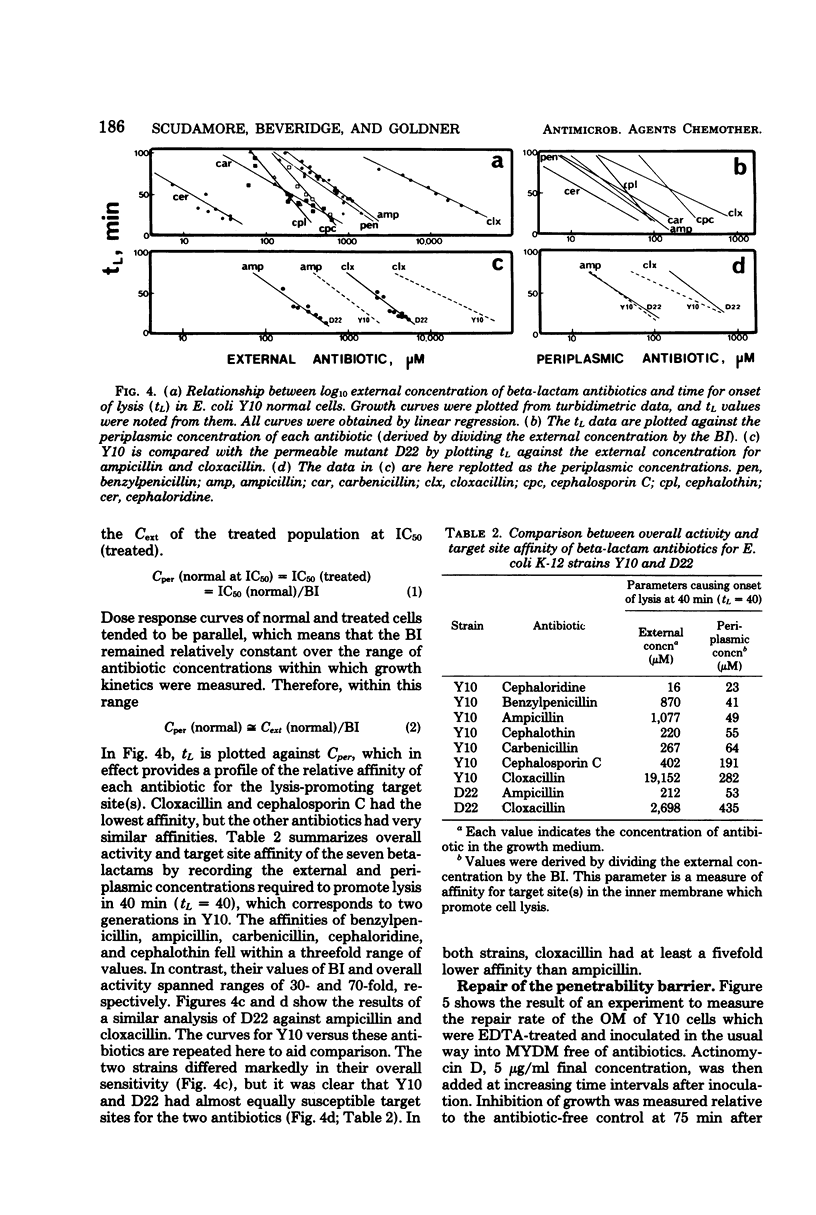
A new technique has been devised to investigate the penetration of antibiotics through the gram-negative outer membrane; the application here was to study intrinsic resistance of Escherichia coli K-12. Exponential cells in broth were briefly treated with 2.5 mM ethylenediaminetetraacetic acid at 5°C to disrupt the outer membrane penetration barrier, and the response of treated and untreated cells to antibiotics was compared by turbidimetry. A barrier index was derived to describe the ability of 7 beta-lactam and 10 other antibiotics to penetrate the outer membrane of strain Y10. There was correlation between the molecular weight and log10 barrier index (r = 0.59, P ≅ 0.01). The envelope mutant D22 (envA) had low barrier indexes for erythromycin, rifampin, ampicillin, and cloxacillin. For the beta-lactams, outer membrane penetration and affinity for inner membrane target site(s) triggering cell lysis were measured as independent components of the overall activity; although penetration and overall activity varied greatly, the affinities of most were within a narrow range.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M. Penicillin binding components of bacterial cells and their relationship to the mechanism of penicillin action. Ann N Y Acad Sci. 1974 May 10;235(0):310–325. doi: 10.1111/j.1749-6632.1974.tb43274.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Jonsson S., Monner D., Normark S., Bloom G. D. Cell-surface alterations in Escherichia coli K-12 with chromosmal mutations changing ampicillin resistance. Ann N Y Acad Sci. 1971 Jun 11;182:342–357. doi: 10.1111/j.1749-6632.1971.tb30670.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formanek H. A three dimensional model of the digestion of peptidoglycan by lysozyme. Biophys Struct Mech. 1977 Dec 27;4(1):1–14. doi: 10.1007/BF00538836. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Effect of EDTA upon bacterial permeability to benzylpenicillin. Biochem Biophys Res Commun. 1965 Sep 22;20(6):688–691. doi: 10.1016/0006-291x(65)90070-7. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Adachi O., Shinagawa E., Ameyama M. Isolation and characterization of outer and inner membranes from Pseudomonas aeruginosa and effect of EDTA on the membranes. J Biochem. 1978 Jan;83(1):171–181. doi: 10.1093/oxfordjournals.jbchem.a131888. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Kent R. L., O'Brien T. F. Characterization and prevalence of the different mechanisms of resistance to beta-lactam antibiotics in clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1974 Dec;6(6):791–801. doi: 10.1128/aac.6.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Normark S., Westling B. Nature of the penetration barrier in Escherichia coli K-12: effect of macromolecular inhibition of penetrability in strains containing the envA gene. J Bacteriol. 1971 Oct;108(1):45–50. doi: 10.1128/jb.108.1.45-50.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Edwards J. R., Wise E. M., Jr In vivo studies on the uptake and binding of beta-lactam antibiotics in relation to inhibition of wall synthesis and cell death. Ann N Y Acad Sci. 1974 May 10;235(0):300–309. doi: 10.1111/j.1749-6632.1974.tb43273.x. [DOI] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Retsema J. A., Ray V. A. Correlation between the binding of beta-lactam antibiotics to Staphylococcus aureus and their physical-chemical properties. Antimicrob Agents Chemother. 1972 Sep;2(3):173–180. doi: 10.1128/aac.2.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Owen P. Bacterial membrane structure. Annu Rev Microbiol. 1976;30:451–482. doi: 10.1146/annurev.mi.30.100176.002315. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- TREFFERS H. P. The linear representation of dosage-response curves in microbial-antibiotic assays. J Bacteriol. 1956 Jul;72(1):108–114. doi: 10.1128/jb.72.1.108-114.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Leive L. Release of lipopolysaccharide in Escherichia coli resistant to the permeability increase induced by ethylenediaminetetraacetate. J Biol Chem. 1970 Mar 10;245(5):1108–1114. [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



