Abstract
BALB/c and C57BL/6 mice differ in their Th1/Th2 lymphocyte and M1/M2 macrophage phenotypes, radiosensitivity, and post-irradiation tumor incidence. In this study we evaluated the effects of repeated low-level exposures to X-rays on the development of artificial tumor colonies in the lungs of animals from the two strains and cytotoxic activities of natural killer (NK) cells and macrophages obtained from these mice. After ten daily irradiations of BALB/c or C57BL/6 mice with 0.01, 0.02, and 0.1 Gy X-rays NK cell-enriched splenocytes collected from the animals demonstrated significant and comparable up-regulation of their anti-tumor cytotoxic function. Likewise, peritoneal macrophages collected from the two irradiated strains of mice exhibited the similarly stimulated cytotoxicities against susceptible tumor cells and produced significantly more nitric oxide. These results were accompanied by the significantly reduced numbers of the neoplastic colonies induced in the lungs by intravenous injection of syngeneic tumor cells. The obtained results indicate that ten low-level irradiations with X-rays stimulate the generally similar anti-tumor reactions in BALB/c and C57BL/6 mice.
Keywords: low fractionated doses of X-rays, radiosensitivity, NK cells, macrophages, tumor colonies, anti-neoplastic activity
INTRODUCTION
Numerous experimental reports indicate that low-level exposures to low-LET ionizing radiation can retard the development of neoplasms in laboratory animals (Cai 1999, Caratero et al. 1998, Cheda et al. 2004b, 2008, Hashimoto et al. 1999, Hosoi and Sakamoto 1993, Ina et al. 2005, Ishii et al. 1996, Ju et al. 1995, Liu 2007, Mitchel et al. 1999, 2003, Nowosielska et al. 2006b, 2011a,b). In these studies the animals were inoculated with tumor cells and the observed tumor-inhibitory effects of the exposures were generally detectable when whole bodies of the subjects were irradiated before the inoculations. In contrast, local irradiations of the growing tumor neither inhibited the inception of metastases nor stimulated lymphocyte migration to the neoplastic tissue (Hashimoto et al. 1999, Safwat 2000). These findings suggest that reactions of the immune system may be involved in the anti-tumor effects of the exposures.
We have corroborated and extended some of the above results demonstrating that the development of the pulmonary tumor colonies in BALB/c mice intravenously (i.v.) injected with syngeneic L1 sarcoma cells was significantly inhibited after single whole body irradiation (WBI) at 0.1 or 0.2 Gy X-rays (Cheda et al. 2004a,b, 2006, Janiak et al. 2006, Nowosielska et al. 2005, 2006b, 2008). Similarly, mice from the same strain exposed to 0.01, 0.02 or 0.1 Gy X-rays daily for ten days had markedly fewer induced ‘metastases’ in the lungs than their sham-irradiated counterparts (Nowosielska et al. 2008, 2011a,b). These effects were accompanied by the significant up-regulation of the anti-tumor cytotoxic reactions of natural killer (NK) lymphocytes (mediated in part by the perforin and/or the Fas receptor ligand pathways) and/or activated macrophages (through the production of nitric oxide) (Cheda et al. 2004b, 2004a, 2005, 2006, 2009, Janiak et al. 2006, Nowosielska et al. 2005, 2006a,b, 2011a,b,). We further demonstrated that both single and fractionated irradiations of BALB/c mice at total absorbed doses of 0.1, 0.2 and 1.0 Gy X-rays significantly stimulated the production of IFN-γ and IL-2 by the NK cell-enriched and total splenocytes, respectively, as well as of IL-1β, IL-12 and TNF-α by activated peritoneal macrophages (Cheda et al. 2008, 2009, Nowosielska et al. 2010, 2011a,b). Collectively, our findings supported the notion that cooperation of macrophages and NK cells may be necessary for the efficient control of the development of both primary and secondary tumors in vivo (Young and Ortaldo 2006).
All our previous examinations were performed on the relatively radiosensitive BALB/c mice.
Obviously, other results may be seen in strains of the differently determined immunological profile and/or radiosensitivity. Hence, in the present study we also employed C57BL/6 mice which, compared to BALB/c mice, exhibit a Th1/M1 (Th lymphocyte/macrophage) response, are more radioresistant, and develop fewer types of cancers following irradiation (Kataoka et al. 2006, Mills et al. 2000, Okayasu et al. 2000, Storer et al. 1988, Wells et al. 2003). Since the latter two differences may result from different responses of the immune systems of the two strains to radiation the aim of the present study was to evaluate the effects of fractionated low-level exposures of C57BL/6 and BALB/c mice to X-rays on the growth of induced tumor colonies and activities of cells involved in the innate anti-tumor immunity.
MATERIAL AND METHODS
Animals and irradiation
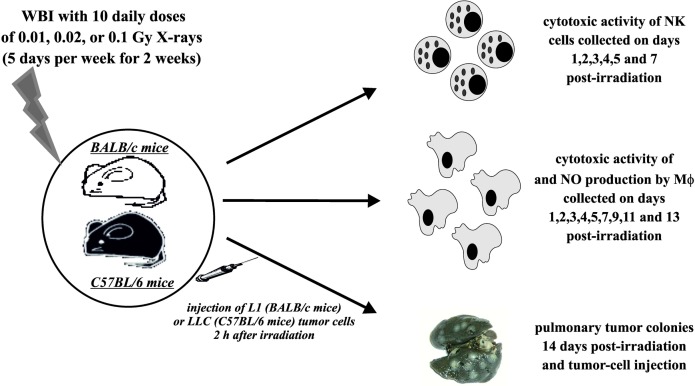
Male BALB/c mice were obtained from the Nofer Institute of Occupational Medicine, Lodz, Poland, and male C57BL/6 mice were obtained from the Institute of Biochemistry and Biophysics, Warsaw, Poland, and at 6–8 weeks of age were used for the experiments (Fig. 1). The animals were exposed daily for 10 days (5 days per week for 2 weeks) to the WBI from the ANDREX X-ray generator (150 kV, 3 mA; Holger Andreasen, Denmark) at 2.16 Gy/hour dose rate so that the absorbed doses per mouse per day equaled to 0.01, 0.02 or 0.1 Gy and the total absorbed doses per mouse equaled to 0.1, 0.2 or 1.0 Gy X-rays, respectively; all the irradiations were performed at the Institute of Nuclear Chemistry and Technology, Warsaw, Poland. Control mice were sham-exposed (generator at the off-mode) in identical conditions. All the preparations and assays described below were performed after completion of the ten-day exposures of the mice to X-rays. The absorbed doses were verified using thermoluminescent dosimeters (TLD; Institute of Nuclear Physics, Krakow, Poland) implanted subcutaneously (s.c.) in the middle abdominal region and removed after completion of the irradiations. The TLDs were then analysed using the Laboratory Reader-Analyser TLRA 94 (Mikrolab, Kraków, Poland) by counting the light signals the number of which is directly proportional to the absorbed dose of X-rays; the proportion was calculated based on the calibration curve prepared earlier after irradiations of TLDs at 0.05, 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 Gy of X-rays. All mice were maintained under specific pathogen-free conditions. The investigations were carried out by permission of the Local Ethical Committee for Experimentation on Animals at the National Institute of Public Health in Warsaw.
FIG. 1.
Outline of the experimental procedures. NK cells – splenocytes enriched for NK lymphocytes; MΦ – activated peritoneal macrophages.
Tumor cells
L1 sarcoma (L1; obtained from the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology, Warsaw, Poland) or Lewis Lung Carcinoma (LLC; obtained from the Polish Academy of Sciences, Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Wroclaw, Poland) cells were used for the induction of pulmonary tumour nodules. YAC-1 lymphoma (obtained from the Polish Academy of Sciences, Ludwik Hirszfeld Institute of Immunology and Experimental Therapy) and P815 mastocytoma (obtained from the Medical University of Warsaw, Poland) cells were used as targets in the NK cell- and macrophage-mediated cytotoxicity assays, respectively. The cells were maintained in a culture medium (CM) composed of the RPMI-1640 medium with L-glutamine (PAA, Pasching, Austria), 10% FBS (PAA), 100 U/ml penicillin (Polfa, Warsaw, Poland) and 100 mg/ml streptomycin (Polfa) in standard conditions (SC): humidified atmosphere of 95% air and 5% CO2 at 37°C.
Preparation of the NK cell-enriched splenocytes
On the selected days after the irradiation mice were killed, single cell-suspensions prepared from the spleens were resuspended in CM and incubated on glass Petri dishes for 40 min in SC; in each case the cells were collected and pooled from at least five mice. Non-adherent cells were then collected and erythrocytes present in the suspension were lysed. After washing and resuspending in CM the cells were passed through a nylon wool column and the wool nonadherent cells were recovered as the NK cell enriched splenocytes (12% as estimated by labelling with the anti-mouse Pan-NK Cells DX5 antibody; Becton Dickinson [BD], Warsaw, Poland) (Cheda et al. 2004b).
NK cell-mediated cytotoxicity assay
Cytolytic activity of the NK cell-enriched splenocytes was measured using the 51Cr-release assay (Cheda et al. 2004b). Briefly, the YAC-1 target cells (T; 106 in 100 ml CM ) were incubated in SC for 1.5 h with 5.55 MBq of sodium chromate (Na251CrO4; Polatom, Otwock-Swierk, Poland). Then, the cells were washed with PBS (Biomed, Lublin, Poland) and added to the NK cell-enriched effector splenocytes (E) at 100:1 E:T cell ratio; five samples were taken for each in vitro experimental group. After the 4-h incubation in SC, aliquots of the cell-free supernatants were harvested and the radioactivity of 51Cr released from T was measured in a γ-counter (Auto-g Cobra II; Canberra-Packard, Warsaw, Poland). The ratio of cytolytic activity was calculated using the formula: % cytotoxicity = [(experimental release – spontaneous release)/(maximum release –spontaneous release)] × 100.
Preparation of peritoneal macrophages
Two days before the collection of peritoneal macrophages mice were intraperitoneally (i.p.) injected with 1 ml of 10% Sephadex G-25 (Pharmacia, Uppsala, Sweden) and peritoneal exudate cells were collected on the selected days after cessation of the irradiations (in each case the cells were collected and pooled from at least five mice), resuspended in CM and incubated on glass Petri dishes for 2 h in SC. The glass-adherent cells with morphological features of a typical macrophage were then harvested and resuspended in CM (Nowosielska et al. 2006b).
Macrophage-mediated cytotoxicity assay
Cytolytic activity of macrophages was measured using the [3H]thymidine-uptake assay (Nowosielska et al. 2006b). Briefly, the P815 target cells (T; 4×106 in 2.5 ml CM) were incubated for 20 h in SC with 0.3 MBq of [3H]thymidine (Polatom). Then, the cells were washed with PBS, added to macrophages (E) at 20:1 E:T ratio and incubated in SC with 50 U/ml IFN-γ (Sigma, Poznan, Poland) and 100 ng/ml LPS (Sigma); five samples were taken for each in vitro experimental group. After the 48-h incubation viable adherent cells (Mφ) were lysed, harvested, and their radioactivity was monitored in a β-counter (Tri-Carb 2100TR Counter, Canberra-Packard). The rate of cytotoxicity was calculated using the formula: [(A −B)/A]×100%, where A indicates isotope disintegrations counted in T cultured alone, and B indicates the counts measured in T cultured in the presence of macrophages.
Production of nitric oxide
Nitric oxide (NO) synthesized in macrophages was quantitated by measuring the level of the nitrite ion (NO2−) in the incubation medium (Farias-Eisner et al. 1994). The cells were suspended in CM, supplemented with 50 U/ml IFN-γ and 100 ng/ml LPS and incubated for 48 h in SC; eight samples were taken for each in vitro experimental group. After that, 100 μl of the supernatant was mixed with 100 μl of the Griess reagent and kept in the dark for 10 min at room temperature (RT). Absorbance at 540 nm was then measured using the SpectraCount™ microplate reader (Canberra Packard).
Tumor colony assay
To obtain the L1 or LLC cells for the assays, 14 days after the s.c. transplantation of 106 L1 or LLC cells to three BALB/c or C57BL/6 mice, respectively, the developed tumors were removed, minced, and incubated for 30 min at RT in 0.25% trypsin- EDTA (Gibco, Warsaw, Poland) and standard DNase I enzyme solution (Sigma). After that, the cells were washed and resuspended in CM. For the assay, 2 h after cessation of the irradiations 2.5x105 L1 or LLC cells per mouse were intravenously (i.v.) injected (24 mice were used per group). Fourteen days later the animals were killed and total numbers of macroscopic colonies were counted on the surface of the dissected lungs (Cheda et al. 2004b).
Statistical analysis
All the assays were repeated two or three times and mean values and SDs were calculated for the results thereof. For statistical analysis of the results, the Mann-Whitney U test for non-parametric trials was used and p values less than 0.05 were regarded as significant. Pearson’s correlation analysis was carried out for the cytotoxic activities or production of NO against dose for each day and correlation coefficients at the significance level of p < 0.05 indicated the degree of linear relationship between the two variables.
RESULTS
In two preliminary experiments conducted on BALB/c mice (one with the fractionated total dose of 0.2 Gy and another with the fractionated total dose of 1 Gy; results not shown) no significant differences in the cytotoxic activity of the NK cell-enriched splenocytes were detected between the control and irradiated groups beyond days 5–7 (and up to day 15) after cessation of the exposures. Hence, in the following assays this activity was estimated only up to day 7 post-exposure.
As shown in Table 1, WBI of BALB/c mice with 10 daily doses of 0.01, 0.02 or 0.1 Gy X-rays led to the significant and comparable (i.e., dose-independent) enhancement of the cytolytic function of the NK cell-enriched splenocytes obtained from all the three groups of the exposed animals. This effect was pronounced already on the first day after cessation of the irradiations and occurred for four days after the exposure ended. Between the fifth and seventh days post-exposure the activity of the NK cell-enriched splenocytes declined to the control level.
Table 1.
Cytotoxic activity [%] of NK cell-enriched splenocytes on various days after repeated WBI of BALB/c or C57BL/6 mice at 0.01, 0.02, and 0.1 Gy X-rays.
| Days post exp. | BALB/c mice | C57BL/6 mice | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Control | 10x 0.01 Gy | 10x 0.02 Gy | 10x 0.1 Gy | Control | 10x 0.01 Gy | 10x 0.02 Gy | 10x 0.1 Gy | |
| 1 | 7.5 ± 0.9 | 13.2* ± 1.5 | 12.9* ± 1.5 | 13.3* ± 1.6 | 10.1 ± 0.9 | 10.5 ± 1.5 | 11.1 ± 1.5 | 10.2 ± 1.6 |
| 2 | 7.1 ± 0.9 | 10.2* ± 1.1 | 11.1* ± 1.2 | 11.5* ± 1.3 | 10.3 ± 0.9 | 10.9 ± 1.1 | 10.3 ± 1.2 | 9.7 ± 1.3 |
| 3 | 7.6 ± 1.0 | 13.6* ± 1.4 | 14.0* ± 1.7 | 13.2* ± 1.5 | 10.6 ± 1.0 | 18.2* ± 1.4 | 14.2* ± 1.7 | 14.9* ± 1.5 |
| 4 | 7.0 ± 0.7 | 11.9* ± 1.4 | 12.3* ± 1.5 | 12.0* ± 1.4 | 10.7 ± 0.7 | 15.2* ± 1.4 | 16.1* ± 1.5 | 19.2* ± 1.4 |
| 5 | 7.0 ± 0.8 | 8.2 ± 1.1 | 8.6 ± 1.1 | 8.9 ± 1.1 | 10.6 ± 0.8 | 15.6* ± 1.1 | 19.2* ± 1.1 | 17.5* ± 1.1 |
| 7 | 6.9 ± 0.8 | 6.6 ± 0.9 | 6.7 ± 0.8 | 6.8 ± 0.7 | 10.1 ± 0.8 | 14.2* ± 0.9 | 15.8* ± 0.8 | 17.0* ± 0.7 |
Control - sham-exposed mice; 10 × 0.01 Gy - mice exposed to ten fractions of 0.01 Gy X-rays; 10 × 0.02 Gy - mice exposed to ten fractions of 0.02 Gy X-rays; 10 × 0.1 Gy - mice exposed to ten fractions of 0.1 Gy X-rays. Days post exp. - days after cessation of irradiations. Mean values obtained from three independent experiments are presented; in each experiment five mice were used per group per each time point.
indicates statistically significant (p<0.05) difference from the control group.
Cytotoxic activity of NK cell-enriched splenocytes collected from C57BL/6 mice after ten daily irradiations at 0.01, 0.02, or 0.1 Gy X-rays was also significantly and comparably stimulated (Table 1). However, the kinetics of this activity was different from that demonstrated in BALB/c mice, i.e., the effect cropped up on the third day after cessation of the exposures and carried on for at least four days to follow.
As demonstrated in Table 2, WBI of BALB/c mice with 10 equal doses of 0.01, 0.02 or 0.1 Gy X-rays resulted in the significant, dose-independent (as indicated by the correlation analysis; Pearson’s correlation coefficients for the cytotoxic activity against the dose for days 1–13 after completion of the irradiation ranged from 0.282 to 0.928 at p > 0.05) stimulation of the cytolytic activity of macrophages incubated with IFN-γ, LPS, and the P815 target cells. Enhanced cytocidal function of Mφ was detected on the second day after cessation of the exposures to X-rays, reached the highest values on the fourth (daily doses of 0.1 Gy) or fifth (daily doses of 0.01 or 0.02 Gy) days, and then, after a transient decline to the control level on the seventh day, was rising again until the 13th (at least) day post-exposure.
Table 2.
Cytotoxic activity [%] of macrophages on various days after repeated WBI of BALB/c or C57BL/6 mice at 0.01, 0.02, and 0.1 Gy X-rays.
| Days post exp. | BALB/c mice | C57BL/6 mice | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Control | 10x 0.01 Gy | 10x 0.02 Gy | 10x 0.1 Gy | Control | 10x 0.01 Gy | 10x 0.02 Gy | 10x 0.1 Gy | |
| 1 | 25.5 ± 1.8 | 28.7 ± 1.4 | 29.2 ± 1.5 | 29.7 ± 1.5 | 30.0 ± 2.1 | 50.1* ± 2.5 | 47.5* ± 2.4 | 52.5* ± 2.6 |
| 2 | 26.0 ± 1.8 | 35.7* ± 1.8 | 36.9* ± 1.8 | 33.6* ± 1.7 | 30.3 ± 2.1 | 44.9* ± 2.2 | 39.8* ± 2.0 | 41.2* ± 2.1 |
| 3 | 25.1 ± 1.8 | 45.9* ± 2.3 | 48.1* ± 2.4 | 42.3* ± 2.1 | 30.4 ± 2.1 | 50.3* ± 2.5 | 35.6 ± 1.8 | 39.7* ± 2.0 |
| 4 | 25.3 ± 1.8 | 51.4* ± 2.6 | 53.7* ± 2.7 | 55.6* ± 2.8 | 30.1 ± 2.0 | 69.6* ± 3.5 | 52.6* ± 2.6 | 66.6* ± 3.3 |
| 5 | 26.1 ± 1.8 | 56.5* ± 2.8 | 58.3* ± 2.9 | 51.9* ± 2.6 | 30.3 ± 2.0 | 47.8* ± 2.4 | 46.7* ± 2.3 | 42.7* ± 2.1 |
| 7 | 25.7 ± 1.8 | 26.5 ± 1.3 | 27.3 ± 1.4 | 25.9 ± 1.3 | 30.5 ± 1.9 | 23.4 ± 1.2 | 22.7 ± 1.1 | 29.3 ± 1.5 |
| 9 | 25.0 ± 1.7 | 31.4* ± 1.6 | 32.1* ± 1.6 | 25.9 ± 1.3 | 30.4 ± 1.9 | 49.6* ± 2.5 | 40.1* ± 2.0 | 48.2* ± 2.4 |
| 11 | 25.1 ± 1.8 | 38.7* ± 1.9 | 39.6* ± 2.0 | 31.3* ± 1.6 | 30.2 ± 2.1 | 50.8* ± 2.5 | 49.4* ± 2.5 | 50.9* ± 2.5 |
| 13 | 25.5 ± 1.6 | 52.1* ± 2.6 | 53.4* ± 2.7 | 50.2* ± 2.5 | 30.1 ± 2.0 | 33.6 ± 2.0 | 39.2* ± 2.0 | 45.3* ± 2.3 |
Control - sham-exposed mice; 10 × 0.01 Gy - mice exposed to ten fractions of 0.01 Gy X-rays; 10 × 0.02 Gy - mice exposed to ten fractions of 0.02 Gy X-rays; 10 × 0.1 Gy - mice exposed to ten fractions of 0.1 Gy X-rays. Days post exp. - days after cessation of irradiations. Mean values obtained from three independent experiments are presented; in each experiment five mice were used per group per each time point.
indicates statistically significant (p<0.05) difference from the control group.
In turn, ten daily exposures of C57BL/6 mice to 0.01, 0.02, or 0.1 Gy of X-rays led to the significant and comparable (Pearson’s correlation coefficients for the cytotoxic activity against the dose for days 1–13 after completion of the irradiation ranged from −0.727 to 0.441 at p > 0.05) stimulation of the cytotoxic activity of Mφ, although the effect seemed to be slightly less pronounced after the daily irradiations at 0.02 Gy compared to 0.01 or 0.1 Gy (Table 2). As in BALB/c mice, the stimulation was also biphasic with a transient decline to the control values on the seventh day, but the boosted cytocidal function of the C57BL/6 Mφ was detectable already on the first day after cessation of the exposures and, past the seventh day nadir, was rising again only until the 11th day post-irradiation.
As shown in Table 3, WBI of BALB/c mice with 10 daily doses of 0.01, 0.02 or 0.1 Gy X-rays resulted in the significant and kinetically comparable (Pearson’s correlation coefficients for the production of NO against the dose for days 1–13 after completion of the irradiation ranged from 0. 091 to 0.806 at p > 0.05) stimulation of the production of NO in macrophages incubated with IFN-γ and LPS. The enhanced secretion of NO was manifested already on the first day after cessation of the exposures, reached the highest levels on the fourth (daily doses of 0.1 Gy) or fifth (daily doses of 0.01 or 0.02 Gy) days post-exposure, declined to the control level on the seventh day (after irradiation with 0.1 Gy daily the decline below the control values occurred on days seventh and ninth post-exposure), and was rising again to the significantly increased levels until at least day 13 post-irradiation.
Table 3.
Production of NO [μM/l] by macrophages on various days after repeated WBI of BALB/c or C57BL/6 mice at 0.01, 0.02, and 0.1 Gy X-rays.
| Days post exp. | BALB/c mice | C57BL/6 mice | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Control | 10x 0.01 Gy | 10x 0.02 Gy | 10x 0.1 Gy | Control | 10x 0.01 Gy | 10x 0.02 Gy | 10x 0.1 Gy | |
| 1 | 10.1 ± 0.9 | 16.2* ± 1.1 | 18.0* ± 1.3 | 19.3* ± 1.3 | 18.5 ± 1.3 | 30.9* ± 1.5 | 29.3* ± 1.5 | 32.4* ± 1.6 |
| 2 | 10.4 ±1.0 | 18.3* ± 1.3 | 24.8* ± 1.7 | 17.2* ± 1.2 | 18.7 ± 1.2 | 27.7* ± 1.4 | 24.6* ± 1.2 | 25.4* ± 1.3 |
| 3 | 10.3 ± 1.0 | 26.3* ± 1.8 | 29.4* ± 2.0 | 25.5* ± 1.5 | 18.8 ± 1.3 | 31.1* ± 1.6 | 21.9 ± 1.1 | 24.5* ± 1.2 |
| 4 | 10.4 ± 1.0 | 23.6* ± 1.6 | 26.5* ± 1.9 | 32.5* ± 2.3 | 18.6 ± 1.1 | 43.0* ± 2.1 | 32.5* ± 1.6 | 41.1* ± 2.1 |
| 5 | 10.7 ± 1.1 | 30.7* ± 2.1 | 36.8* ± 2.5 | 28.1* ± 1.9 | 18.7 ± 1.2 | 29.5* ± 1.5 | 28.8* ± 1.4 | 26.4* ± 1.3 |
| 7 | 11.2 ± 1.1 | 11.3 ±0.8 | 9.9 ± 0.7 | 8.9 ± 0.7 | 18.8 ± 1.2 | 14.4 ± 0.7 | 14.0 ± 0.7 | 18.1 ± 0.9 |
| 9 | 11.5 ± 1.0 | 20.3* ±1.3 | 15.1* ± 1.0 | 8.4 ± 0.6 | 18.8 ± 1.3 | 30.6* ± 1.5 | 24.8* ± 1.2 | 29.7* ± 1.5 |
| 11 | 10.6 ± 0.9 | 21.0* ±1.5 | 18.2* ± 1.3 | 12.5 ± 0.8 | 18.6 ± 1.3 | 31.3* ± 1.6 | 30.5* ± 1.5 | 31.4* ± 1.6 |
| 13 | 11.0 ± 1.1 | 33.9* ± 2.0 | 33.1* ± 2.3 | 23.4* ± 1.5 | 18.6 ± 1.2 | 20.7 ± 1.0 | 24.2* ± 1.2 | 28.0* ± 1.4 |
Control - sham-exposed mice; 10 × 0.01 Gy - mice exposed to ten fractions of 0.01 Gy X-rays; 10 × 0.02 Gy - mice exposed to ten fractions of 0.02 Gy X-rays; 10 × 0.1 Gy - mice exposed to ten fractions of 0.1 Gy X-rays. Days post exp. - days after cessation of irradiation. Mean values obtained from three independent experiments are presented; in each experiment five mice were used per group per each time point.
indicates statistically significant (p<0.05) difference from the control group.
Likewise, ten daily exposures of C57BL/6 mice to 0.01, 0.02, or 0.1 Gy of X-rays led to the significant and comparable (Pearson’s correlation coefficients for the production of NO against the dose for days 1–13 after completion of the irradiation ranged from 0.102 to 0.913 at p > 0.05) stimulation of NO in Mφ. The effect was detectable already on the first day post-exposure and also followed the biphasic pattern: elevated secretion of NO reached the highest levels on the fourth day, declined to below the control levels on the seventh day, and then rose again to reach the second peak on the 11th day post-irradiation. Notably, in both strains of the mice the kinetics of the stimulated production of NO in the activated macrophages closely followed the biphasic changes in the cytocidal activities of these cells.
As shown in Table 4, WBI of BALB/c mice with 10 daily doses of 0.01, 0.02, and 0.1 Gy X-rays prior to i.v. injection of L1 cells resulted in the significantly reduced number of the developed tumor colonies in the lungs (expressed as percentages of the control values obtained in the sham-exposed animals). Since in all the irradiated groups of the mice there was an approx. 45% reduction in the number of the colonies, the effect seemed to be dose-independent.
Table 4.
Relative numbers [% of the control value] of the pulmonary L1 sarcoma or Lewis Lung Carcinoma cell colonies in BALB/c or C57BL/6 mice, respectively, exposed to repeated WBI at 0.01. 0.02. and 0.1 Gy X-rays.
| Group | BALB/c mice | C57BL/6 mice |
|---|---|---|
| Control | 100.0 ± 25.0 | 100.0 ± 22.4 |
| 10x 0.01 Gy | 60.2*± 14.4 | 82.5* ± 25.7 |
| 10x 0.02 Gy | 50.4*± 12.9 | 73.9* ± 21.3 |
| 10x 0.1 Gy | 54.7*± 15.1 | 65.9* ± 18.3 |
Control - sham-exposed mice; 10 × 0.01 Gy - mice exposed to ten fractions of 0.01 Gy X-rays; 10 × 0.02 Gy - mice exposed to ten fractions of 0.02 Gy X-rays; 10 × 0.1 Gy - mice exposed to ten fractions of 0.1 Gy X-rays. Mean values obtained from two independent experiments are presented; in each experiment experimental groups consisted of 24 mice each.
indicates statistically significant (p<0.05) difference from the control group.
In contrast, although ten daily exposures of C57BL/6 mice to 0.01, 0.02, or 0.1 Gy of X-rays also markedly inhibited the development of the pulmonary tumor colonies produced by injection of the syngeneic LLC cells, the effect seemed to be dose-dependent: the strongest reduction was detected after irradiations of the mice at 0.1 Gy daily (about 34%), while in the animals exposed daily to 0.01 or 0.02 Gy the number of the colonies decreased by about 18% and 26%, respectively.
DISCUSSION
Based on their post-irradiation survival rate and incidence of radiogenic neoplasms BALB/c and C57BL/6 mice are regarded as radiosensitive or radioresistant, respectively (Storer et al. 1988). Indeed, Ponnaiya et al. (1997) demonstrated that primary mammary epithelial cells collected from BALB/c mice and exposed in vitro to 3.0 Gy of 137Cs γ-rays at 0.74 Gy/min. dose rate exhibited a marked increase in the frequency of radiation-induced genomic instability, whereas no such effect was observed in the same cells collected from C57BL/6 mice; in the latter animals frequency of the radiation-induced chromatid-type aberrations after the rapid clearance of initial aberrations remained at or near that detected in non-irradiated controls. Also, mammary epithelium of BALB/c mice appears to be inherently more sensitive to the transforming effect of ionizing radiation (as measured by the induction of the ductal dysplasia) than the same tissue in C57BL/6 mice (Ullrich et al. 1996, Ullrich and Ponnaiya 1998). Moreover, cells from radiosensitive, cancer-prone BALB/c mice showed inefficient non-homologous end joining of the γ-ray-induced double-strand breaks as compared with cells from the C57BL/6 and other commonly used strains of mice (Okayasu et al. 2000).
In terms of immune responses C57BL/6 mice are regarded as a prototypical Th1 strain, whereas BALB/c mice tend to display typical Th2 responses: T lymphocytes from the former strain produce IFN-γ, which activates macrophages to produce NO, while T lymphocytes from the latter strain produce macrophage-suppressive interleukins – IL-4, IL-5, and IL-10 (Mills et al. 2000). Moreover, Mills et al. (2000) proposed to divide macrophages into M1 and M2 subtypes, which predominately express the nitric oxide synthase (iNOS) or arginase pathways of the arginine metabolism, respectively. These investigators also indicated that C57BL/6 mice express the Th1/M1- (pro-inflammatory) and BALB/c mice – the Th2/M2 (anti-inflammatory) phenotypes. However, it was also indicated that the M1 and M2 macrophage characteristics represent extremes of a phenotypic continuum not directly related to the clonally separable Th1 and Th2 lymphocytes (Coates et al. 2008, Mills et al. 2000)
Since cytotoxic macrophages and/or NK cells constitute important components of the innate anti-neoplastic immune system (Nathan 1991, Farias-Eisner et al. 1994, Liu et al. 1994, Moretta et al. 1994, Barao and Ascensao 1998, Al-Sarireh and Eremin 2000) and in view of the above described differences between radiosensitive BALB/c and radioresistant C57BL/6 mice, we sought in the present study to compare the effects of fractionated low-level exposures to X-rays on the development of induced tumor colonies and anti-neoplastic activities of activated macrophages and NK lymphocytes in the two strains of the animals.
The results demonstrate that ten daily exposures of BALB/c and C57BL/6 mice to 0.01, 0.02 or 0.1 Gy X-rays significantly stimulated anti-neoplastic functions of the NK cell-enriched splenocytes and the in vitro activated macrophages obtained from both strains and that such effects coincided with the radiation-induced suppression of the induced tumor colonies in the lungs of the animals. There were, however, few differences in reactions of the BALB/c and C57BL/6 mice to the exposures: in the former animals stimulation of the cytotoxic activity of the NK cell-enriched splenocytes occurred between the first and fourth days after cessation of the irradiations, while in C57Bl/6 mice similar effects were detectable later, i.e., between days three and seven post-exposure. In contrast, the low-level X-ray-induced up-regulation of the cytotoxicity of Mφ occurred earlier and was slightly more pronounced in the latter compared to the former mice; however, while the cytotoxicity of the macrophages from C57BL/6 mice began to fall after days four and 11 post-exposure, the same function of Mφ from BALB/c mice tended to be still on the rise at those times. With respect to the tumor colonies in the lungs, the post-exposure reduction in their numbers was more pronounced in BALB/c than C57BL/5 mice and the effect appeared to be dose-independent, whereas in the latter mice the reduction was less marked (especially following the lowest dose) and clearly increased with the rise of the absorbed dose.
Apart from these differences, responses of the two strains of mice to repeated low-level irradiations with X-rays appeared to be pretty comparable, especially in terms of the biphasic stimulation of the activities of macrophages. Indeed, decline of the boosted cytotoxicity of and NO synthesis in these cells exactly at the same time (i.e., on the seventh day) post-irradiation followed by the second wave of stimulation observed in both strains is surprising and difficult to explain. Notably, very similar kinetics and similar differences between BALB/c and C57BL/6 mice in terms of the production of NO by Mφ likely supports the described tumoricidal role of this molecule (Nathan 1991, Cui et al. 1994, Farias-Eisner et al. 1994, Jenkins et al. 1995, Xie and Fidler 1998).
Based on the results of other authors (Coates et al. 2008) it can be speculated that the in vivo irradiations employed in the present study and/or the in vitro manipulations of the assayed cells might have caused shifts in the phenotypes of activated macrophages along the M1-M2 and the M2-M1 spectra. Indeed, as demonstrated by Coates et al. (2008) unstimulated bone marrow macrophages obtained from C57BL/6 mice exhibit M2 rather than M1 characteristics which is even more pronounced after exposure of the mice at 4 Gy γ-rays; these cells assume M1 phenotype only after in vitro stimulation with LPS and IFN-γ. Similarly, Mills et al. (2000) showed that BALB/c macrophages, which have intrinsic M2 phenotype, can also be shifted by exposure to LPS and IFN-γ towards the M1 response, although the effect is less pronounced than in the C57BL/6 macrophages. In fact, in the present study the LPS and IFN-γ-stimulated Mφ collected from C57BL/6 mice produced slightly greater amounts of NO than the same cells obtained from BALB/c mice. Moreover, macrophages from both low-dose X-ray irradiated and sham-exposed BALB/c (Nowosielska et al. 2006a,b) and C57BL/6 (data not published) mice and cultured without LPS and IFN-γ produced negligible amounts of NO, whereas addition of these classic pro-inflammatory stimuli to the incubation medium boosted the secretion of NO, which was significantly more pronounced in the irradiated compared to the control mice. These observations of the stimulatory effects of the low-level exposures to X-rays contrast with the findings of Coates et al. (2008) who reported that WBI of C57BL/6 mice at 4 Gy γ-rays enhanced the M2 characteristics of bone marrow macrophages obtained from these animals.
We demonstrated previously that single exposures of BALB/c mice to low doses of X-rays significantly stimulated tumoricidal functions of activated macrophages and that the enhanced activity of these cells may, to an even larger extent than NK cells, account for the tumor-inhibitory effect of such exposures (Cheda et al. 2004b, Nowosielska et al. 2006b). The present results demonstrate that both cell types may be responsible for the anti-metastatic effects of fractionated low-level exposures of both radiosensitive (BALB/c) and radioresistant (C57BL/6) mice to X-rays. Indeed, cooperation of macrophages, NK lymphocytes, and probably other cells of the anti-tumor surveillance system along with their cytocidal factors is necessary for the efficient control of the development of both primary and secondary tumors in vivo. Thus, despite the fact that C57BL/6 and BALB/c mice vary in their Th phenotypes, transitions across the M1-M2 continuum of macrophage responses as well as other in vivo stimuli may explain the similar in the two strains final anti-tumor effect of the ten daily irradiations with low doses of X-rays.
Although no directly comparable data are available, a few reports have indicated that the effect of the low-level irradiation of mice is independent of the strain used. Indeed, as shown by Kataoka et al. (2006) the spleens of sham irradiated BALB/c mice contained more plasma cells than the spleens of C57BL/6J mice, but after irradiations at 0.25 or 0.5 Gy X-rays no differences in the spleen morphology and histology were detected between the two strains. However, the same authors demonstrated that four hours post-irradiation the number of the splenic plasma cells increased in the irradiated versus sham-exposed animals from both strains. Furthermore, no increases in the frequencies of both chromatid and chromosome aberrations were found in the bone marrow cells obtained from BALB/cJ or C57BL/6J mice exposed to 0.05 Gy of 137Cs γ-rays (Rithidech et al. 2012). Instead, a significant reduction in some types of abnormalities (i.e., abnormal cells, chromatid and chromosome breaks) was detected in these cells at one and six months post-irradiation as compared to the corresponding rates observed in the counterpart cells obtained from the sham-exposed control animals of the two strains.
Taken together, our data show that despite some differences between the radiosensitive BALB/c and radioresistant C57BL/6 mice in the NK cell- and macrophage-mediated responses to repeated low-level irradiations with X-rays, the final tumor-inhibitory effects of such exposures were comparable in the two strains. The results also suggest that similar anti-metastatic immune mechanisms may operate in the irradiated BALB/c and C57BL/6 mice. Whether the observed variations in the kinetics and magnitude of the stimulation of the functions of macrophages and NK cells derived from BALB/c and C57BL/6 mice relate to the higher post-radiation tumor proneness of the former compared to the latter and/or to the differently genetically determined default M1/M2 phenotypes in the two strains remains to be elucidated in future studies.
Acknowledgments
This study was supported by grants No. 2P05D05028 and No. N40400532/0161 from the Polish Ministry of Science and Higher Education.
REFERENCES
- Al-Sarireh B, Eremin O. Tumour-associated macrophages (TAMS): disordered function, immune suppression and progressive tumour growth. J R Coll Surg Edinb. 2000;45:1–16. [PubMed] [Google Scholar]
- Barao I, Ascensao JL. Human natural killer cells. Arch Immunol Ther Exp. 1998;46:213–229. [PubMed] [Google Scholar]
- Cai L. Research of the adaptive response induced by low-dose radiation: Where have we been and where should we go? Hum Exp Toxicol. 1999;18:419–425. doi: 10.1191/096032799678840291. [DOI] [PubMed] [Google Scholar]
- Caratero A, Courtade M, Bonnet L, Planel H, Caratero C. Effect of a continuous g irradiation at a very low dose on the life span of mice. Gerontology. 1998;44:272–276. doi: 10.1159/000022024. [DOI] [PubMed] [Google Scholar]
- Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat Environ Biophys. 2008;47:275–283. doi: 10.1007/s00411-007-0147-7. [DOI] [PubMed] [Google Scholar]
- Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Single or fractionated irradiations of mice with low doses of X-rays stimulate innate immune mechanisms. Int J Low Radiation. 2009;6:325–342. [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Lisiak E, Marciniak M, Nowosielska EM, Janiak MK. Inhibition of the development of pulmonary tumour nodules and stimulation of the activity of NK cells and macrophages in mice by single low doses of low-LET radiation. Int J Low Radiation. 2004a;1:171–179. [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska EM, Marciniak M, Janiak MK. Single Low Doses of X-Rays Inhibit the Development of Experimental Tumor Metastases and Trigger the Activities of NK Cells in Mice. Radiat Res. 2004b;161:335–340. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Nowosielska EM, Janiak MK. Stimulatory effect of a single low-level irradiation with X-rays on functions of murine peritoneal macrophages. Nukleonika. 2005;50(Suppl. 2):S13–S16. [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Nowosielska EM, Janiak MK. Immune mechanism of the retarded growth of tumor nodules in mice exposed to single low-level irradiations with X-rays. Centr Eur J Immunol. 2006;31:44–50. [Google Scholar]
- Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implicationsfor genotype-dependent bystander signaling. Cancer Res. 2008;68:450–456. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- Cui S, Reichner JS, Mateo RB, Albina JE. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994;54:2462–2467. [PubMed] [Google Scholar]
- Farias-Eisner R, Sherman MP, Aeberhard E, Chaudhuri G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci USA. 1994;91:9407–9411. doi: 10.1073/pnas.91.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–724. [PubMed] [Google Scholar]
- Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26:177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by lifelong low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163:153–158. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hosoi Y, Yamada S, Ono T, Sakamoto K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat Res. 1996;146:582–585. [PubMed] [Google Scholar]
- Janiak MK, Wrembel-Wargocka J, Cheda A, Nowosielska EM, Lisiak E, Bilski M. Modulation of the antitumour functions of murine NK cells and macrophages after single low-level exposures to X-rays. Int J Low Radiation. 2006;3:178–191. [Google Scholar]
- Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Role of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju GZ, Liu SZ, Li XY, Liu WH, Fu HQ. Effect of high versus low dose radiation on the immune system. In: Hagen U, Harder D, Jung H, Streffer C, editors. Radiat Res 1895–1995. Proc of the Tenth Int Congress of Radiaton Research. Würzburg, Germany: 1995. pp. 709–714. [Google Scholar]
- Kataoka T, Mizuguchi1 Y, Notohara K, Taguchi T, Yamaoka K. Histological changes in spleens of radiosensitive and radioresistant mice exposed to low-dose X-ray irradiation. Physiol Chem Phys & Med NMR. 2006;38:21–29. [PubMed] [Google Scholar]
- Liu SZ, Han ZB, Liu WH. Changes in lymphocyte reactivity to modulatory factors following low dose ionizing radiation. Biomed Environ Sci. 1994;7:130–135. [PubMed] [Google Scholar]
- Liu SZ. Cancer Control Related to Stimulation of Immunity by Low-Dose Radiation. Dose Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt J M, Heilman MJ, Hill AM. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- Mitchel RE, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBAH mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moretta L, Ciccone E, Poggi A, Mingari MC, Moretta A. Origin and functions of human natural killer cells. Int J Clin Lab Res. 1994;24:181–186. doi: 10.1007/BF02592459. [DOI] [PubMed] [Google Scholar]
- Nathan C. Mechanisms and modulation of macrophage activation. Behrings Inst Mitt. 1991;88:200–207. [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Modulation of the growth of pulmonary tumour colonies in mice after single or fractionated low-level irradiations with X-rays. Nukleonika. 2008;53(Suppl. 1):S9–S15. [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Immunological mechanism of the low-dose radiation-induced suppression of cancer metastases in a mouse model. Dose-Response. 2010;8:209–226. doi: 10.2203/dose-response.09-016.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Anti-neoplastic and immunostimulatory effects of low-dose X-ray fractions in mice. Int J Radiat Biol. 2011a;87:202–212. doi: 10.3109/09553002.2010.519422. [DOI] [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Stimulation of the natural anti-tumor cells by single and fractionated irradiations of mice with low doses of X-rays. Health Physics. 2011b;100:283–285. doi: 10.1097/hp.0b013e3182080e68. [DOI] [PubMed] [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Janiak MK. A single low-dose irradiation with X-rays stimulates NK cells and macrophages to release factors related to the cytotoxic functions of these cells. Centr Eur J Immunol. 2006a;31:51–57. [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Lisiak E, Janiak MK. Low-level exposures to ionising radiation modulate the anti-tumour activity of murine NK cells. Nukleonika. 2005;50(Suppl. 2):S21–S24. [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Lisiak E, Janiak MK. Enhanced cytotoxic activity of macrophages and suppressed tumor metastases in mice irradiated with low doses of X- rays. J Radiat Res. 2006b;47:229–236. doi: 10.1269/jrr.0572. [DOI] [PubMed] [Google Scholar]
- Okayasu R, Suatomi K, Yu Y, Silver A, Bedford JS, Cox R, Ullrich RL. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-Induced Chromosomal Instability in BALB/c and C57BL/6 Mice: The Difference Is as Clear as Black and White. Radiat Res. 1997;147:121–125. [PubMed] [Google Scholar]
- Rithidech KN, Udomtanakunchai C, Honikel LM, Whorton EB. No evidence for the in vivo induction of genomic instabilityby low doses of 137cs g rays in bone marrow cells of Balb/CJ and C57BL/6J mice. Dose-Response. 2012;10:11–36. doi: 10.2203/dose-response.11-002.Rithidech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat A. The immunology of low-dose total-body irradiation: more questions than answers. Radiat Res. 2000;153:599–604. doi: 10.1667/0033-7587(2000)153[0599:tioldt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Storer JB, Mitchell TJ, Fry RJ. Extrapolation of the relative risk of radiogenic neoplasms across mouse strains and to man. Radiat Res. 1988;114:331–53. [PubMed] [Google Scholar]
- Ullrich RL, Ponnaiya B. Radiation-induced instability and its relation to radiation carcinogenesis. Int J Radiat Biol. 1998;74:747–754. doi: 10.1080/095530098141023. [DOI] [PubMed] [Google Scholar]
- Ullrich RL, Bowles ND, Satterfield LC, Davis CM. Strain-dependent susceptibility to radiation-induced mammary cancer is a result of differences in epithelial cell sensitivity to transformation. Radiat Res. 1996;146:353–5. [PubMed] [Google Scholar]
- Wells CA, Ravasi T, Faulkner GJ, Carninci P, Okazaki Y, Hayashizaki Y, Sweet M, Brandon J, Wainwright BJ, Hume DA. Genetic control of the innate immune response. BMC Immunol. 2003;4:5. doi: 10.1186/1471-2172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Fidler IJ. Therapy of cancer metastasis by activation of the inducible nitric oxide synthase. Cancer Met Rev. 1998;17:55–75. doi: 10.1023/a:1005956721457. [DOI] [PubMed] [Google Scholar]
- Young HA, Ortaldo J. Cytokines as critical co-stimulatory molecules in modulating the immune response of natural killer cells. Cell Res. 2006;16:20–24. doi: 10.1038/sj.cr.7310004. [DOI] [PubMed] [Google Scholar]