Abstract
Low-dose ionizing radiation (LDR) may lead to suppression of smoking-related lung cancer. We examined the effects of a known cigarette smoke carcinogen Benzo[a]pyrene (B[a]P) alone or in combination with fractionated low-dose gamma radiation (60 – 600 mGy total dose) on the induction of lung neoplasms in the A/J mouse. Our results show that 600 mGy of gamma radiation delivered in six biweekly fractions of 100 mGy starting 1 month after B[a]P injection significantly inhibits the development of lung adenomas per animal induced by B[a]P. Our data also indicated that the six biweekly doses suppressed the occurrence of spontaneous hyperplastic foci in the lung, although this suppression failed to reach statistical significance when analyzed as average foci per lung possibly related to the small sample sizes used for the control and test groups.
Keywords: Low-dose gamma-radiation, Benzo[a]pyrene, lung cancer
INTRODUCTION
Lung cancer is the leading cause of cancer mortality in both men and women in the United States and will soon reach epidemic proportion worldwide (American Cancer Society, 2012). Thus, lung cancer prevention is currently an active research area (Belinsky et al. 2003; Lyon et al. 2009). The most important risk factor for lung cancer is tobacco smoking (Sanders and Scott 2008); it is estimated to account for up to 90% of lung cancer risk in men and up to 80% in women (Walser et al. 2008). Cigarette smoke contains approximately 5000 reactive chemical compounds and carcinogens that can lead to the development of lung cancer by damaging DNA and inducing inflammation through the recruitment of inflammatory cells (Yao and Rahman 2009). One of these compounds is Benzo[a]pyrene (B[a]P), a polycyclic aromatic hydrocarbon (PAH). Metabolites of B[a]P (e.g. benzo[a]pyrenediolexposide, BPDE) are mutagenic and carcinogenic. B[a]P causes DNA damage in lung cells consistent with DNA damage observed in malignant lung cancers (Denissenko et al. 1996), and its metabolites elicit a number of toxic effects in target cells such as DNA adduct formation, cytotoxicity of lymphocytes, and production of proinflammatory mediators such as certain cytokines (Wojdani et al. 1984; Umannova, et al. 2008). Studies conducted at our Institute have revealed an important role of epigenetic changes caused by BPDE exposure that are associated with neoplastic transformation of human lung epithelial cells and presumably also lung cancer development (Tellez et al. 2011).
The A/J mouse strain because of its known susceptibility to lung tumor induction by PAHs such as B[a]P, is commonly used to study lung cancer development after carcinogen exposure (Hecht et al. 1994; Malkinson 1992). In addition, the time course for development and progression of lung cancer (hyperplastic foci progressing to adenoma and finally carcinoma) can be easily quantified (Lyon et al. 2009).
Although it is common knowledge that exposure to very high doses of radiation can induce cancer; a correlation between low doses of radiation and lung cancer has yet to be demonstrated. Recently scientists have shown that low-dose radiation can yield beneficial biological effects suggesting the potential of using low doses of radiation to combat cancer formation and reduce tumor prevalence and metastasis (Sakamoto et al. 1997). Researchers have investigated the effects of low-dose radiation, given as a single dose or fractionated or protracted over time, with regard to altering cancer development or resolution while others have focused on the effects of low-dose radiation on the systemic immune system (Liu 2003, 2007; Sakai et al. 2003; Sakai 2006; Thompson et al. 2008; Nowosielska 2010). The purpose of this study was to examine the effect of a known cigarette smoke carcinogen, alone or delivered in combination with low doses of low linear-energy-transfer (LET) gamma radiation, on the development of lung cancer in the A/J mouse. Our hypothesis is that low-dose gamma rays protect from B[a]P-induced lung cancer. In testing this hypothesis, we investigated the development of lung carcinogenesis 46 weeks after treatment of mice with B[a]P and the ability of fractionated gamma radiation (60 – 600 mGy total dose) to suppress cancer development.
MATERIALS AND METHODS
Animals:
Female, 10 week-old, A/J mice purchased from Jackson Laboratories (Bar Harbor, ME), were separated into the following groups: B[a]P only, B[a]P + radiation, radiation only, and age-matched unirradiated, untreated controls for a 46 week carcinogenesis experiment (n= 8–18 per group). At 46 weeks, mice were humanely euthanized by i.p. injection of a lethal dose of Euthasol.
Chemicals:
B[a]P (Sigma), at a concentration of 100 mg/kg body weight, was dissolved in 0.2 ml of tricaprylin (Glyceryltrioctanoate, Sigma) vehicle prior to interperitoneal (i.p.) injection.
Irradiation:
Mice were exposed to six biweekly whole body irradiations (WBI) at a dose rate of 1.07 mGy/s (10 mGy target dose) or 1.33 mGy/s (100 mGy target dose) from the Gammacell 1000 irradiator (137Cs source) (Best Theratronics, Ontario, CA) for a total dose of 60 and 600 mGy, respectively. To achieve low doses, mice were exposed individually in polypropylene tubes set inside a stainless steel sample canister lined with lead foil (7.4 mm thickness). Experimental doses received were confirmed by nanoDots (Optically Stimulated Luminescence Technology, 1 cm2, Landauer, Inc., Glenwood, IL) adhered to the outside of the tubes containing the mice. Initial dosimetry was performed using nanoDots inserted into the chests of mouse carcasses.
Histology
To estimate tumor burden, gross lung tumors were counted at necropsy. Lungs were inflated with neutral buffered formalin at a constant hydrostatic pressure of 25 cm for 6 hours and fixed further by immersion in formalin for >48 hours. Left lung lobes were systematically trimmed in a dorsoventral-transverse direction at 3–4 mm intervals with the first slice randomly positioned within the cranial 4 mm of tissue to yield 3–5 slices per lobe. A single slice along the axial airway of each of the right lung lobes was also made. Trimmed tissue was histoprocessed routinely, and 5 μm-thick paraffin sections were mounted and stained with hematoxylin and eosin for light-microscopic identification and enumeration of hyperplastic foci, adenomas, and carcinomas.
Statistical Analyses
Tumor counts are given as means ± standard error (SE). Comparisons between groups were performed using the Wilcoxon Rank Sum Test except where otherwise stated.
RESULTS
Repeated 100 mGy gamma irradiation in B[a]P treated mice suppresses adenoma development
Various groups have previously demonstrated that animals exposed to single or fractionated low doses of X-rays or gamma-rays exhibit an attenuated growth of implanted cancerous tumors, and lung metastases are reduced when animals are exposed to radiation prior to inoculation with tumor cells (Hosoi and Sakamoto 1993; Cai 1999; Cheda et al. 2004; Nowosielska 2010). In order to ascertain whether low dose radiation could also inhibit lung carcinogenesis initiated by a PAH contained in cigarette smoke, we investigated the potential for suppression of cancer progression of B[a]P-induced lung tumors in mice exposed to repeated low doses of gamma radiation (Figure 1).
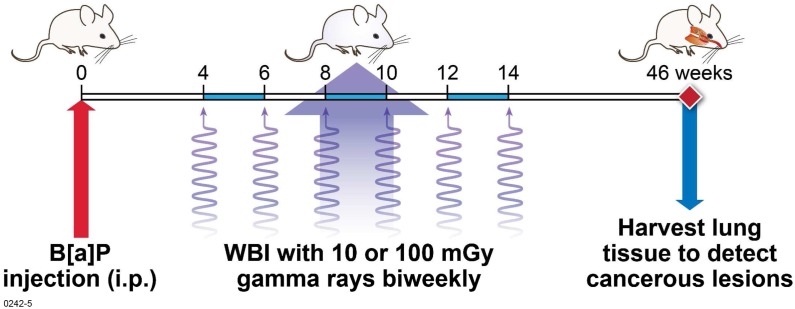
FIGURE 1.
Study design. Mice were given a single dose of B[a]P four weeks prior to exposure to gamma radiation. Fractionated whole-body gamma-ray doses of 10 mGy or 100 mGy were then given once every 2 weeks until week 14 post-B[a]P injection for cumulative dose of 60 mGy and 600 mGy, respectively. At 46 weeks, lungs were harvested and lesions were enumerated grossly. In addition, lungs were fixed and tissue sections were prepared for histological examination.
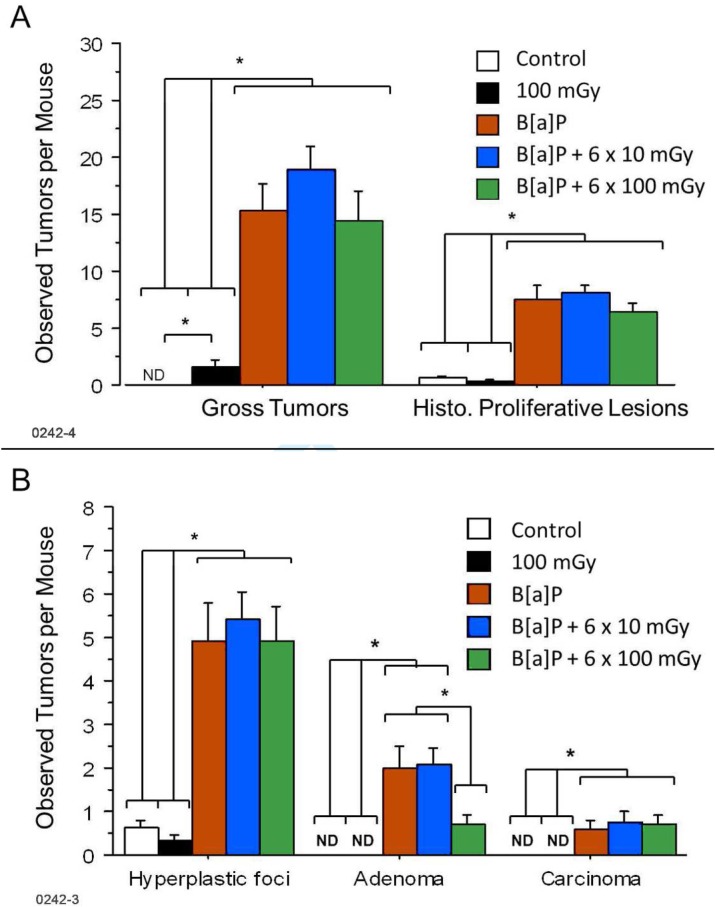
As expected, B[a]P treated mice exhibited a significantly higher number of lesions compared to control mice or mice receiving radiation only (p < .05, Figure 2A). The tumors observed grossly represented larger hyperplastic foci, adenomas, and carcinomas as well as non-proliferative lesions, such as inflammatory or fibrotic or non-lesions: multifocal pallor, atelectasis, and/or hyperinflation. These observations were proportional to those performed by histological analysis. In each B[a]P treated group, the number of hyperplastic foci greatly exceeded adenomas which in turn exceeded carcinomas representing the natural progression of lung cancer (Figure 2B). In contrast, exposure of B[a]P treated mice to six fractions of 100 mGy doses of gamma radiation (600 mGy total) was associated with a significant reduction of adenomas compared to B[a]P alone (p = .04). Interestingly, as indicated in Table 1, a single histoproliferative lesion (hyperplastic focus) was found in 33 ± 11% of mice in the 600 mGy radiation group whereas 63 ± 17% of the age-matched, untreated control group had such a lesion (n = 8); however, further testing is needed to confirm this finding as the small group size of the age matched control mice did not allow for statistical significance to be achieved when evaluated on the basis of average foci per animal (p = .18). A more detailed analysis of these data (Scott et al. 2012) in the context of the hormetic relative risk (HRR) model (Scott et al. 2009; Scott 2011) demonstrated a significant gamma-ray protective effect against spontaneous hyerplastic foci. No adenomas or carcinomas were detected in the untreated control group or the group treated with six fractions of 100 mGy dose (Table 1).
FIGURE 2.
Lung tumors from mice injected i.p. with B[a]P four weeks prior to repeated irradiations with 10, and 100 mGy gamma-rays were grossly counted upon necropsy at 46 weeks post injection and histologically verified as proliferative lesions (A). Proliferative lesions (tumors) were further classified as hyperplastic foci, adenomas, or carcinomas (B). Mean values ± SE obtained from a single experiment is presented. Each experimental group contained 15–18 mice (n = 15–18), control group contained 8 mice (n = 8). Experimental groups are as described in materials and methods section. ND = not detected; * = p < 0.05 with the indicated groups being significantly different than each other on the same day of analysis.
TABLE 1.
Tumor Incidence (% of mice with the indicated lesions) and standard errora
| Group | No. Mice | % Mice with 1 or more Hyperplastic focus | % Mice with 1 or more Adenoma | % Mice with 1 or more Carcinoma |
|---|---|---|---|---|
| Control | 8 | 62.5±17.1 | 0.0 | 0.0 |
| 100 mGy | 18 | 33.3±11.1 | 0.0 | 0.0 |
| B[a]P | 12 | 91.7±8.0 | 75.0±12.5 | 41.7±14.2 |
| B[a]P + 10 mGy | 15 | 100.0 | 86.7±8.8 | 46.7±12.9 |
| B[a]P + 100 mGy | 14 | 92.9±6.9 | 57.1±13.2 | 50.0±13.4 |
Standard error calculated assuming a binomial distribution of lesions
We investigated the distribution of lung tumors (adenomas and carcinomas only) by exposure group. Our previously introduced HRR model incorporates a protection factor (PROFAC), which is the population average of individual-specific protection factors, profac, against lung tumor formation given that radiation has activated the body’s natural anticancer mechanisms. The parameter PROFAC represents the on-average probability of lung tumor prevention when natural protection mechanisms have been activated in every individual (Scott et al. 2009; Scott 2011). With the HRR model, lung cancer relative risk (RR) is given by RR = 1−B(x)PROFAC, where the benefit function B(x) represents the probability that the body’s natural defenses are activated by a low dose x of radiation.
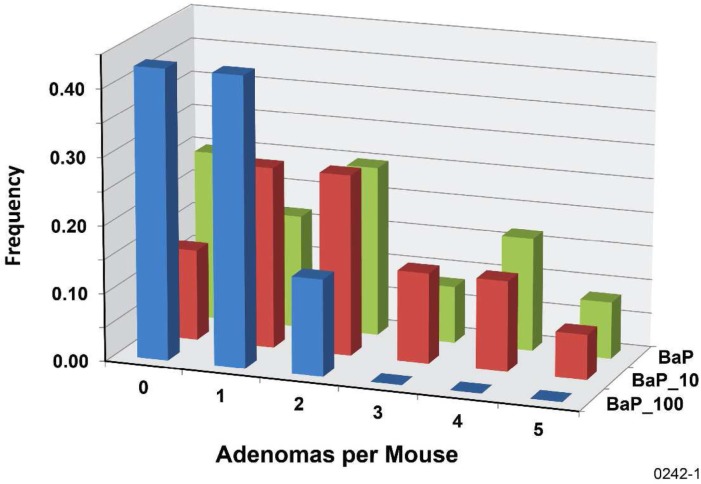
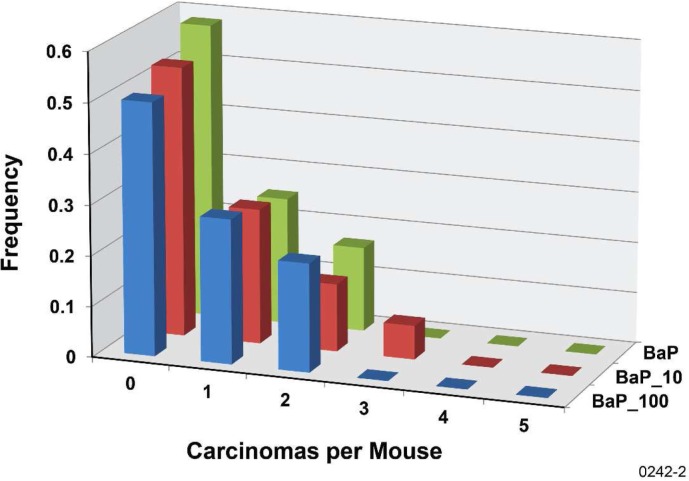
To characterize the level of protection against B[a]P-induced lung tumors that is associated with n gamma-ray fractions, we introduce the differential protection factor, PROFAC{N+}, for protecting from cases of N or more lung tumors. Thus, PROFAC{1+} applies to protection from 1 or more lung tumors. PROFAC{2+} applies to protecting from 2 or more lung tumors, etc. Table 2 shows results obtained for PROFAC{1+}, PROFAC{2+}, and PROFAC{3+} for protection from B[a]P-induced lung adenomas by six fractions of 100 mGy gamma-rays. In each case B(x) was assumed to be 1 (e.g., protective mechanisms activated in each animal). Where values of PROFAC{N+} minus two standard errors was > 0, the PROFAC value reported was considered significantly > 0 (interpreted to indicate a significant protective effect). Thus, only protection against 2 or more and against 3 or more adenomas per animal were considered significant (p < .05). There was no indication of a gamma-ray protective effect by the six fractions of 10 mGy dose from adenomas or carcinomas (Figures 3 and 4).
TABLE 2.
PROFAC{N+} estimates for suppression of N or more B[a]P-induced adenomas via six biweekly 100-mGy, gamma-ray fractions to female A/J mice
| “N ” | PROFAC{N+} | Standard Error | Significantly > 0? |
|---|---|---|---|
| 1 | 0.24 | 0.23 | no |
| 2 | 0.76 | 0.17 | yesb |
| 3 | >0.79a | <0.23a | yesb |
Based on subjectively assigning 1 case among 14 animals when actually none had 3 or more adenomas per lung.
Based on PROFAC{N+} minus 2 standard errors being > 0.
FIGURE 3.
Frequency distribution of adenomas per mouse for the B[a]P-exposure-only group (BaP), for the group exposed to B[a]P and 10-mGy gamma-ray fractions (BaP_10), and for the group exposed to B[a]P and 100-mGy gamma-ray fractions (BaP_100).
FIGURE 4.
Frequency distribution of carcinomas per mouse for the B[a]P-exposure-only group (BaP), for the group exposed to B[a]P and 10-mGy gamma-ray fractions (BaP_10), and for the group exposed to B[a]P and 100-mGy gamma-ray fractions (BaP_100).
DISCUSSION
As expected, B[a]P and/or specific metabolites (e.g., BPDE) induced lung cancer in this study detected as hyperplastic lesions, adenomas, and carcinomas. In contrast, mice exposed to radiation alone and unexposed control mice developed no adenomas or carcinomas that would indicate a progression to a more malignant phenotype. Treatment with six fractions of 10 mGy gamma irradiation did not result in a reduction of B[a]P-induced hyperplastic foci, adenomas, or carcinomas. The six fractions of 10 mGy gamma rays also did not alter the distribution of the tumors per mouse from what was found for the B[a]P-only exposure. However, repetitive exposure to six fractions of 100 mGy gamma radiation reduced the number of adenomas in B[a]P treated mice. Seventy-five ± 13% of B[a]P treated mice developed one or more adenomas compared to 57.1 ± 13% when six fractions of 100 mGy gamma rays were administered (fractionated) in addition to B[a]P (Table 1).
When investigating the distribution of B[a]P-induced lung adenomas we found a clear beneficial effect of the six fractions of 100 mGy gamma-ray exposure related to reducing the multiplicity of these lesions (3 or more) in the mouse lung. The lack of a gamma-ray protective effect against carcinomas may relate to the 46 week follow-up time used in the study design. We cannot rule out the possible occurrence of a protective effect against carcinomas for much longer follow-up times.
As a result of low-dose, gamma-ray exposure, spontaneous hyperplastic foci prevalence was reduced from non-irradiated controls from 0.63 to 0.33 (Figure 2B, Table 1), although not statistically significant due to the small sample sizes used. This finding is currently under further investigation. Interestingly, a reduction in spontaneous adenoma formation (PRO-FAC{1+}= 0.209 to 0.321) was observed in RFMf/Un mice when subjected to single whole-body doses of gamma radiation ranging from 100 up to 1500 mGy (Ullrich and Storer 1979). In humans, long-term residential radon exposure has been associated with a reduction rather than an increase in sporadic lung cancers for a range of average radon levels (Cohen 1997, Thompson et al. 2008, Scott 2011). A PROFAC{1+} > 0.5 has been implicated for the population studied by Thompson et al. in their 2008 paper (Scott 2011). Previously, protective effects of protracted, low-dose, low-LET gamma radiation has also been shown against high-LET, alpha-radiation-induced lung cancer in rats (Scott et al. 2008). A gamma-ray PROFAC{1+} close to 1 was reported for preventing lung tumor cases in rats exposed to low doses (< 200 mGy) of highly-damaging alpha radiation where not more than 1 alpha-radiation-induced lung tumor per animal or not more than 1 spontaneous lung tumor per animal was expected (Scott et al. 2008; Sanders 2012). In contrast, low-dose gamma rays did not protect (PROFAC{1+} = 0) from lung tumor induction (one or more per animal) by very high doses (> 5000 mGy) of alpha radiation where multiple tumors per animal may have occurred among long-term survivors because of immune system suppression (Scott et al. 2009). The data were not analyzed to see if low dose gamma rays protected from multiple tumors per animal. Gamma-ray protection (PROFAC{1+} > 0.8) against alpha-radiation-induced (from inhaled plutonium-239) and smoking-related lung tumors has also been reported for humans (Scott 2007; Scott et al. 2009). The indicated data relate to chronic exposure of the lung at low rates over many years to both alpha and gamma radiations.
B[a]P primarily targets the lung when administered i.p., however we also observed lesions not of pulmonary origin. Seven out of 77 total mice were found to have lesions (hyperplasias, adenomas, carcinomas, or in combination) in tissues of the stomach, pancreas, and ovaries. These observations are consistent with those previously documented in which B[a]P induced injection site tumors and tumors in the abdomen and pancreas of mice injected i.p. (Hecht et al. 1994, Balansky et al. 2006). As there were so few mice observed to have these lesions, no analysis was performed to examine the effect of radiation.
In conclusion, results from this study suggests that six fractions of 100 mGy but not 10 mGy gamma radiation significantly reduces the multiplicity of adenomas but not carcinomas induced by B[a]P. Although not statistically significant, radiation also appears to decrease the number of cases of spontaneous hyperplastic foci by a similar degree as was reported by other researchers for gamma-ray prevention of cases of spontaneous lung cancer in mice (Ullrich and Storer, 1979). The mechanisms behind this phenomenon have yet to be fully elucidated, although there is growing evidence for a hierarchy of protective mechanisms being involved (Scott et al. 2009). Taken together, these findings support the hypothesis that low-LET radiation (e.g., gamma rays) given at low doses and dose rates can prevent lung cancer induction by agents such as cigarette smoke carcinogens.
Acknowledgments
We extend our gratitude to Nicole Kikendall, Mackey Makvandi, Elizabeth Maloy, and Ayako Monier, and the necropsy department at Lovelace Respiratory Research Institute for their wonderful technical assistance. This work was supported by the Office of Science (BER), U.S. Department of Energy, Grant No. DE-FG02-09ER64783.
REFERENCES
- American Cancer Society Cancer Facts and Figures 2012. 2012. Available at http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012.
- Balansky R, D’Agostini F, Ganchev G, Izzotti A, Di Marco B, Lubet RA, Zanesi N, Croce CM, De Flora S. Influence of FHIT on benzo[a]pyprene-induced tumors and alopecia in mice: Chemoprevention by budesonide and N-acetylcysteine. PNAS. 2006;103(20):7823–7828. doi: 10.1073/pnas.0601412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, March TH, Baylin SB. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Research. 2003;63:7089–7093. [PubMed] [Google Scholar]
- Cai L. Research of the adaptive response induced by low-dose radiation: where have we been and where should we go? Hum Exp Toxicol. 1999;18(7):419–425. doi: 10.1191/096032799678840291. [DOI] [PubMed] [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska EM, Marciniak M, Janiak MK. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat Res. 2004;161(3):335–340. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Lung cancer rate vs. mean radon level in U.S. counties of various characteristics. Health Phys. 1997;72:114–119. doi: 10.1097/00004032-199701000-00016. [DOI] [PubMed] [Google Scholar]
- Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26(2):177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Isaacs S, Trushin N. Lung tumor induction in A/J mice by the tobacco smoke carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene: a potentially useful model for evaluation of chemopreventive agents. Carcinogenesis. 1994;15(12):2721–2725. doi: 10.1093/carcin/15.12.2721. [DOI] [PubMed] [Google Scholar]
- Liu S. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlin Biol Toxicol Med. 2003;1(1):71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Cancer control related to stimulation of immunity by low-dose radiation. Dose-Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon ML, Klinge S, Do KC, Grimes MJ, Thomas CL, Damiani LA, March TH, Stidley CA, Belinsky SA. Rosiglitazone prevents the progression of preinvasive lung cancer in a murine model. Carcinogenesis. 2009;30(12):2095–2099. doi: 10.1093/carcin/bgp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52(9 Suppl):2670s–2676s. [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Anti-neoplastic and immunostimulatory effects of low-dose X-ray fractions in mice. Int J Radiat Biol. 2010;87(2):202–212. doi: 10.3109/09553002.2010.519422. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, Oda T, Iwasaki T, Fujita K, Yamada T, Tanooka H. Suppression of carcinogenic process in mice by chronic low dose rate gamma-irradiation. Int J Low Radiat. 2003;1(1):142–146. [Google Scholar]
- Sakai K. Enhancement of bio-protective functions by low dose/dose-rate radiation. Dose-Response. 2006;4(4):327–332. doi: 10.2203/dose-response.06-115.Sakai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Myogin M, Hosoi Y, Nemoto K, Takai Y, Kakuto Y, Yamada S, Watabe M. Fundamental and clinical studies on cancer control with total or upper half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;9:161–175. [Google Scholar]
- Sanders CL, Scott BR. Smoking and hormesis as confounding factors in radiation pulmonary carcinogenesis. Dose-Response. 2008;6:53–79. doi: 10.2203/dose-response.06-003.Sanders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Potential treatment of inflammatory and proliferative diseases by ultra-low doses of ionizing low-LET radiation. Dose-Response. 2012;10(4):610–625. doi: 10.2203/dose-response.12-017.Sanders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Low-dose radiation-induced protective process and implications for risk assessment, cancer prevention, and cancer therapy. Dose-Response. 2007;5(2):131–149. doi: 10.2203/dose-response.05-037.Scott. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT scans may reduce rather than increase the risk of cancer. J Am Physicians Surg. 2008;13(1):8–11. [Google Scholar]
- Scott BR, Belinsky SA, Leng S, Lin Y, Wilder JA, Damiani LA. Radiation-stimulated epigenetic reprogramming of adaptive-response genes in the lung: An evolutionary gift for mounting adaptive protection against lung cancer. Dose-Response. 2009;7(2):104–131. doi: 10.2203/dose-response.08-016.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Residential Radon Appears to Prevent Lung Cancer. Dose-Response. 2011;9:444–464. doi: 10.2203/dose-response.11-027.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Bruce V, Gott KM, March T, Wilder J. Small gamma-ray doses prevent rather than increase lung tumors in mice. Dose-Response. 2012;10(4):527–540. doi: 10.2203/dose-response.12-035.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez CS, Juri DE, Bernauer AM, Thomas CL, Damiani LA, Tessema M, Leng S, Belinsky SA. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71(8):3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Nelson DF, Popkin JH, Popkin Z. Case-control study of lung cancer risk from residential radon exposure in Worchester County, Massachusetts. Health Phys. 2008;94(3):228–241. doi: 10.1097/01.HP.0000288561.53790.5f. [DOI] [PubMed] [Google Scholar]
- Ullrich RL, Storer JB. Influence of gamma irradiation on the development of neoplastic disease in mice. II. Solid tumors. Radiat Res. 1979;80(2):317–324. [PubMed] [Google Scholar]
- Umannova L, Machala M, Topinka J, Novakova Z, Milcova A, Kozubik A, Vondrácek J. Tumor necrosis factor-alpha potentiates genotoxic effects of benzo[a]pyrene in rat liver epithelial cells through upregulation of cytochrome P450 1B1 expression. Mutat Res. 2008;640(1–2):162–169. doi: 10.1016/j.mrfmmm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, Sharma S, Dubinett SM. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5(8):811–815. doi: 10.1513/pats.200809-100TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdani A, Attarzadeh M, Wolde-Tsadik G, Alfred LJ. Immunocytotoxicity effects of polycyclic aromatic hydrocarbons on mouse lymphocytes. Toxicology. 1984;31(3–4):181–189. doi: 10.1016/0300-483x(84)90100-8. [DOI] [PubMed] [Google Scholar]
- Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9(4):375–383. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]