Abstract
The current radiation safety paradigm using the linear no-threshold (LNT) model is based on the premise that even the smallest amount of radiation may cause mutations increasing the risk of cancer. Autopsy studies have shown that the presence of cancer cells is not a decisive factor in the occurrence of clinical cancer. On the other hand, suppression of immune system more than doubles the cancer risk in organ transplant patients, indicating its key role in keeping occult cancers in check. Low dose radiation (LDR) elevates immune response, and so it may reduce rather than increase the risk of cancer. LNT model pays exclusive attention to DNA damage, which is not a decisive factor, and completely ignores immune system response, which is an important factor, and so is not scientifically justifiable. By not recognizing the importance of the immune system in cancer, and not exploring exercise intervention, the current paradigm may have missed an opportunity to reduce cancer deaths among atomic bomb survivors. Increased antioxidants from LDR may reduce aging-related non-cancer diseases since oxidative damage is implicated in these. A paradigm shift is warranted to reduce further casualties, reduce fear of LDR, and enable investigation of potential beneficial applications of LDR.
Keywords: Radiation safety, Low dose radiation, LNT model, Immune system, Antioxidant stimulation, Aging-related diseases
INTRODUCTION
Whereas the carcinogenic nature of high dose radiation is well established, the health effects of low dose radiation are still being debated. The current radiation safety paradigm is based on the linear no-threshold (LNT) premise that even the smallest amount of radiation may cause DNA damage and mutations increasing the risk of cancer. An analysis of the historical foundation of the LNT model shows that the no-threshold model was adopted in the 1950s due to carcinogenic concerns following the observation of excess leukemias in atomic bomb survivors, but without much supporting data at low doses since most of the radiobiological data available at the time was for high doses, e.g. observed increase in leukemias in atomic bomb survivors and observed increase in mutations in drosophila subjected to radiation (Calabrese, 2009). The decision to adopt the LNT model may also have been influenced by the political movements of that time period to stop the development of nuclear weapons (Jaworowski, 2010b). Recent measurements have shown a U-shaped dose response curve for X-ray induced mutations in drosophila (Ogura et al., 2009; Koana and Tsujimura, 2010) invalidating one of the original justifications for adopting the LNT model.
The use of a no-threshold model results in efforts and expenditures that generally do not reduce cancer incidence significantly, but may increase it. Cancer incidence is known to be affected by many factors, with some factors that increase the risk, e.g. smoking (Hymowitz, 2011), obesity (Basen-Engquist and Chang, 2011), alcohol (Testino et al., 2011), and infection (Sell, 2011), and other factors that decrease the risk, e.g. physical activity (Alberts et al., 2008) and vaccination (Frazer et al., 2011). The effect of such factors on cancers can be quite significant. An estimate of the fraction of cancers that can be attributed to sub-optimal past exposures of 14 lifestyle and environmental risk factors indicates 42.7% of cancers in the UK in 2010 may be attributed to these factors (Parkin et al., 2011). Such analysis can enable us to design an optimum allocation of available resources among the different risk factors in order to maximize the overall reduction of cancers. The use of a no-threshold model for one of the factors can result in a lopsided allocation of resources to remedy that factor to an extreme level, resulting in a large deviation from this optimum allocation of resources and hence leading to a sub-optimal reduction in cancers. Thus, using a no-threshold model for remedying a carcinogenic factor is in general not a conservative approach.
Though these general arguments invalidate the use of a no-threshold model for low dose radiation (and other carcinogens), the LNT model has become firmly established in radiation protection policies around the world. Recent reports from advisory bodies such as International Commission on Radiological Protection (ICRP, 2007), National Research Council (NRC, 2006) and National Council on Radiation Protection & Measurements (NCRP, 2001) have re-affirmed the use of the LNT model. There is continuing support for the use of the LNT model in publications (Nussbaum, 1998; Kellerer, 2000; Preston, 2003; Martin, 2005; Brenner and Sachs, 2006; Little et al., 2009). The government regulatory agencies continue to use the LNT model for regulatory purposes, generally following the guidelines set by the above advisory bodies.
The support for the use of the LNT model is however not universal. The validity and wisdom of using the LNT model has been questioned by many scientists over the years, because of radiobiological data that is inconsistent with the LNT model, e.g. (Luckey, 1980; Hickey et al., 1983; Luckey, 1991; Thomas, 1994; Jaworowski, 1997; Cohen, 2002; Feinendegen, 2005; Cook and Calabrese, 2006; Cohen, 2007; Mitchel, 2007; Jaworowski, 2008; Scott, 2008; Averbeck, 2009; Cuttler and Pollycove, 2009; Tubiana et al., 2009; Jaworowski, 2010a; Sanders, 2010; Calabrese, 2011; Scott, 2011). The French Academy of Sciences has recommended that the possibility of beneficial effects of low dose radiation should be investigated (Tubiana, 2005). A re-evaluation of the current approach to radiation safety has been recommended (Mitchel, 2007) and an alternative radiation safety approach has been proposed that raises recommended dose limits considerably from the present values (Allison, 2011). Some scientists have even suggested utilizing the hormetic effects (e.g. cancer preventive effects) of low dose radiation, taking a stand completely opposite to that of the current radiation safety paradigm (Hickey et al., 1983; Luckey, 1991; Cook and Calabrese, 2006). Reduction of many diseases and conditions has been reported from the use of low dose radiation in controlled animal studies, e.g. chemical induced brain damage (Kojima et al., 1999), metastasis (Hashimoto et al., 1999), autoimmune diseases (Tanaka et al., 2005), thymic lymphoma (Ina et al., 2005), diabetes (Nomura and Sakai, 2006), atopic dermatitis and tumor metastasis (Takahashi and Kojima, 2006), collagen-induced arthritis (Nakatsukasa et al., 2008), tumor growth (Hayase et al., 2008), diabetes related nephropathy (Nomura et al., 2011), diabetes related cardiac damage (Zhang et al., 2011), prion infection in brain (Plews et al., 2010), and atherosclerosis (Mitchel et al., 2011). Human studies have also shown reduction of some diseases using low dose radiation, e.g. hypertension, diabetes, and pain (Yamaoka and Komoto, 1996), bronchial asthma (Mitsunobu et al., 2003), degenerative joint and spine diseases (Becker, 2004), and rheumatic diseases (Falkenbach et al., 2005). The current vast difference of opinion in the scientific community on the health effects of low dose radiation in humans may be resolved by performing prospective studies. Considering the current radiation safety regulations based on the LNT model, and the fear of low dose radiation among the general public, such studies in humans are not feasible. If the reported beneficial effects of low dose radiation are true, the current paradigm may be causing considerable harm to human health by preventing the study of these effects. Thus, it is important to evaluate the validity of the current radiation safety paradigm.
In this paper, arguments will be presented to justify a paradigm shift in radiation safety by showing that the present radiation safety paradigm has fundamental flaws and so is not scientifically justifiable, has likely led to missed opportunities in reducing cancer deaths among atomic bomb survivors, has prevented the study of beneficial effects of low dose radiation, and has likely prevented progress in reducing aging-related diseases including cancer.
FUNDAMENTAL FLAWS OF THE CURRENT RADIATION SAFETY PARADIGM
Exclusive attention to mutations as the cause of cancer
The basic premise of the current radiation safety paradigm and the consequent LNT model is that even a single ray of ionizing radiation can result in a base change leading to a mutation that could cause cancer (Hall and Giaccia, 2006). How important are mutations in the pathogenesis of clinical cancer?
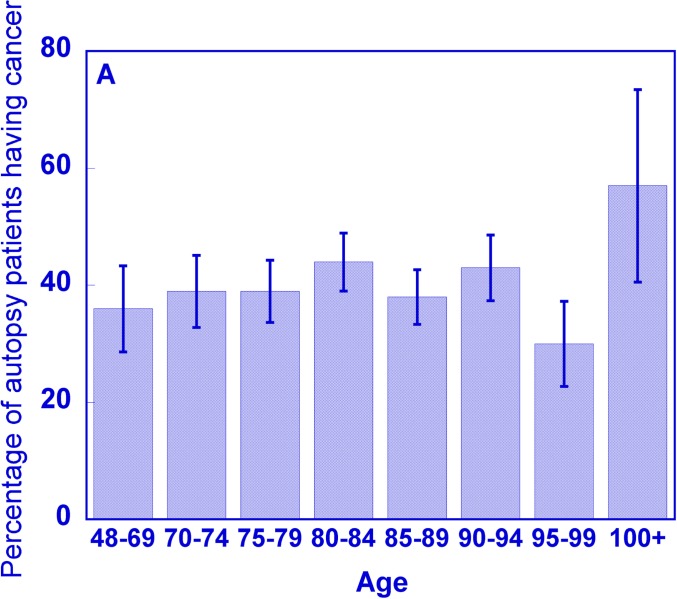
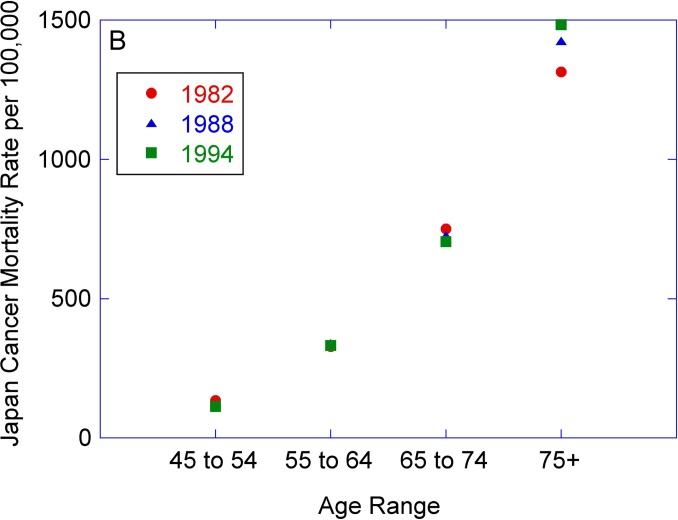
Figure 1A shows the percentage of patients having cancer cells (mutations indicative of cancer) as determined from full body autopsies in a geriatric hospital in Japan during 1982–1994 (Imaida et al., 1997). It is seen that the percentage of patients having cancer cells is ∼40% and is relatively unchanged for the age ranges covering 48 to 94 years. The cancer mortality rate for a similar age range in Japan has on the other hand increased by a factor of ∼10 (see Fig. 1B) (WHO, 2011), indicating that the presence of cancer cells is not a decisive factor in the occurrence of clinical cancer.
FIGURE 1A.
Percentage of patients from a geriatric hospital in Japan who had cancer as determined by autopsy during 1982–1994, as a function of age. Data from (Imaida et al., 1997).
FIGURE 1B.
Cancer mortality rate per 100,000 in Japan as a function of age (WHO, 2011).
A similar pattern is seen in an autopsy study of Hungarian men (Soos et al., 2005), where the percentage of patients having prostate cancer cells has increased by a factor of ∼2 between the ages of ∼50 and ∼70 whereas the prostate cancer mortality rate has increased by a factor of ∼38 between these ages (WHO, 2011). A third example is that in a recent review, the presence of occult cancer is reported to be quite high for cancers of the prostate (30–70%), thyroid (36–100%), and breast (7–39%) for specified age groups, whereas the lifetime risk of death or metastatic disease from these cancers is quite low at 4%, 0.1%, and 4% respectively (Welch and Black, 2010). These examples show that mutations, though essential, are not the determinant factors in clinical cancer.
Ignoring the influence of immune system response in carcinogenesis
A second feature of the current radiation safety paradigm is that it completely ignores the effects of the different bodily defense mechanisms in estimating the cancer risk from low dose radiation. Let us consider the importance of immune system response (which is just one of the body’s defense mechanisms) in the occurrence of clinical cancer. The incidence of cancers in kidney disease patients has been estimated at various stages of their disease treatment (Vajdic and van Leeuwen, 2009a). The cancer incidence for kidney transplant patients (in whom the immune system was suppressed) was observed to be higher by a factor of ∼2.4 in comparison to kidney dialysis patients, indicative of the importance of the immune system in keeping the occult cancers in check. This increase included not only cancers known to be caused by viruses, but also cancers not viral in origin. The immune system can play a key role in maintaining cancers in an equilibrium state preventing occult cancers from becoming clinical cancers (Koebel et al., 2007). If the immune system were suppressed in the general population, and resulted in an increase in cancer similar to kidney transplant patients (i.e. by a factor of ∼2.4), essentially the whole population would face clinical cancer, since the lifetime risk of cancer incidence is ∼40%. What appears to separate those who have cancer from those who do not is not the presence of cancer cells (or carcinogenic viruses) but the immune system response. The increased risk of cancer from immune suppression in organ transplant patients was reported more than forty years ago (Allison, 1970).
A considerable amount of additional evidence is available indicative of the importance of the immune system response in modulating the carcinogenic process. AIDS patients, whose immune system is suppressed, also have higher levels of cancer incidence in a manner similar to kidney transplant patients (Vajdic and van Leeuwen, 2009b). The age-related decline in immune system (Weinberger et al., 2008) may be mainly responsible for the age-related increase in cancer incidence rather than the mutations (Jones, 2011). Increase in lung cancer and other cancers among smokers is attributed to the adverse effect of cigarette smoke on the immune system (Stampfli and Anderson, 2009). The lower immune response may also be responsible for the higher recurrence rate of prostate cancer and poorer survival among smokers (Kenfield et al., 2011a).
Since suppression of the immune system has been observed to correlate with increased cancers, it would be logical to infer that improving the immune system response may reduce the cancer incidence. Moderate exercise is known to stimulate the immune system (Martin et al., 2009) and is known to reduce the incidence of many types of cancers (Warburton et al., 2006; John et al., 2010). Higher immune response is correlated with longer survival in pancreatic cancer patients (Hamanaka et al., 2003). An overactive immune system as indicated by allergies has been associated with reduced incidence of some types of cancers and overall cancer rates (Wang and Diepgen, 2005). Increased immune response was indicative of lower ovarian cancer risk in a prospective study (Pinheiro et al., 2010). Success has been reported in treating cancers with immunotherapy, e.g. with Ipilimumab, which improves the immune response by overcoming T-cell suppression (Weber, 2007).
All these data point to an extremely important role played by deficiencies in the immune system in the pathogenesis of clinical cancer, with oncogenic mutations in the cancer cells playing an essential but not the decisive role. The immune system is however not a perfect defense against cancer, and cancer can develop in spite of the fully functioning immune system (Dunn et al., 2004). The LNT model completely ignores the immune system response in its estimation of cancer risk.
Since the immune system is so crucial in modulating the carcinogenic process, let us consider how radiation affects the immune system. Low dose radiation has been observed to stimulate the immune system (Hashimoto et al., 1999; Yu et al., 2004; Ina et al., 2005; Liu, 2007). Low dose radiation also stimulates other aspects of the bodily defense mechanisms, e.g. it increases antioxidant levels reducing the endogenous DNA damage, increases DNA repair capacity, and increases apoptosis of damaged cells (Feinendegen, 2005). High dose radiation, on the other hand, suppresses the immune capacity (Kennedy, 1965; Anderson and Lefkovits, 1979; Celer, 1990; Liu, 2003; Kusunoki and Hayashi, 2008).
An analogy with exercise may be helpful in understanding the biphasic dose response of the immune system to radiation. Whereas moderate exercise is known to stimulate the immune system, extreme exercise has an immune suppressive effect (Nieman, 2003; Radak et al., 2008; Martin et al., 2009). The benefits of moderate exercise in reducing the incidence of many diseases including cancer have been well documented (Warburton et al., 2006). For patients diagnosed with breast cancer and prostate cancer, vigorous physical activity has been linked to decreased mortality from the diseases (Holick et al., 2008; Kenfield et al., 2011b). On the other hand, extreme exercise such as marathon or ultra-marathon running is associated with increased upper respiratory tract infections indicative of the suppression of the immune system (Peters and Bateman, 1983), and increased intensity of training is associated with an increased number of melanoma markers in marathon runners (Richtig et al., 2008).
Ignoring the large variation in cancer rates in specifying no threshold
A third aspect of the current radiation safety paradigm and the consequent LNT model is that even the smallest dose of radiation is claimed to increase the risk of cancer, as there is no threshold. Meaningful measurement of the small predicted increase in cancer from the low dose radiation would be possible only if the cancer rates are stable, and have small measurement errors. It is well known that cancer incidence rates are highly variable as they depend on a large number of factors including age, tobacco use, ionizing radiation, chemicals, infections, alcohol, diet, exercise, etc. Separating the effect of one factor such as radiation in the presence of all the other variables would be subject to large errors and uncertainties. The measured cancer incidence rate is also affected by the sensitivity of the technologies used for screening for different types of cancers, and the extent of the screening. Over short periods of time most of these variables may be expected to have changed by a smaller amount. Hence, we may be able to obtain an indication of the stability of the cancer rates and uncertainties in their measurements by studying differences in cancer rates between successive years.
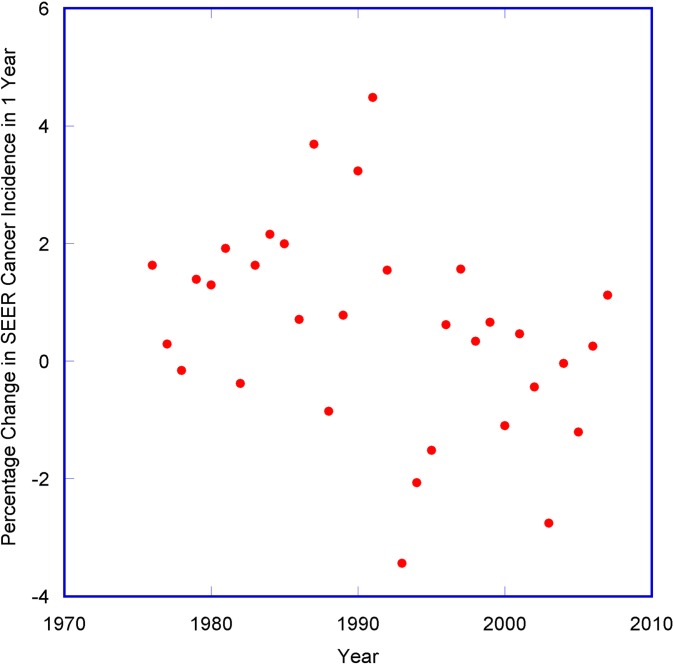
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute collects and publishes cancer incidence data from population-based cancer registries covering a large segment of the population in the USA (Altekruse et al., 2010). A large variation (much higher than standard errors) has been observed in the SEER age-adjusted cancer incidence rates between successive years, ranging from −3.4% to +4.5% (See Fig. 2). This variation is indicative of the effect of the multiple factors that can change the cancer rates between successive years. When calculating the lifetime risk of cancer for any cohorts, these variations can compound and become substantial. Hence there can be large uncertainties in estimating low dose cancer risk factors from carcinogens like radiation using long term studies. Indeed, the 95% confidence interval (C.I.) of cancer risk factor for low dose radiation spans a wide range of 400 to 1600 excess solid cancers per 100 mSv per 100,000 (for males), in the recent BEIR VII report (NRC, 2006). The uncertainty in the recommended risk factor has actually worsened when compared to the range of 420 to 1040 given in the BEIR V report (for 90% C.I.) 16 years earlier (NRC, 1990). Such a large and increasing uncertainty in the basic parameter of the LNT model after more than fifty years of study indicates the relationship between low dose radiation and cancer is very tenuous indeed, and low dose radiation may not be a relevant factor in carcinogenesis.
FIGURE 2.
Percentage change in SEER age-adjusted cancer incidence rates in 1 year.
In the above analysis, I have described three fundamental flaws of the current radiation safety paradigm:
The paradigm pays exclusive attention to DNA damage and mutations which are not decisive factors in clinical cancer.
The paradigm completely ignores the effect of the immune system response which is an extremely important factor modulating the occurrence of clinical cancer. The effect of radiation on immune system response is not linear, as low dose radiation stimulates the immune system, and high dose radiation suppresses it.
The paradigm and the consequent LNT model ignore the large variability in cancer rates in specifying no threshold. The lifetime risk measurements are likely to have large errors arising from the variability in the confounding factors and cancer rates from year to year. In view of this, specifying no threshold and implying that the smallest calculated increment in cancer rate is significant, is not credible.
Notwithstanding these fundamental flaws, we have been using the current radiation safety paradigm during the past five decades to guide us in our use of radiation. Were there any adverse health consequences from following this flawed paradigm?
FAILURE OF THE CURRENT RADIATION SAFETY PARADIGM
Our current radiation safety paradigm (exclusive attention to mutations, and ignoring the importance of bodily defense mechanisms including the immune system, as signified by the adoption of the LNT extrapolation model) may have led to missed opportunities in reducing cancer deaths in populations exposed to high doses of radiation such as the atomic bomb survivors in Japan. The importance of the immune system in holding cancers in check was noted as early as the 1970s, from the observed dramatic increase in cancers in organ transplant patients whose immune systems were suppressed, e.g. (Allison, 1970; Hoover and Fraumeni, 1973). The effect of exercise on enhancing different aspects of the immune system has been known for more than a century (Gleeson, 2000). Increased exercise had been correlated with decreased growth of tumors in animal models (Rusch and Kiline, 1944; Rashkis, 1952; Newton, 1965). Improved immune response (leukocytosis) from exercise had been observed in atomic bomb survivors (Belsky et al., 1972). Therefore, data was available in the 1970s to infer the beneficial effects of exercise in improving the immune system response and in reducing cancer. However, during this period, radiation-induced mutations became the primary focus in the carcinogenic process as signified by the dominance of the LNT model in radiation safety policies. The possibility of reducing cancers in the atomic bomb survivors through lifestyle modification (by educating them on the importance of the immune system and encouraging more physical exercise) was missed. A recent prospective study of the effects of lifestyle on cancer among atomic bomb survivors has shown that the group that exercised had between 15% and 35% less cancer mortality with 95% confidence compared to the group that did not exercise, indicating the effectiveness of exercise in reducing cancers in this population group (Mine et al., 2011). If we had conducted a study such as this in the 1970s, demonstrated the importance and effectiveness of exercise in reducing cancers, and initiated an education and support program for the atomic bomb survivors to exercise regularly, it may have resulted in significantly decreased cancer incidence and mortality in the radiated population. Instead, the LNT model has led us to a fatalistic and pessimistic approach towards population groups subjected to high doses of radiation, where we monitor their health closely, but take little action to reduce their risk of developing clinical cancer, for example by boosting their immune system with exercise. It is ironic that lack of action due to our radiation safety paradigm may have resulted in increased cancer deaths in the most radiated population group, whom the radiation safety paradigm should have protected. The radiation safety paradigm may have failed in its primary responsibility of protecting the health of the radiated population. It is imperative we consider a shift in the current radiation safety paradigm to include consideration of biological defense mechanisms in order to reduce further casualties in population groups that have been subjected to high dose radiation. Exercise intervention should be prescribed to the atomic bomb survivors and other radiated population groups (e.g. Chernobyl liquidators) to reduce their cancer risk in a pilot study, and if the intervention is found to be beneficial, should be expanded to the whole group. A shift in the present radiation safety paradigm can also enable study of the many reported beneficial effects of low dose radiation.
POTENTIAL BENEFICIAL EFFECTS OF LOW DOSE RADIATION
The biological effects of low dose radiation have been found to be similar to that of moderate exercise in animal studies, in that they stimulate the production of antioxidants (Yamaoka et al., 1991; Kojima et al., 1999; Khassaf et al., 2001; Ji, 2002), have a similar effect on cancer-initiation related parameters such as DNA damage, double-strand breaks, and apoptosis, (De Lisio et al., 2011; Phan, 2011), and enhance the immune system response (Nieman, 2003; Farooque et al., 2011). Several different classes of beneficial health effects of low dose radiation may be envisioned based on these observed biological effects.
The first class of potential beneficial effects of low dose radiation is its cancer preventive or therapeutic effect, since low dose radiation stimulates the bodily defense mechanisms including the immune system (Feinendegen, 2005). Low dose radiation has been observed to have a cancer preventive effect in controlled animal studies (Ullrich and Storer, 1979; Ito et al., 2007; Nowosielska et al., 2010; Phan, 2011). Low dose radiation has been shown to be an effective method of treating cancer by boosting the immune system in animal and human studies with little adverse side effects (Sakamoto, 2004; Farooque et al., 2011). Several retrospective human studies have shown a reduced cancer incidence from low dose radiation, however such studies are subject to many confounding factors, and so they are not discussed here. Prospective cancer prevention studies have been suggested (Cameron, 2002) but would require subjecting an asymptomatic population group to low dose radiation to measure the change in cancer rates. Considering the present radiation safety regulations and the widespread fear of radiation, such a prospective study is neither feasible nor advisable.
A second class of beneficial effects of low dose radiation may be in the control of aging-related non-cancer diseases. Oxidative damage has been implicated in the pathogenesis of many of these aging-related diseases and conditions, e.g. Alzheimer’s disease (Martins et al., 1986; Bonda et al., 2010), arthritis (Vasanthi et al., 2009), cataract (Spector, 1995), diabetes (Henriksen et al., 2011), heart disease (Heitzer et al., 2001), osteoporosis (Baek et al., 2010), Parkinson’s disease (Zhang et al., 1999), and stroke (Nanetti et al., 2011). Elevating antioxidant levels in the relevant organs may be helpful in reducing the oxidative damage and the impact of such diseases. In animal models, increased amount of antioxidants has led to a reduction of some of the non-cancer diseases, e.g. (Jung et al., 2001; Redout et al., 2010). Application of this idea in humans has however been problematic. Antioxidant therapies have failed to reduce diabetes (Sheikh-Ali et al., 2011), cardiovascular disease (Kris-Etherton et al., 2004) and stroke (Schurks et al., 2010) in controlled clinical trials, though oxidative damage has been identified as playing a key role in these. Bioavailability of the antioxidants in the relevant organs may be a factor, as the administered antioxidants are distributed throughout the body. Increased administration of antioxidants may be considered for improving bioavailability. However, excessive levels of antioxidants can interfere with essential cellular signaling mechanisms and so may be harmful (Halliwell, 2011). Use of antioxidants more than seven times per week has been associated with a doubling of prostate cancer risk when compared to the population not using any antioxidant supplements in a large study (Lawson et al., 2007). Antioxidant supplements have been observed to prevent the health-promoting effects of exercise (Ristow et al., 2009). A compilation of a large number of randomized clinical trials on the effects of antioxidant supplements has shown a slight increase in mortality in the groups using antioxidant supplements (Bjelakovic et al., 2007). Whereas externally administered antioxidants have been observed to not be beneficial and potentially harmful, endogenous production of antioxidants, such as from moderate exercise, has been found to be beneficial (Briones and Touyz, 2009).
Low dose radiation is known to elevate the antioxidant levels in many organs in animal studies, e.g. (Yamaoka et al., 1991; Kojima et al., 1999; Pathak et al., 2007), and so may play a role in reducing the impact of the diseases and conditions caused by oxidative damage. Animal studies have shown low dose radiation reduces several non-cancer diseases, e.g. diabetes and diabetes related complications (Nomura and Sakai, 2006; Wang et al., 2008; Nomura et al., 2011), autoimmune diseases (Tanaka et al., 2005), arthritis (Nakatsukasa et al., 2008), brain injury from chemical induced oxidative damage (Kojima et al., 1999) and prion infection in the brain (Plews et al., 2010). Human studies with low dose radiation have also shown a reduction in some non-cancer diseases, e.g. asthma (Mitsunobu et al., 2003), and rheumatic diseases (Falkenbach et al., 2005). Reduced non-cancer mortality has been reported in atomic bomb survivors exposed to low dose radiation for the period 1950–67 (but not in a later period) indicative of a non-cancer disease preventive effect of low dose radiation in the near term (Preston et al., 2003; Luckey, 2008). In a study of fluorspar miners exposed to high dose radiation to the lungs from radon, the miners had higher mortality from lung cancer as expected, but the standardized mortality ratio for non-cancer diseases was 0.74 (95% CI: 0.70–0.77) (Villeneuve et al., 2007). Thus, there is suggestive evidence in epidemiological studies for the beneficial effect of low dose radiation for non-cancer diseases. Considering that currently there is no method of preventing, curing or controlling some of these diseases (e.g. Alzheimer’s), the effect of low dose radiation on the non-cancer diseases should be studied systematically, to determine if low dose radiation has a beneficial effect for any of these diseases. The beneficial effects may become evident in a short period of time in terms of reduced sickness and mortality. By not studying the health effects of low dose radiation systematically because of the LNT model based fear, we may have missed an opportunity to reduce non-cancer diseases.
A third class of beneficial effects of low dose radiation may be the reduction of adverse side effects of standard cancer therapies. Radiation-induced free radicals and the oxidative damage they cause are predominantly responsible for the biological damage and adverse side effects from the high-dose radiation used in radiotherapy (Lawenda et al., 2008). Likewise, for many chemotherapy agents, oxidative damage to normal tissues is responsible for the adverse effects (Chen et al., 2007). Elevating the level of antioxidants in the normal tissues prior to the cancer therapies may be helpful in reducing the damage and the side effects. However, systemic administration of antioxidants may reduce the effectiveness of the therapies, and so is not recommended (Lawenda et al., 2008). The advantage of low dose radiation is that it can be applied selectively to normal tissues while minimally exposing the tumors, thus limiting the primary adaptive response to normal tissue only. For example, prior low dose radiation of normal cells surrounding the tumor volume has been tested in a canine model to demonstrate reduction of skin and mucous membrane side effects of radiation therapy by inducing an adaptive response in the normal cells (Blankenbecler, 2010). Another advantage of using low-dose radiation is that it has been shown to stimulate the immune system leading to improved tumor control in animal studies (Wu et al., 2008). It may also lead to increased apoptosis of pre-cancerous cells through inter-cellular communication (Portess et al., 2007). Thus pre-treatment of normal tissues with low dose radiation may reduce the adverse side effects from cancer therapies, without reducing the effectiveness of the therapies, and may even enhance their effectiveness.
A fourth class of beneficial effects of low dose radiation may be to impart the beneficial effects of exercise to persons with disabilities, painful conditions or weakness that prevent or discourage them from exercising. Though the benefits of appropriate exercise for such populations have been well documented, compliance with exercise programs due to pain is a considerable problem as there are high dropout rates (Richards and Scott, 2002).
The use of low dose radiation contrasts sharply with traditional approaches for these illnesses and conditions. Many of the standard drugs and treatments are effective in controlling the symptoms of the diseases, e.g. coronary plaque formation, hormonal imbalance, hypertension, tumors, etc. without addressing the underlying causes of the aging-related diseases which appear to be oxidative stress and/or deficiencies in the immune system. Thus the diseases and the symptoms tend to recur, e.g. blockage of arteries, second cancers, etc. Since the administered drugs are distributed throughout the body and act on normal tissues and organs in addition to the targeted organs, many of the drugs have serious side effects. Standard cancer therapies also have immediate and long lasting adverse side effects (Cukier, 2005). Even targeted therapies can have serious side effects when continued over a long period of time (Appleby et al., 2011). On the other hand, low dose radiation has beneficial biological effects similar to moderate exercise, as it elevates antioxidant levels and addresses the underlying cause of the diseases and conditions, and it can be applied to any organ or tissue locally minimizing the effects on other organs and tissues, if so desired. Low dose radiation may be useful in reducing the recurrence of the diseases by reducing oxidative damage after the symptoms have been addressed through traditional means.
DISCUSSION
It is clear from the discussions above that there is no justification for continuing the use of the current radiation safety paradigm, as it was established based on premises that have turned out to be false, is fundamentally flawed, has no scientific foundation, has likely led to missed opportunities in reducing cancer deaths among atomic bomb survivors, has led to an irrational fear of low dose radiation, has prevented the study of potentially beneficial applications of low dose radiation, and has likely prevented progress in reducing aging-related diseases, side effects of cancer therapies, and diseases in the infirm. We need to shift to a paradigm that recognizes adaptive response and the potential for beneficial effects of low dose radiation. Making this change is not going to be easy, as the new paradigm is contrary to the recommendations of most advisory bodies, present government regulations, and the public perception of the effects of low dose radiation. Any attempts to rescind the current regulations may be viewed with suspicion by the public because of the widespread belief in the LNT model. A sustained educational campaign should be initiated to correct the current misconceptions in the scientific community and the public about the pathogenesis of clinical cancer and the health effects of low dose radiation. Demonstration of beneficial health effects of low dose radiation through pilot clinical trials may be helpful in reducing the fear of low dose radiation, and enabling the paradigm shift.
To guide us in this change, the scientific community should form new advisory bodies that use experimental evidence (including the observed beneficial effects of low dose radiation) in recommending radiation safety policies rather than the unproven LNT extrapolation hypothesis advocated by most of the present advisory bodies. Such advisory bodies can also guide us in studying and implementing the beneficial applications of low dose radiation.
The last paradigm shift in radiation safety occurred in the 1950s. Prior to this period, the main concern with radiation use was skin erythema (Sinclair, 1981), and it was considered as appropriate to treat many diseases and conditions with radiation, including for children, e.g. (Mottram and Hill, 1949). In the 1950s, following the observation of increased leukemias in atomic bomb survivors, genetic effects became the dominant concern leading to the adoption of the current radiation safety paradigm, as the advisory bodies such as NCRP and ICRP reduced the radiation dose limits. This was not because of any observed harm from low dose radiation but because of general public concerns (Sinclair, 1981). Realization that the current paradigm may have led to missed opportunities in preventing cancer deaths among atomic bomb survivors may again raise public concerns and scrutiny by the media, and facilitate another paradigm shift in radiation safety in the near future.
SUMMARY AND CONCLUSIONS
The current radiation safety paradigm using the LNT model was introduced following the observation of linear dose dependence of leukemias in atomic bomb survivors for high dose radiation, observation of linear dose dependence of mutations in drosophila for high dose radiation, linking the two, and extrapolating linearly to low doses since cancer is such a feared disease. The LNT model pays exclusive attention to DNA damage, which is not a decisive factor in clinical cancer as observed in autopsy studies, while ignoring the immune system response, which plays a major role in keeping occult cancers in check, as indicated by the huge increase in cancers in organ transplant and AIDS patients. The large temporal and regional variations in cancer rates have been ignored in setting a zero threshold in the LNT model, implying the smallest changes in projected cancer rates are significant. The large expenditures for dose reduction based on the LNT model may not result in any measurable reduction in cancers. In view of the large number of factors that have measurable effects on cancer rates, an optimum allocation of available resources between the different risk factors can result in maximizing the reduction in cancers. The lopsided allocation of resources based on the LNT model deviates from this optimum allocation and so would result in a sub-optimal reduction in cancers. Thus, the use of a no-threshold model is not a conservative approach to radiation safety.
Our misunderstanding of the pathogenesis of clinical cancer and the resultant radiation safety paradigm paying exclusive attention to mutations may have resulted in missed opportunities in preventing cancer deaths in atomic bomb survivors and in other radiated populations. The importance of the immune system in preventing cancers was known in the 1970s from the increased cancers in organ transplant patients in whom the immune system had been suppressed. The increase in immune response from exercise has been known for more than a century. A recent prospective study of the effect of lifestyle on cancers has shown the effectiveness of exercise in reducing cancers in atomic bomb survivors. An exercise intervention study in the 1970s could have resulted in reduced cancer deaths among atomic bomb survivors. However, the focus of the radiation safety paradigm in that time period was on reducing mutations as the cause of cancer, and so missed the opportunity to reduce cancers in this population group. The current radiation safety paradigm has likely failed in its primary responsibility of reducing radiogenic cancers. A paradigm shift is warranted to reduce further casualties.
The biological effects of low dose radiation are similar to that of moderate exercise in that they both lead to slightly increased production of free radicals stimulating the body’s defensive mechanisms such as increased antioxidant capacity and the immune system. Thus, low dose radiation may be expected to reduce rather than increase cancers, since the immune system plays an extremely important role in preventing cancers. This has been observed in many controlled animal studies. The increased antioxidants may also help to reduce aging-related diseases since oxidative damage has been implicated in many of these diseases. External administration of antioxidants has failed to reduce diseases in clinical trials, possibly because there may not have been sufficient bioavailability of the antioxidants in the relevant organs to reduce the oxidative damage. Administration of large doses of antioxidants can interfere with important cellular signaling mechanisms, resulting in worsening of health and so is not recommended. Thus we are at an impasse in dealing with the underlying cause of aging-related diseases. Low dose radiation may provide a solution to this impasse by endogenous production of antioxidants in the relevant organs. Controlled animal studies have shown reduction of many of these diseases using low dose radiation, and some human studies have also shown promising results. Low dose radiation can be used to supplement traditional treatments which address the symptoms of the diseases, in order to reduce the recurrence of the diseases. The increased antioxidants from low dose radiation may also reduce the adverse side effects of cancer therapies, since many of these are caused by oxidative damage also. Another potential application of low dose radiation may be in providing the benefits of exercise to the infirm that are unable to exercise.
The current situation with respect to these different diseases and conditions is far from satisfactory, as there is no effective way to prevent the cancers, aging-related diseases such as Alzheimer’s, adverse side effects of cancer therapies, and inactivity related diseases in the infirm. Pilot clinical trials should be conducted to study the effects of low dose radiation for these diseases and conditions to identify the beneficial ones for potential use. The current radiation safety paradigm and regulations, and the consequent fear of low dose radiation have discouraged such studies, preventing progress in dealing with these unsolved problems in human health.
A paradigm shift is warranted to reduce further casualties and improve human health. Since the proposed changes are completely contrary to the recommendations of most of the current advisory bodies, it may be preferable to form new advisory bodies with a fresh perspective to guide us in these changes. Realization by the media and the public that the present paradigm, by its inaction, may have missed opportunities in reducing cancer deaths among radiated populations may lead to increased public concerns and closer scrutiny, and hasten the process of dismantling the current radiation safety paradigm in the near future.
Acknowledgments
This research was supported in part by the Office of Science (BER), U.S. Department of Energy, under Award No. DE-SC0001196. The views and opinions expressed herein are those of the author and do not necessarily reflect those of his employer or the funding agency.
The author would like to thank: Drs. Samuel Litwin and Karthik Devarajan for many useful discussions; Dr. Matthew K. Robinson for a critical review of the manuscript; Drs. Rosaleen Parsons and Jian Q. Yu for their reviews and suggestions; and Ms. Maryann Krajkowski for her editorial input.
REFERENCES
- Alberts DS, Hess LM, Thomson CA, Chen Z. The Role of Diet, Physical Activity and Body Composition in Cancer Prevention - Fundamentals of Cancer Prevention. Springer; Berlin Heidelberg: 2008. [Google Scholar]
- Allison AC. Tumour development following immunosuppression. Proc R Soc Med. 1970;63:1077–80. doi: 10.1177/003591577006301055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison W. Risk perception and energy infrastructure, Written evidence submitted to UK Parliament [Online] 2011. Available: http://www.publications.parliament.uk/pa/cm201012/cmselect/cmsctech/writev/risk/m04.htm [Accessed Jan 8 2012].
- Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Cronin K, Chen H, Feuer E, Stinchcomb D, Edwards BK, editors. National Cancer Institute; Bethesda, MD: 2010. SEER Cancer Statistics Review, 1975–2007, based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Online] Available: http://seer.cancer.gov/csr/1975_2007/ [Accessed Apr 23 2010]. [Google Scholar]
- Anderson RE, Lefkovits I. In vitro evaluation of radiation-induced augmentation of the immune response. Am J Pathol. 1979;97:456–72. [PMC free article] [PubMed] [Google Scholar]
- Appleby L, Morrissey S, Bellmunt J, Rosenberg J. Management of treatment-related toxicity with targeted therapies for renal cell carcinoma: evidence-based practice and best practices. Hematol Oncol Clin North Am. 2011;25:893–915. doi: 10.1016/j.hoc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Averbeck D. Does scientific evidence support a change from the LNT model for low-dose radiation risk extrapolation? Health Phys. 2009;97:493–504. doi: 10.1097/HP.0b013e3181b08a20. [DOI] [PubMed] [Google Scholar]
- Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87:226–35. doi: 10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13:71–6. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K. One century of radon therapy. International Journal of Low Radiation. 2004;1:333–357. [Google Scholar]
- Belsky JL, Ishimaru T, Oashi T, Robertson TL, Taniguchi B. Leukocyte response to exercise in atomic bomb survivors. Radiat Res. 1972;50:699–707. [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Blankenbecler R. Low-dose pretreatment for radiation therapy. Dose Response. 2010;8:534–42. doi: 10.2203/dose-response.10-033.Blankenbecler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59:290–4. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Sachs RK. Estimating radiation-induced cancer risks at very low doses: rationale for using a linear no-threshold approach. Radiat Environ Biophys. 2006;44:253–6. doi: 10.1007/s00411-006-0029-4. [DOI] [PubMed] [Google Scholar]
- Briones AM, Touyz RM. Moderate exercise decreases inflammation and oxidative stress in hypertension: but what are the mechanisms? Hypertension. 2009;54:1206–8. doi: 10.1161/HYPERTENSIONAHA.109.136622. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Improving the scientific foundations for estimating health risks from the Fukushima incident. Proc Natl Acad Sci U S A. 2011;108:19447–8. doi: 10.1073/pnas.1117296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. The road to linearity: why linearity at low doses became the basis for carcinogen risk assessment. Arch Toxicol. 2009;83:203–25. doi: 10.1007/s00204-009-0412-4. [DOI] [PubMed] [Google Scholar]
- Cameron JR. A prospective study should be performed to test the hypothesis that an increase in background radiation to residents in the gulf states will increase their longevity. For the proposition. Med Phys. 2002;29:1511–2. doi: 10.1118/1.1489045. [DOI] [PubMed] [Google Scholar]
- Celer V. Suppressive effects of ionizing radiation on immunoproductive cells in laboratory mice. Vet Med (Praha) 1990;35:495–500. [PubMed] [Google Scholar]
- Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–56. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- Cohen B. The Cancer Risk from Low-Level Radiation. In: Tack D, Gevenois P, editors. Radiation Dose from Adult and Pediatric Multidetector Computed Tomography. Berlin: Springer-Verlag; 2007. [Google Scholar]
- Cohen BL. Cancer risk from low-level radiation. AJR Am J Roentgenol. 2002;179:1137–43. doi: 10.2214/ajr.179.5.1791137. [DOI] [PubMed] [Google Scholar]
- Cook R, Calabrese EJ. The importance of hormesis to public health. Environ Health Perspect. 2006;114:1631–5. doi: 10.1289/ehp.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukier D. Coping with chemotherapy and radiation. New York: McGraw-Hill; 2005. [Google Scholar]
- Cuttler JM, Pollycove M. Nuclear energy and health: and the benefits of low-dose radiation hormesis. Dose Response. 2009;7:52–89. doi: 10.2203/dose-response.08-024.Cuttler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisio M, Phan N, Boreham DR, Parise G. Exercise-induced protection of bone marrow cells following exposure to radiation. Appl Physiol Nutr Metab. 2011;36:80–7. doi: 10.1139/H10-087. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Falkenbach A, Kovacs J, Franke A, Jorgens K, Ammer K. Radon therapy for the treatment of rheumatic diseases—review and meta-analysis of controlled clinical trials. Rheumatol Int. 2005;25:205–10. doi: 10.1007/s00296-003-0419-8. [DOI] [PubMed] [Google Scholar]
- Farooque A, Mathur R, Verma A, Kaul V, Bhatt AN, Adhikari JS, Afrin F, Singh S, Dwarakanath BS. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther. 2011;11:791–802. doi: 10.1586/era.10.217. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Frazer IH, Leggatt GR, Mattarollo SR. Prevention and treatment of papillomavirus-related cancers through immunization. Annu Rev Immunol. 2011;29:111–38. doi: 10.1146/annurev-immunol-031210-101308. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Special feature for the Olympics: effects of exercise on the immune system. Overview: exercise immunology. Immunol Cell Biol. 2000;78:483–4. doi: 10.1111/j.1440-1711.2000.t01-13-.x. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol Sci. 2011;32:125–30. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–24. [PubMed] [Google Scholar]
- Hayase H, Ohshima Y, Takahashi M, Kojima S. The enhancement of Th1 immunity and the suppression of tumour growth by low-dose γ-radiation. International Journal of Low Radiation. 2008;5:275–289. [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial Dysfunction, Oxidative Stress, and Risk of Cardiovascular Events in Patients With Coronary Artery Disease. Am Heart Assoc. 2001. [DOI] [PubMed]
- Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey RJ, Bowers EJ, Clelland RC. Radiation hormesis, public health, and public policy: a commentary. Health Phys. 1983;44:207–19. doi: 10.1097/00004032-198303000-00001. [DOI] [PubMed] [Google Scholar]
- Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–86. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- Hoover R, Fraumeni JF., Jr Risk of cancer in renal-transplant recipients. Lancet. 1973;2:55–7. doi: 10.1016/s0140-6736(73)93256-x. [DOI] [PubMed] [Google Scholar]
- Hymowitz N. Smoking and cancer: a review of public health and clinical implications. J Natl Med Assoc. 2011;103:695–700. doi: 10.1016/s0027-9684(15)30408-9. [DOI] [PubMed] [Google Scholar]
- ICRP The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Imaida K, Hasegawa R, Kato T, Futakuchi M, Takahashi S, Ogawa K, Asamoto M, Yamamoto T, Suzuki K, Inagaki T, Shinagawa N, Shirai T. Clinicopathological analysis on cancers of autopsy cases in a geriatric hospital. Pathol Int. 1997;47:293–300. doi: 10.1111/j.1440-1827.1997.tb04496.x. [DOI] [PubMed] [Google Scholar]
- Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by lifelong low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163:153–8. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Ito M, Shibamoto Y, Ayakawa S, Tomita N, Sugie C, Ogino H. Low-dose whole-body irradiation induced radioadaptive response in C57BL/6 mice. J Radiat Res (Tokyo) 2007;48:455–60. doi: 10.1269/jrr.07022. [DOI] [PubMed] [Google Scholar]
- Jaworowski Z. Beneficial effects of radiation and regulatory policy. Australas Phys Eng Sci Med. 1997;20:125–38. [PubMed] [Google Scholar]
- Jaworowski Z. The paradigm that failed. International Journal of Low Radiation. 2008;5:151–155. [Google Scholar]
- Jaworowski Z. Observations on the Chernobyl Disaster and LNT. Dose Response. 2010a;8:148–71. doi: 10.2203/dose-response.09-029.Jaworowski. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski Z. Radiation hormesis—a remedy for fear. Hum Exp Toxicol. 2010b;29:263–70. doi: 10.1177/0960327110363974. [DOI] [PubMed] [Google Scholar]
- Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- John EM, Koo J, Horn-Ross PL. Lifetime physical activity and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1276–83. doi: 10.1158/1055-9965.EPI-09-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. Failing to adapt – the ageing immune system’s role in cancer pathogenesis. Reviews in Clinical Gerontology. 2011;21:209–218. [Google Scholar]
- Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett. 2001;304:157–60. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- Kellerer AM. Risk estimates for radiation-induced cancer—the epidemiological evidence. Radiat Environ Biophys. 2000;39:17–24. doi: 10.1007/pl00007679. [DOI] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011a;305:2548–55. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011b;29:726–32. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. Radiosensitivity of the immune response to sheep red cells in the mouse, as measured by the hemolytic plaque method. The Journal of immunology. 1965;94:715–722. [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–5. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Koana T, Tsujimura H. A U-shaped dose-response relationship between x radiation and sex-linked recessive lethal mutation in male germ cells of Drosophila. Radiat Res. 2010;174:46–51. doi: 10.1667/RR2085.1. [DOI] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Kojima S, Matsuki O, Nomura T, Yamaoka K, Takahashi M, Niki E. Elevation of antioxidant potency in the brain of mice by low-dose gamma-ray irradiation and its effect on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced brain damage. Free Radic Biol Med. 1999;26:388–95. doi: 10.1016/s0891-5849(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–41. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol. 2008;84:1–14. doi: 10.1080/09553000701616106. [DOI] [PubMed] [Google Scholar]
- Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–83. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, Leitzmann MF. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–64. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251:6–12. doi: 10.1148/radiol.2511081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SZ. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinearity Biol Toxicol Med. 2003;1:71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SZ. Cancer control related to stimulation of immunity by low-dose radiation. Dose Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey TD. Hormesis with ionizing radiation. Boca Raton, Fla: CRC Press; 1980. [Google Scholar]
- Luckey TD. Radiation hormesis. Boca Raton, Fla: CRC Press; 1991. [Google Scholar]
- Luckey TD. Atomic bomb health benefits. Dose Response. 2008;6:369–82. doi: 10.2203/dose-response.08-009.Luckey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CJ. The LNT model provides the best approach for practical implementation of radiation protection. Br J Radiol. 2005;78:14–6. doi: 10.1259/bjr/31745335. [DOI] [PubMed] [Google Scholar]
- Martin SA, Pence BD, Woods JA. Exercise and respiratory tract viral infections. Exerc Sport Sci Rev. 2009;37:157–64. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RN, Harper CG, Stokes GB, Masters CL. Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer’s disease may reflect oxidative stress. J Neurochem. 1986;46:1042–5. doi: 10.1111/j.1471-4159.1986.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Mine M, Yokota K, Shibata Y. Effects of Lifestyle on the mortality in Nagasaki A-bomb survivors, Poster POS10-5. 14th International Congress of Radiation Research; Warsaw, Poland. 2011. p. 121. [Google Scholar]
- Mitchel RE. Cancer and low dose responses in vivo: implications for radiation protection. Dose Response. 2007;5:284–91. doi: 10.2203/dose-response.07-014.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel RE, Hasu M, Bugden M, Wyatt H, Little MP, Gola A, Hildebrandt G, Priest ND, Whitman SC. Low-dose radiation exposure and atherosclerosis in ApoE/mice. Radiat Res. 2011;175:665–76. doi: 10.1667/RR2176.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunobu F, Yamaoka K, Hanamoto K, Kojima S, Hosaki Y, Ashida K, Sugita K, Tanizaki Y. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res (Tokyo) 2003;44:95–9. doi: 10.1269/jrr.44.95. [DOI] [PubMed] [Google Scholar]
- Mottram ME, Hill HA. Radiation therapy of ringworm of the scalp. Calif Med. 1949;70:189–93. [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res (Tokyo) 2008;49:381–9. doi: 10.1269/jrr.08002. [DOI] [PubMed] [Google Scholar]
- Nanetti L, Raffaelli F, Vignini A, Perozzi C, Silvestrini M, Bartolini M, Provinciali L, Mazzanti L. Oxidative stress in ischaemic stroke. Eur J Clin Invest. 2011. [DOI] [PubMed]
- NCRP . Report No. 136 - Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing Radiation. Bethesda, MD: National Committee on Radiation Protection and Measurements; 2001. [Google Scholar]
- Newton G. Tumor Susceptibility in Rats: Role of Infantile Manipulation and Later Exercise. Psychol Rep. 1965;16:127–32. doi: 10.2466/pr0.1965.16.1.127. [DOI] [PubMed] [Google Scholar]
- Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep. 2003;2:239–42. doi: 10.1249/00149619-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Nomura T, Li XH, Ogata H, Sakai K, Kondo T, Takano Y, Magae J. Suppressive Effects of Continuous Low-Dose-Rate gamma Irradiation on Diabetic Nephropathy in Type II Diabetes Mellitus Model Mice. Radiat Res. 2011. [DOI] [PubMed]
- Nomura T, Sakai K. Suppressive effect of low-dose X-irradiation on type I diabetes non-obese diabetic (NOD) mice. Int. J. Low Radiation. 2006;2:28–33. [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Immunological mechanism of the low-dose radiation-induced suppression of cancer metastases in a mouse model. Dose Response. 2010;8:209–26. doi: 10.2203/dose-response.09-016.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Health effects of exposure to low levels of ionizing radiation : BEIR V, National Research Council (U.S.). Committee on the Biological Effects of Ionizing Radiations. Washington, D.C.: National Academy Press; 1990. [Google Scholar]
- NRC . Health risks from exposure to low levels of ionizing radiation : BEIR VII Phase 2, National Research Council (U.S.). Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. Washington, D.C.: National Academies Press; 2006. [PubMed] [Google Scholar]
- Nussbaum RH. The linear no-threshold dose-effect relation: is it relevant to radiation protection regulation? Med Phys. 1998;25:291–9. doi: 10.1118/1.598210. [DOI] [PubMed] [Google Scholar]
- Ogura K, Magae J, Kawakami Y, Koana T. Reduction in mutation frequency by very low-dose gamma irradiation of Drosophila melanogaster germ cells. Radiat Res. 2009;171:1–8. doi: 10.1667/RR1288.1. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 105 Suppl. 2011;2:S77–81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak CM, Avti PK, Kumar S, Khanduja KL, Sharma SC. Whole body exposure to low-dose gamma radiation promotes kidney antioxidant status in Balb/c mice. J Radiat Res (Tokyo) 2007;48:113–20. doi: 10.1269/jrr.06063. [DOI] [PubMed] [Google Scholar]
- Peters EM, Bateman ED. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J. 1983;64:582–4. [PubMed] [Google Scholar]
- Phan N. 2011. Understanding The Biological Effects And Cancer Risk Of Medical Diagnostic Computed Tomography. PhD Thesis, McMaster University. [Google Scholar]
- Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, Cramer DW. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:1595–601. doi: 10.1158/1055-9965.EPI-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plews M, Simon SL, Boreham DR, Parchaliuk D, Wyatt H, Mantha R, Frost K, Lamoureux L, Stobart M, Czub S, Mitchel RE, Knox JD. A radiation-induced adaptive response prolongs the survival of prion-infected mice. Free Radic Biol Med. 2010;49:1417–21. doi: 10.1016/j.freeradbiomed.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Portess DI, Bauer G, Hill MA, O’Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–53. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- Preston RJ. The LNT model is the best we can do—today. J Radiol Prot. 2003;23:263–8. doi: 10.1088/0952-4746/23/3/303. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rashkis HA. Systemic stress as an inhibitor of experimental tumors in Swiss mice. Science. 1952;116:169–71. doi: 10.1126/science.116.3007.169. [DOI] [PubMed] [Google Scholar]
- Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038–47. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
- Richards SC, Scott DL. Prescribed exercise in people with fibromyalgia: parallel group randomised controlled trial. BMJ. 2002;325:185. doi: 10.1136/bmj.325.7357.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtig E, Ambros-Rudolph CM, Trapp M, Lackner HK, Hofmann-Wellenhof R, Kerl H, Schwaberger G. Melanoma markers in marathon runners: increase with sun exposure and physical strain. Dermatology. 2008;217:38–44. doi: 10.1159/000121473. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch H, Kiline B. The Effect of Exercise on the Growth of a Mouse Tumor. Cancer Research. 1944;4:116–118. [Google Scholar]
- Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity Biol Toxicol Med. 2004;2:293–316. doi: 10.1080/15401420490900254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Radiation hormesis and the linear-no-threshold assumption. Heidelberg: Springer; 2010. [Google Scholar]
- Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. Residential Radon Appears to Prevent Lung Cancer. Dose-Response. 2011;9:444–464. doi: 10.2203/dose-response.11-027.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. It’s time for a new low-dose-radiation risk assessment paradigm—one that acknowledges hormesis. Dose Response. 2008;6:333–51. doi: 10.2203/dose-response.07-005.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. Infection, stem cells and cancer signals. Curr Pharm Biotechnol. 2011;12:182–8. doi: 10.2174/138920111794295675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Ali M, Chehade JM, Mooradian AD. The antioxidant paradox in diabetes mellitus. Am J Ther. 2011;18:266–78. doi: 10.1097/MJT.0b013e3181b7badf. [DOI] [PubMed] [Google Scholar]
- Sinclair WK. Radiation protection: the NCRP guidelines and some considerations for the future. Yale J Biol Med. 1981;54:471–84. [PMC free article] [PubMed] [Google Scholar]
- Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Eur Urol. 2005;48:739–44. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Spector A. Oxidative stress-induced cataract: mechanism of action. Faseb J. 1995;9:1173–82. [PubMed] [Google Scholar]
- Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat Res. 2006;165:337–42. doi: 10.1667/rr3501.1. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tago F, Fang SP, Shimura N, Kojima S. Repeated 0.5-Gy gamma-ray irradiation attenuates autoimmune manifestations in MRL-lpr/lpr mice. Int J Radiat Biol. 2005;81:731–40. doi: 10.1080/09553000500519790. [DOI] [PubMed] [Google Scholar]
- Testino G, Ancarani O, Scafato E. [Alcohol consumption and cancer risk] Recenti Prog Med. 2011;102:399–406. doi: 10.1701/955.10455. [DOI] [PubMed] [Google Scholar]
- Thomas R. The US radium luminisers: a case for a policy of ‘below regulatory concern’. J Radiol Prot. 1994;14:141–153. [Google Scholar]
- Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the joint report of the Academie des Sciences (Paris) and of the Academie Nationale de Medecine. Int J Radiat Oncol Biol Phys. 2005;63:317–9. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich RL, Storer JB. Influence of gamma irradiation on the development of neoplastic disease in mice. II. Solid tumors. Radiat Res. 1979;80:317–24. [PubMed] [Google Scholar]
- Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009a;125:1747–54. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- Vajdic CM, van Leeuwen MT. What types of cancers are associated with immune suppression in HIV? Lessons from solid organ transplant recipients. Curr Opin HIV AIDS. 2009b;4:35–41. doi: 10.1097/coh.0b013e328319bcd1. [DOI] [PubMed] [Google Scholar]
- Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis. 2009;12:29–33. doi: 10.1111/j.1756-185X.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Morrison HI, Lane R. Radon and lung cancer risk: an extension of the mortality follow-up of the Newfoundland fluorspar cohort. Health Phys. 2007;92:157–69. doi: 10.1097/01.HP.0000239127.43136.89. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Li XK, Sakai K, Lu C. Low-dose radiation and its clinical implications: diabetes. Hum Exp Toxicol. 2008;27:135–42. doi: 10.1177/0960327108090752. [DOI] [PubMed] [Google Scholar]
- Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60:1098–111. doi: 10.1111/j.1398-9995.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–9. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–72. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–84. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- WHO . Number of Registered Deaths [Online] World Health Organization; 2011. Available: http://apps.who.int/whosis/database/mort/table1.cfm [Accessed June 26, 2011]. [Google Scholar]
- Wu N, Jin SZ, Pan XN, Liu SZ. Increase in efficacy of cancer radiotherapy by combination with whole-body low dose irradiation. Int J Radiat Biol. 2008;84:201–10. doi: 10.1080/09553000801902133. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Edamatsu R, Mori A. Increased SOD activities and decreased lipid peroxide levels induced by low dose X irradiation in rat organs. Free Radic Biol Med. 1991;11:299–306. doi: 10.1016/0891-5849(91)90127-o. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Komoto Y. Experimental study of alleviation of hypertension, diabetes and pain by radon inhalation. Physiol Chem Phys Med NMR. 1996;28:1–5. [PubMed] [Google Scholar]
- Yu HS, Song AQ, Lu YD, Qiu WS, Shen FZ. Effects of low-dose radiation on tumor growth, erythrocyte immune function and SOD activity in tumor-bearing mice. Chin Med J (Engl) 2004;117:1036–9. [PubMed] [Google Scholar]
- Zhang C, Jin S, Guo W, Li C, Li X, Rane MJ, Wang G, Cai L. Attenuation of diabetes-induced cardiac inflammation and pathological remodeling by low-dose radiation. Radiat Res. 2011;175:307–21. doi: 10.1667/RR1950.1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–9. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]