Abstract
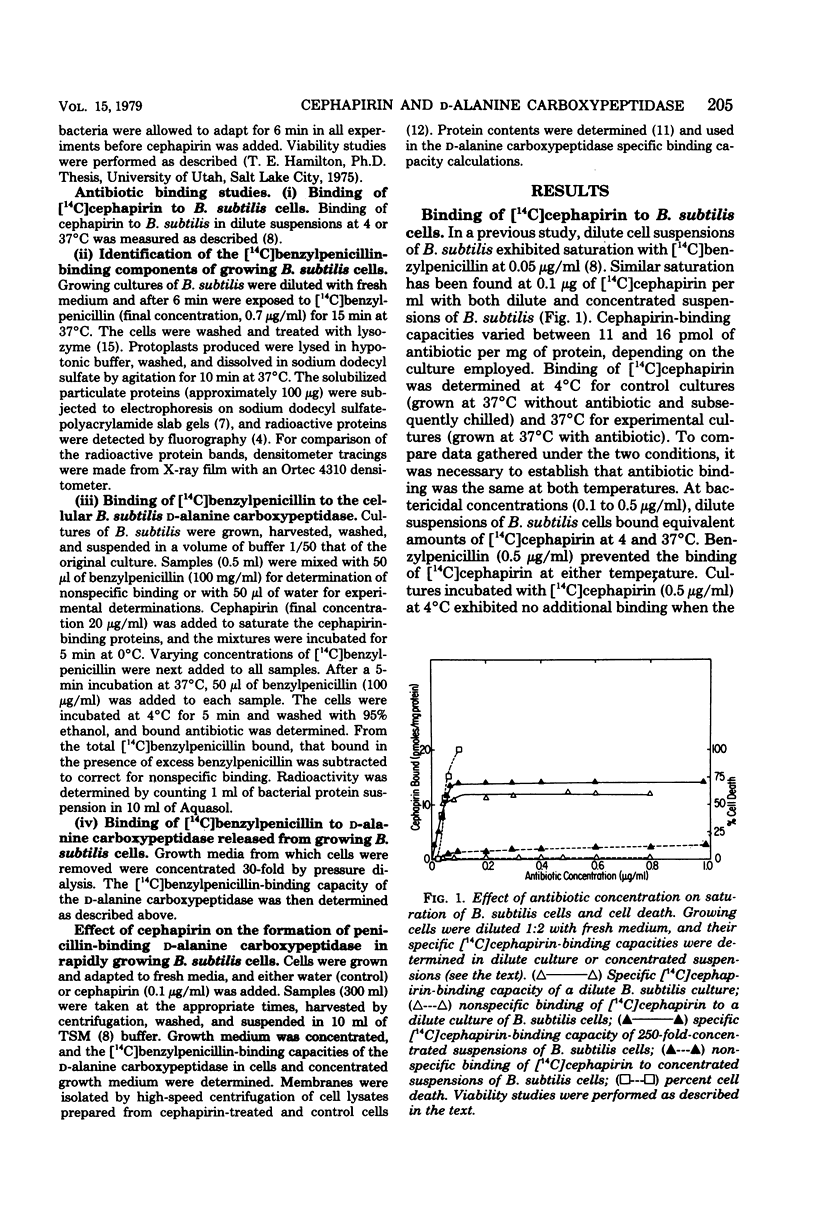
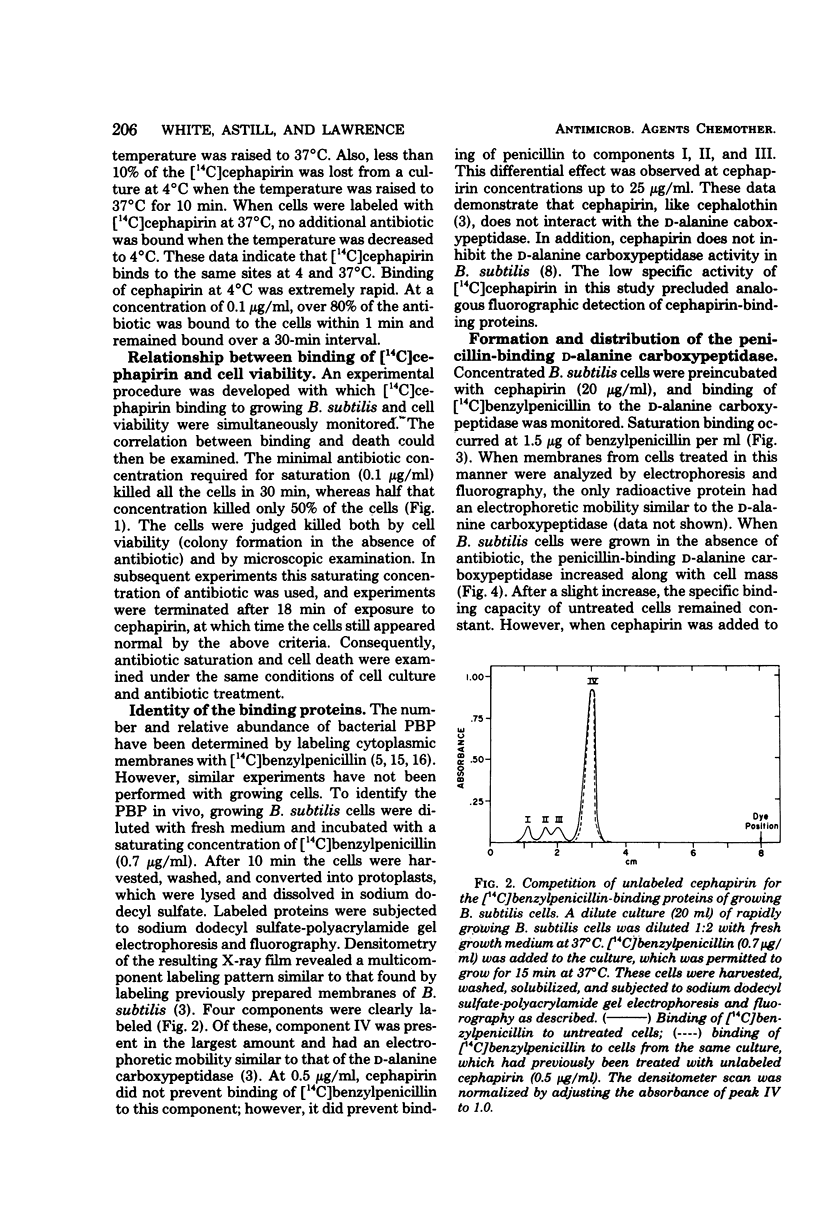
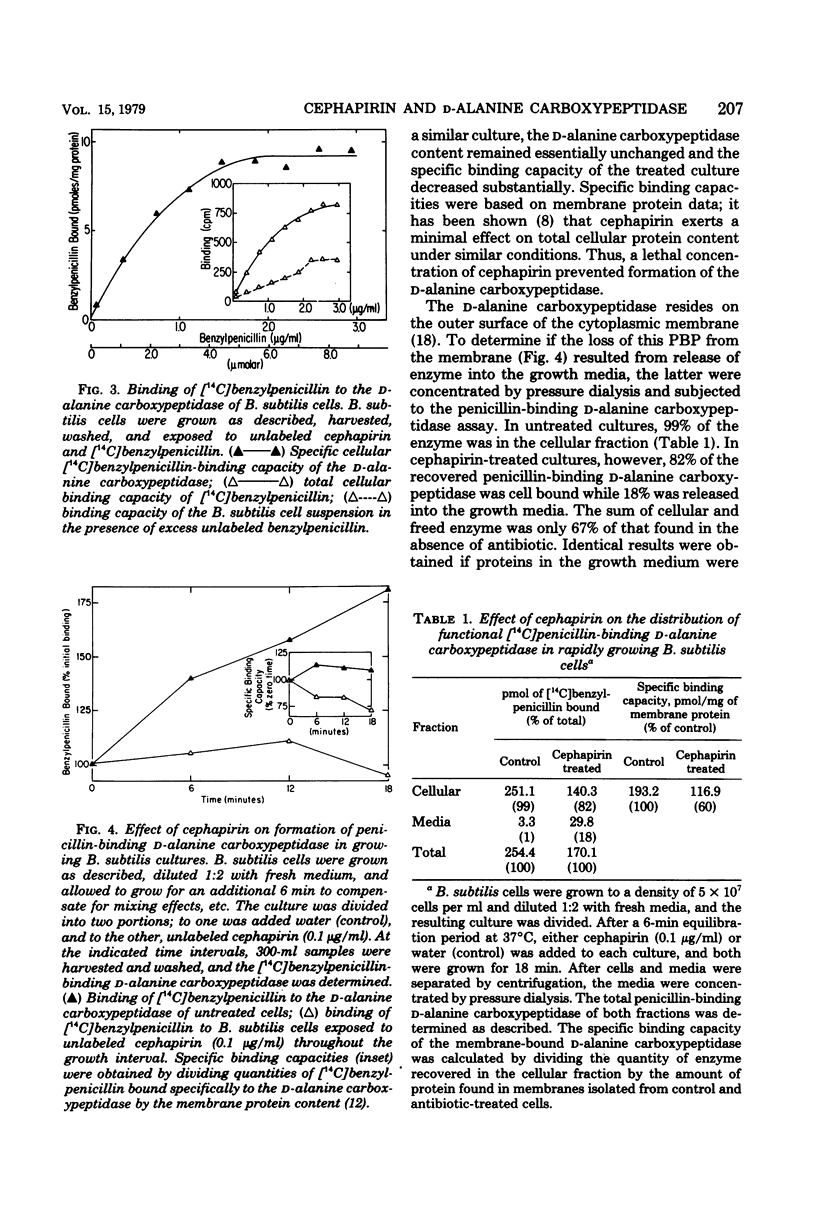
Cephapirin was utilized to examine the interaction of β-lactam antibiotics with growing Bacillus subtilis cells and the biological effects simultaneously produced. Saturation binding and quantitative cell death were observed at the cephapirin concentration of 0.1 μg/ml. Cephapirin bound to all penicillin-binding proteins except the d-alanine carboxypeptidase. A specific [14C]benzylpenicillin-binding assay was developed for the d-alanine carboxypeptidase. At the lowest saturating concentration of antibiotic (0.1 μg/ml), cephapirin inhibited formation of the d-alanine carboxypeptidase. Upon incubation with cephapirin, 18% of the membranous d-alanine carboxypeptidase was released into the media. The data suggest that β-lactam antibiotics may affect the formation of bacterial cytoplasmic membranes in addition to their effect on cell wall synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M. Penicillin binding components of bacterial cells and their relationship to the mechanism of penicillin action. Ann N Y Acad Sci. 1974 May 10;235(0):310–325. doi: 10.1111/j.1749-6632.1974.tb43274.x. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER P. D. Site of action of radiopenicillin. Bacteriol Rev. 1956 Mar;20(1):28–48. doi: 10.1128/br.20.1.28-48.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hamilton T. E., Lawrence P. J. The formation of functional penicillin-binding proteins. J Biol Chem. 1975 Aug 25;250(16):6578–6585. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence P. J. Penicillin: reversible inhibition of forespore septum development in Bacillus megaterium cells. Antimicrob Agents Chemother. 1974 Dec;6(6):815–820. doi: 10.1128/aac.6.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. The inhibition of mucopeptide synthesis by benzylpenicillin in relation to irreversible fixation of the antibiotic by staphylococci. Biochem J. 1967 Apr;103(1):90–102. doi: 10.1042/bj1030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Membrane synthesis in synchronous cultures of Bacillus subtilis 168. J Bacteriol. 1973 Oct;116(1):397–409. doi: 10.1128/jb.116.1.397-409.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Blumberg P. M., Strominger J. L. Inhibition of the Bacillus subtilis membrane-bound D-alanine carboxypeptidase by 6-aminopenicillanic acid covalently coupled to sepharose. J Bacteriol. 1974 Feb;117(2):783–785. doi: 10.1128/jb.117.2.783-785.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



