Abstract
Ultra-low doses and dose- rates of ionizing radiation are effective in preventing disease which suggests that they also may be effective in treating disease. Limited experimental and anecdotal evidence indicates that low radiation doses from radon in mines and spas, thorium-bearing monazite sands and enhanced radioactive uranium ore obtained from a natural geological reactor may be useful in treating many inflammatory conditions and proliferative disorders, including cancer. Optimal therapeutic applications were identified via a literature survey as dose-rates ranging from 7 to 11μGy/hr or 28 to 44 times world average background rates. Rocks from an abandoned uranium mine in Utah were considered for therapeutic application and were examined by γ-ray and laser-induced breakdown fluorescence spectroscopy. The rocks showed the presence of transuranics and fission products with a γ-ray energy profile similar to aged spent uranium nuclear fuel (93% dose due to β particles and 7% due to γ rays). Mud packs of pulverized uranium ore rock dust in sealed plastic bags delivering bag surface β,γ dose-rates of 10–450 μGy/h were used with apparent success to treat several inflammatory and proliferative conditions in humans.
Keywords: Hormesis, Therapy, Radioactive, Rocks
INTRODUCTION
Radiation standards in 1934 were 1 mSv/day for the NCRP [National Council of Radiation Protection & Measurements] and 2 mSv/day for the ICRP [International Commission on Radiological Protection]. Neither dose limit was associated with a measurable increased risk of cancer or any other disease (Taylor 1980). Low doses of γ-and x-rays and low dose-rates from β- and γ-rays exhibit significant hormetic effects based on many observations in epidemiological and experimental studies (Luckey 2008a; Sanders 2010). Protective adaptive response mechanisms are activated by low linear-energy-transfer (LET) radiation doses < 100 mSv (or combined low- and high-LET doses in the indicated range) that can result in reduction in the level of inflammatory and proliferative diseases (Dauer et al. 2010). Low doses of ionizing radiation may be useful in preventing cancers in high-risk populations, such as in heavy cigarette smokers, as well as in curing early stage cancers (Scott and Di Palma 2006; Sanders 2008).
Natural radiation sources are available that may have therapeutic application. A natural nuclear reactor was revealed in Oklo in Gabon, Africa in an area containing a 70% uranium oxide ore seam up to meter(s) thick. Both fission products and transuranic radionuclides were found at this site. Overall, the isotopic composition of the Gabon uranium ore resembled that of aged spent nuclear fuel (Cowan 1976; Meshik 2004). A natural nuclear reactor(s) appears also to have been operational in a high uranium sandstone formation of the Colorado Plateau.
A variety of developing, but rudimentary, low-dose radiation therapy strategies have been evaluated with respect to treating chronic inflammatory and proliferative diseases using natural radiation sources (Yamaoka et al. 2004;Falkenbach et al. 2005; Takatori et al. 2010; Lewis 2011). These include radon therapy in abandoned mines and spas, therapy using thorium-bearing monazite sand, ultra-low doses of x-ray and γ-ray exposures and β/γ exposures from uranium-bearing rocks resembling aged spent nuclear fuel.
METHODS AND RESULTS
Radioactive sandstone rocks for use in low-dose radiation therapy were obtained from an abandoned uranium mine near Monticello in San Juan County, UT. Ore from this mine contained the highest concentration of uranium (up to 87% U3O8). The ore was contained within a matrix of calcareous sandstones (filling interstices in the sandstone) and conglomerates colored dark gray to black. Small flat rocks from the mine were examined separately or were pulverized into a fine dust placed in heavy plastic bags as ‘mud packs’ of sizes that ranged from about 10 to 30 cm square. The packs helped to minimized dose in-homogeneity.
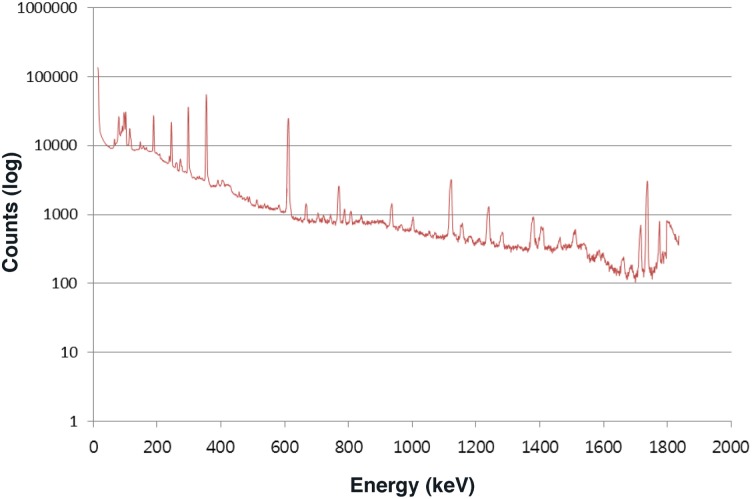
Beta/gamma dose-rates for the radiation sources were measured in Pritchett, Colorado with an ‘Inspector Alert’ nuclear radiation monitor manufactured by International Medcom (United States) and differential γ-ray dose-rates were measured in Korea with a γ-detector which had a photon energy range of 30 keV to 1.2 meV. The natural background γ dose-rate at 91 cm above the ground was 0.28 μGy/h in Daejeon, Korea and 0.55 μGy/h in Pritchett, Colorado. Gamma-ray spectroscopy was performed on the surface area of a small flat rock (8 × 5 × 0.6 cm) that had a surface γ-dose-rate of 11μGy/h. The spectrum was quite different from that seen with typical uranium ore samples (Figure 1). Presumptive radionuclides detected in the Utah rock included 214Bi, 214Pb, 125Xe, 226Ra, 133Ba, 196Au, 111mCd, 114In, 237Pu and 242Am. This made the rocks considerably more radioactive than typical uranium-bearing mine samples. Laser-induced breakdown fluorescence spectroscopy was used for elemental analysis on a small flat rock (7 × 5 × 1 cm) that had a dose rate of 9.8 μGy/h. The entire rock had a high vanadium content. One circular area had a high uranium content which was associated with a small amount of americium (spectral lines 356.916 nm, 367.312 nm, and 466.279 nm).
FIGURE 1.
Surface γ-ray spectroscopy of a small flat rock from a uranium mine in Utah; the surface γ-dose-rate was 11μGy/h.
Beta/gamma dose-rates for the surface of rocks and mud packs ranged from ∼10 to 500 μGy/hr. The γ-ray surface dose-rates ranged from ∼1 to 70μGy/h. The γ-ray dose rate in air was determined at intervals up to 28 cm from the rock surface for six rocks which had surface dose-rates of 9.8 to 43 μGy/hr. The mean half-value distance for γ-rays in air was about 1.5 cm with 10% of surface dose-rate found at about 8.5 cm and 2.5% of the surface dose found at 20-28 cm from the rock surface. The dose-rate distribution with increasing distance from the rock surface is not what one would expect from a single γ-ray photon because it represents a composite of hundreds of different γ-ray energies.
The differential air dose contribution by radiation types for rocks and mud packs were 93% β and 7% γ at their surface. No significant differences were noted in β/γ differential air dose contributions among the rocks or packs. The maximum range of β-particles, with energies > 0.8 MeV, in soft tissue is about 1/2 their energy in MeV given as range in cm. Thus, a 2.3 MeV β-particle has a range of about 1.1 cm and a 1.1 MeV β-particle has a range of about 0.5 cm in soft tissue. The vast majority of β-energy from the rock samples would be absorbed by the first cm of skin.
All experimental dose-rates from the rocks or mud packs were taken at their surface or at intervals of distance in air from their surface. Dose-rates were either combined β + γ radiation or γ radiation alone. Dose was determined as a simple measure of dose-rate in μGy/h x time in hours.
Two cases closely observed by the author were successfully treated (with their consent) by uranium ore mud packs:
Case 1: A dozen 3-7 mm warts on a 5 × 8 cm skin patch near the knee, had been repeatedly treated about every six months, with cryosurgery for the last 10 years. The warts were treated with a 12 cm square mud pack for a few hours a day for six continuous weeks; the dose-rate was 70 μGy/hr and the total dose was 15 mGy. The warts and cryosurgical scars disappeared by 6 weeks and have not returned after 24 months. The skin was healthy and normal following treatment. Treatment was not been repeated.
Case 2: Two raised lesions of seborrheic keratoses on the left shoulder about 5 cm apart were treated with a 1cm thick 7 × 15 cm flat radioactive rock for a few hours a day for four consecutive weeks; the dose-rate was 250 μGy/h and the total dose was 19 mGy. The lesions disappeared and have not returned after 44 months. The skin was healthy and normal following treatment. Treatment was not repeated.
A more recent case is presented of a 50 year-old woman who was diagnosed with adenocarcinoma of the left breast by needle biopsy on September 2011. A PET scan showed no metastatic disease and no lymph node involvement. She declined chemotherapy, radiation therapy or surgery. In the spring of 2012, the patient developed a cough and breathing difficulty that was evaluated by x-ray, showing multiple nodules in both lungs consistent with metastatic disease. The primary tumor size at this time was a firm mobile mass of 10 cm in diameter. She went to Pritchett, Colorado in early April 2012 where she received near continuous exposures to the front and back of the chest from mud packs delivering an average of about 100 μGy/hr at the pack surface. Total dose to the chest after 7 weeks of continuous therapy was ∼150 mGy. The size of the primary tumor decreased about 40% in diameter at this time with improvement in breathing and cough. Observations a few weeks later by a different physician indicated that the primary tumor was ‘dead’ being comprised of necrotic tissue. The patient subsequently received high dose radiotherapy to the head for brain metastases and is currently receiving low dose iv chemotherapy along with near continuous treatment with mud packs.
Case studies have also been provided by Jay Gutierrez (personal communication, 2012) from individuals seen at his radon clinic in Pritchett, Colorado. A medical doctor (MD) is also associated with the clinic. Among medical conditions claimed by Gutierrez to have positive responses to low-dose radiation therapy using radioactive rocks/packs are cancer, ragweed allergy, Dupuytren’s contracture, Meniere’s disease, wet retinopathy, rheumatoid arthritis and circulatory failure associated with diabetes. Total doses used were estimated to be in the range 10–500 mGy, chronically given over weeks to months. Sufficient medical records and dose details are not available to adequately document these cases.
DISCUSSION
Cellular Mechanisms of Radiation Hormesis
Early 20th century radioprotection limits were based on the maximum permissible dose that implied the existence of a threshold. The LNT assumption was introduced later as a substitute and applies to stochastic effects such as cancer and genetic changes. The LNT assumption suggests that carcinogenic and other stochastic health effects, do not exhibit a threshold, and are cumulative over a lifetime. A single-hit (to cell nucleus) model of radiation-induced mutations (which can lead to cancer) provided the rationale for the LNT assumption, based initially on observations of mutations in fruit fly germ cells (Muller 1927). Muller’s work involved very high X-ray doses so that no conclusive results could be obtained related to low radiation doses and he ignored data that showed a threshold at what was then considered low doses (Calabrese 2011), but were quite high compared to today’s definition of low dose ( < 100 mGy). Koana et al. (2004) found a threshold at about 1000 mGy for somatic mutations in fruit flies. In a later study, the frequency of sex-linked recessive lethal mutations in fruit fly germ cells was significantly lowered from the un-irradiated control group by a γ-ray dose of only 500 μGy (Ogura 2009). This observation can be considered to be a protective bystander effect, since at the indicated dose fewer than one electron track (from ionizing events) per cell would be expected. The protection relates to an adaptive response occurring as a result of the mild radiation stress imposed.
DNA damage and other stresses can trigger a highly conserved, anti-cancer, anti-aging survival response (adaptation) that suppresses metabolism and growth and boosts defenses that maintain the integrity of the cell (Hoeijmakers 2009). Protective cellular responses to ionizing radiation include DNA repair, intracellular metabolic redox reactions, cell cycle checkpoint controls, intra- and inter-cellular signaling cascades, apoptosis and mitotic linked cell death. These mechanisms are activated by low radiation doses that result in non-linear responses, producing an adaptive response that reduces the spontaneous or background level of cell transformations, cancer, heart disease or other diseases (Dauer et al. 2010).
Thresholds and Hormesis in Radiation Carcinogenesis
Natural background radiation varies by geographic location up to nearly three orders of magnitude (0.5 to 300mSv/y). No increase in mortality from diseases has been observed for people living in high background dose regions (Mortazavi and Karam 2005). Data on deaths from all causes and cancers among workers in the nuclear industry were evaluated by the International Agency for Research on Cancer (Vrijheid et al. 2007). The low SMRs for all-cancer mortality (0.74) and all-cause mortality (0.62) are examples of radiation hormesis rather than a HWE (Healthy Worker Effect) (Kojiro 1999; Fornalski and Dobrzynski 2009, 2010). These data are consistent with the earlier findings (Luckey 2007; 2008b; Rockwell and Muckerheide, 2008) that workers chronically exposed to low dose radiation exhibit significantly lower SMRs for all mortality and cancer mortality than in unexposed control groups. A study of 250,000 nuclear workers found an average mortality of 67 ± 13% compared to the control group (Luckey 2007, 2008a). A decreased cancer mortality was also observed in radiotherapy patients in organs outside high dose therapy regions of the body (Luckey 2008b). An environmental survey of the US indicates that overall cancer mortality would be negligible at an annual whole-body, presumably low LET radiation dose of 7 mGy (Frigerio et al. 1973).
Cancer was significantly reduced (SMR ∼0.80) in 240,000 Chernobyl emergency workers who received a dose of 100 mSv (Jaworowski 2010). Several 60Co orphan sources were inadvertently recycled into 20,000 tons of structural steel which was used to construct about 200 residential, industrial and school buildings in 1982 housing 10,000 residents of Taiwan. The average cumulative dose for the exposed residential population was about 50 mSv (Chang et al. 1997); the average dose-rate was estimated at11 μSv/h. Only seven fatal cancers were observed out of an expected 232 (SMR = 0.03) (Chen et al. 2004). A latter paper showed an observed cancer incidence of 95 out of an expected 115 (SIR = 0.8) which was significantly less than expected (Hwang et al. 2006); this paper used 10 year lagging (throwing away radiation dose) for solid cancers, resulting in a misrepresentation of the true dose and cancer risk and also did not provide any SMR values. SIR values that are considerably higher than SMR values for cancer may represent, in part, a therapeutic effect of low dose radiation on cancer progression.
Young adult beagle dogs have been exposed to a variety of α, β, γ radionuclides by ingestion and inhalation, thresholds from 0.5 to 20 Gy were found for leukemia, bone tumor and lung tumor formation in lifespan studies carried out during the last fifty years (Raabe 2010). The threshold dose for bone cancer in radium dial painters was 10 Gy; a large majority of painters received less dose and ended up living longer than the unexposed control population (Sanders 2010). The threshold for lung tumors in rats, dogs and Mayak workers exposed to alpha radiation plutonium-239 aerosols ranged from 0.4 to 0.8 Gy (Sanders and Lundgren 1995; Sanders 2008; Tokarskaya et al. 1997) when combined with chronic gamma irradiation. Low LET gamma rays appear to activate natural protection against high-LET alpha-radiation-induced lung cancer from plutonium-239 and also radon-progeny. Greater than 80% of plutonium-239 alpha-radiation-induced rat lung cancers were prevented by chronic, low dose-rate gamma-ray exposure. Interestingly, lifetime exposure to residential radon at the Environmental Protection Agency’s action level of 4 pCi L−1 appears to be associated with on average a > 60% reduction in lung cancer cases from associated low LET radiations (Cohen 1995, 1997). The threshold for lung cancer in humans exposed to low LET radiation ranges from 1–2 Gy (Sanders and Scott 2008).
Optimal Beneficial Doses and Dose-Rates
Very small doses and dose-rates of radiation often exhibit significant health hormetic effects based on observations in epidemiological and experimental studies (Sanders 2010). Low doses and dose rates of ionizing radiation have been defined by UNSCEAR and BEIR VII as those below 100–200 mGy and below 50–100 mGy/min, respectively. Uniform, whole-body, continuous, low-LET radiation exposure was estimated to cause no excess risk of radiation-induced cancer at dose-rates < 150 mSv/y in humans (Keirim-Markus 2002). For the system studied, the adaptive response operates within these dose and dose-rate limits.
An inverse dose-rate effect has been observed with low LET radiations for radioadaptive cellular and therapy mechanisms (Gridley et al. 2005; Leonard 2007). A low dose/dose-rate microdosimetry model for radiation hormesis has been proposed (Feinendegen 2003) based on observations in mammalian cells. Cellular lesions are eliminated by the disappearance of genomically damaged cells at doses < 10 mGy while repair systems are activated at > 10 mGy. An adaptive response is seen in mammalian cells between a dose range of < 1 mGy and 100 mGy for a single low-LET exposure (Mitchel 2010). The consequences of oxyradicalcaused cell damage is reduced once a cell has sensed the radiation by an electron or photon traversal.
The adaptive response causes genomic instability related outcomes, such as cell transformation and chromosomal aberration formation, to decrease below the normal background or spontaneous levels following exposure to ultra-low doses and dose-rates. Cellular hormesis responses from natural and anthropogenic sources of radiation are similar (Pollycove and Feinendegen 2003). However, dose-rates from anthropogenic radiation sources are typically much higher than from natural sources (Ulsh 2010).
Bio-positive effects were estimated to be between 1 mSv and 1000 mSv/y (Luckey, 2008a; Gregoire and Cleland, 2006). Radiation protocols showing evidence of radiation hormesis for γ-dose-rates are found in the 1-50 μSv/hr dose-rate range. Optimal bio-positive effects were estimated to be at a dose-rate of 100 mGy/yr or 11 μGy/hr given as a continuous exposure (Cuttler and Pollycove 2009). Luckey (2008a) estimated the optimal radiation level as 60 mGy/yr or 6.9 μGy /hr. Luckey (2008b) also estimated that 50 mSv/y would reduce cancer mortality to near zero and that the elimination of cancer deaths would increase lifespan by about 10 years.
Are there optimal photon energies that stimulate hormetic reactions? A few studies have examined the role of photon energy. Experimental evidence is limited. Lower energy x-rays were more efficient in inducing genomic instability than γ-rays while higher energy γ-rays and 60 kvp x-rays were more efficient in activating the Protective Apoptosis Mediated (PAM) response than 28 kvp x-rays (Scott 2005). If you could chronically deliver the right gamma ray energy spectrum to critical cellular sites at the same time, then you might expect to see more significant positive biomedical effects, both in prevention and in therapy for inflammatory and proliferative diseases, at ultra-low dose- rates.
Tumor Cell Response at Low Doses
Low dose radiation-induced enhancement of apoptosis and self-destruction of transformed or pre-cancerous cells represents a potential control system during carcinogenesis (Bauer, 2007). Tumor cell apoptosis is stimulated by < 10 mGy of low-LET radiation (Cotter 2009). Very low priming doses increase latency for cancer induction in experimental animals (Wolff 1996; Tapio and Jacob 2007). Very low dose-rates cause cell transformation at 2 mGy/day (Elmore et al. 2009), simulate apoptosis in mice with knock-out gene at 1.2 mGy/hr (Ina and Sakai 2005), suppress thymic lymphoma at 1.2 mGy/hr (Ina et al. 2005), increase lifespan at 25–50 × background dose (Caratero et al. 1998), and suppress methylcholanthrene-induced skin tumors at 1.2 mGy/hr (Sakai et al. 2003).
Low dose radiotherapy modulates the immune-inflammatory response (Rodel et al. 2007). A dose of 200 mGy enhanced phagocytosis by macrophages and increased CD8+ T cell production in mice (Pandey et al. 2005). LDR (Low Dose Radiotherapy) up-regulated genes that encode cytokines at doses as low as 100 mGy (Barcellos-Hoff 1998). Continuous gamma ray exposure (100 mGy per year) prolonged lifespan, suppressed B-cell lymphoma formation and increased CD49+ cell production in mice (Lacoste-Collin et al. 2007). A single dose of 100–200 mGy x-rays to mice reduced lung metastases from implanted syngeneic L1 sarcoma cells, while also increasing NK cell numbers (Cheda et al. 2004).
Single daily doses of 330 μGy at 700 μGy/hr in mice to a total dose of 146 mGy delivered over a period of 90 weeks inhibited tumorigenesis (Mitchel et al. 2008). A dose of 10 mGy increased cancer latency and decreased cancer incidence in cancer prone mice (Mitchel et al. 2003). Low doses of TBI (Total Body Irradiation) significantly delayed SaI tumor cell growth in mice (Anderson et al. 1982). Lower doses delivered over an extended period of time may preferentially sensitize tumor cells, while inducing radio-resistance in normal cells due to the radio-adaptive response. Continuous administration of ultra-low level radiation with either 125I seeds or whole body exposure to 137Cs γ-rays significantly increased the efficacy of HDR (High Dose Radiotherapy) for implanted human malignant glioma cells in athymic mice (Williams et al. 1998). Low dose TBI reduced the incidence of spontaneous lymphoma in mice (Ishii et al. 1996).
A threshold dose-rate effect was seen for skin tumor by localized β-irradiation (Ootsuyama and Tanooka 1991). Exposure of mouse skin to 500 mGy β-irradiation at 24 hr before treatment with methyl-nitro-nitroso guanidine, reduced papilloma formation by five-fold but had no significant effect on carcinoma formation (Mitchel et al. 1999). Administration of tritium to mice in drinking water protected against thymic lymphoma formation up to a dose-rate of 900 mGy/day (Yamamoto et al. 1998). A dose of 100 mGy of 6 MeV x-rays given 24 hours before start of radiotherapy with 48 Gy in 16 × 3 Gy fractions to dogs with oral cancer caused a cytoprotective effect to surrounding normal tissues (Blankenbecler 2010).
Geology and Radioactivity of Uranium-Bearing Sandstone
Uranium mine tailings are not considered to be significantly radioactive. However, there are exceptions (McLeary 2004; Chareyron 2008; Sengiyumva 2010). Uraniferous mineralization consists primarily of the oxides, uraninite and pitchblende (Augustithis, 1995). The 238U/235U ratio has generally been considered invariant in nature with a value of 137.8. However, two modal values of the isotopic ratio exist with a significant relative difference of 0.03% in uranium ores with the lower mode being found in some mines of the Colorado Plateau. This could be attributed to separation from 238U and depletion of 235U by in situ geological nuclear reactions (Cowan and Adler 1976). Uranium deposits form when groundwater with leached uranium is reduced to precipitate uraninite. This is a possible mechanism by which 235U can be fractionated from 238U in ground waters at low temperature in the redox state transition of uranium (U6+ ↔ U4+) (Stirling et al. 2007). Sufficient separation and concentration of 235U to ∼3% level required to sustain a nuclear reaction appeared to have occurred in the uranium-bearing rocks examined in this study. Water also serves as a neutron moderator.
The contribution from actinides and their daughter products to beta decay in CANDU (CANadianDeuteriumUranium) reactor spent fuel becomes significant after 200 years and is dominant at times greater than 300 years, at which time the radiation dose is predominantly from beta decay (Garisto et al. 2009). Very low level levels of transuranics were also found in pitchblende and uraninite ores from Canada and Belgium Congo (Levine and Seaborg 1951; Ridenour 1961). The rocks from Utah also exhibit predominantly beta decay. The presence of ‘excess’ radioactivity, transuranics and fission products and β, γ dose-distribution in the Utah rocks indicates a probably origin from an in situ nuclear reaction hundreds to thousands of years ago.
Low Dose Radiotherapy for Inflammatory and Proliferative Diseases
A pooled analysis of twenty-eight radon epidemiological studies indicates that radon does not cause lung cancer up to a lung dose of 150 mSv (Fornalski and Dobrzynski 2011). The lung cancer rate in the lowest radon states was nearly four times greater than predicted by the LNT while the lung cancer rate in the highest radon states was one-seventh of the LNT prediction (Rockwell and Muckerheide 2008). The low-LET component from radon progeny was probably responsible for the strong hormetic effect described for lung cancer by Cohen (1995) in an ecological radon study and by Thompson et al (2008) in a case-control residential radon study.
Radon therapy is widely available in Europe to treat a variety of chronic inflammatory and painful diseases. These include rheumatoid arthritis, lupus, scleroderma, ankylosing spondylitis, asthma, bronchitis and psoriasis (Erickson 2007). Falkenbach et al (2005) described five trials of radon therapy for rheumatoid arthritis, three of which were double-blind that showed beneficial effects. Radon therapy was also effective in treating osteoarthritis (Yamaoka et al. 2004), bronchial asthma (Mitsunobu et al. 2003), and dyslipidemia associated with cardiovascular disease (Iashina et al. 2011). The standard uranium mine therapy for the Free Enterprise Radon Health Mine in Boulder, Montana recommends a stay of 40 hours in the mine spread over 10 consecutive days. The average mine radon concentration is 1200 pCi/l. This gives a cumulative lung dose of ∼6 mSv (150 μSv/h). The dose schedule in the Free Enterprise mine has been found effective for treating a variety of inflammatory diseases in several thousand people during the last several decades. The bio-positive effects of radon therapy typically last from 6 to 12 months (Lewis 2011). Similar radiation doses are given in European radon therapy protocols. In comparison, the radioactive rocks from Utah gave β/γ-dose-rates that ranged from 21 to 450 μGy/h and γ-dose-rates that ranged from ∼1 to 30μGy/h at the source surface.
India has about 30% of the world’s thorium reserves including monazite-bearing beach sands. Monazite contains 2–7% thorium by weight with nearly all thorium comprised of 232Th. Thoriated gas mantles are widely used in India for lighting both outdoors and indoors resulting in annual effective doses of 2 and 8 mSv, respectively (Ramachandran 2010). People in parts of Iran use the ash from burned thorium-containing mantles for healing of skin wounds. Radioactive lantern mantle ash enhanced the healing of excision wounds in the skin of rats (Mortazavi et al. 2009). The Kerala and Orissa monazite-bearing beach sands of India give absorbed γ-dose-rates in air that range to over ten times background rate in most other areas of India (Mahur et al. 2009; Rao et al. 2009).
A group of four patients with advanced cancer and two patients with severe rheumatoid arthritis and dermatomyositis were exposed to radon and γ-rays from monazite sand for one hour, three times weekly, for a period of 3 to 36 consecutive months. Radon (200 pCi/l) delivered a dose to the lung of 25 μSv/h while monazite delivered 40 μSv/hr from γ-rays. The weekly dose was ∼200 μSv and the monthly dose was ∼1 mSv. All patients had failed orthodox therapy. In each case bio-positive changes were noted, including a decrease in tumor marker antigens, improved tumor control, and improved appetite, muscle strength and exercise ability (Takatori et al. 2010). Advanced cancer patients are currently being treated using thin silicon plates (50 X 50 cm) containing concentrated monazite which give about 2000μSv/h from beta and gamma radiations (Takatori, personal communication).
Prior to and into World War II, Roentgen radiation was used to treated a variety of infections (Kelley 1942). Single and fractionated doses of x-rays of less than 1 Gy were successfully used to cure diphtheria, tuberculosis and gas gangrene, and limit inflammation of arthritis, rheumatism and bronchitis (Cuttler 2004, 2008; Calabrese and Dhawan, 2012) and ulcerative dermatitis (Mitchel et al. 2007). Radiotherapy with fractions of mostly 0.3–1.0 Gy and a total dose of 3–12 Gy exerted anti-inflammatory and analgesic effects for painful degenerative disorders. Relatively low dose radiotherapy for joint inflammation was an effective and less toxic alternative to steroids and low dose chemotherapy drugs in treating arthritis (von Pannewitz 1933; Seegenschmiedt et al. 2000; Micke and Seegenschmiedt 2002; Niewald et al. 2008). Low doses of radiation were effective in treating ulcerative colitis if given chronically over a longer period of time (Mitchel et al. 2007). Low dose TBI may control or even cure AIDS (Shen et al. 1989, 1997)
Low dose radiation alone or in combination with other agents [e.g., agents that shut down cancer cell survival signaling pathways] has potential use in cancer therapy. Patients with multiple myeloma, lymphoma and nasal carcinoma underwent tumor regression after receiving LDI radiotherapy to a total dose of 1.5 Gy (Cuttler and Polycove 2009). Implanted 125I seeds giving 40–70 mGy/hr, significantly improved survival in glioblastoma patients also given external beam radiotherapy (Scharfen et al. 1992). Fractionated whole-body doses (TBI) of 100 mSv or half-body doses (HBI) of 150 mSv delivered three times or two times a week, respectively, for a total dose of 1.5 Sv, significantly improved survival of patients with non-Hodgkin’s lymphoma (Choi et al. 1979; Sakamoto 2004). Low dose TBI decreased lung metastases (Hosoi and Sakomato 1993). Radiation doses delivered in this study are one to two orders of magnitude lower than those used to treat cancer patients with TBI or HBI, given at dose-rates that are lower by four orders of magnitude. Low LET radiation inhibits the development of spontaneous and artificial metastases in humans and laboratory animals. This suggests that γ-irradiation may be used to treat and cure cancer and prevent cancer metastases (Nowosielska et al. 2010).
Evidence obtained from the review of low dose radiobiological studies in this paper and limited anecdotal observations in individuals given low dose exposure to enhanced radioactive uranium ore, radon and monazite sands should provide motivation to pursue controlled experimental studies in animals and humans to evaluate their possible therapeutic utilization in treating a wide variety of conditions associated with inflammation and cell proliferation. Low-dose radiation was successful in treating warts, a model of virus-induced benign tumor, and seborrheic keratosis, a model of squamous metaplasia. A small dose of radiation may be administered in a continuous fashion or at regular intervals for a long time. However, radiation protocols currently lack any standard dose quantization, dose fractionation and duration of a treatment course. Mathematical models, computer simulations and clinical trials are warranted to exploit the potential of low dose radiation therapy to control and cure chronic and complicated diseases. It appears that low-dose radiation therapy may have far reaching effects in controlling and curing many diseases throughout the world. The application of ‘natural’ low dose sources of ionizing radiation may be a particularly low cost, common sense method for treating inflammatory and proliferative diseases without apparent side-effects.
REFERENCES
- Anderson RE, Tokuda S, Williams WL, Warner NL. Radiation-induced augmentation of the response of A/J mice to SaI tumor cells. Amer J Path. 1982;108:24–37. [PMC free article] [PubMed] [Google Scholar]
- Augustithis SS. Atlas of the textural patterns of ore minerals and metallogenic processes. De Gruyter; Berlin: 1995. pp. 233–236. [Google Scholar]
- Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res. 1998;150(Suppl.):S109–S120. [PubMed] [Google Scholar]
- Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int J Radiat Biol. 2007;83:873–888. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- BEIR . Health risks from exposure to low levels of ionizing radiation: BEIR VII, Phase 2 Committee to assess health risks from exposure to low levels of ionizing radiation. National Academy of Sciences, National Academies Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- Blankenbecler R. Low-dose pretreatment for radiation therapy. Dose-Response. 2010;8:534–542. doi: 10.2203/dose-response.10-033.Blankenbecler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E. Muller’s Nobel lecture on dose-response for ionizing radiation: Ideology orscience? Arch Toxicol. 2011;85:1495–1498. doi: 10.1007/s00204-011-0728-8. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Dhawan G. The role of x-rays in the treatment of gas gangrene: Ahistorical assessment. Dose-Response. 2012;10(4):626–643. doi: 10.2203/dose-response.12-016.Calabrese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caratero A, Courtade M, Bonnet L, Planel H, Caratero C. Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology. 1998;44:272–276. doi: 10.1159/000022024. [DOI] [PubMed] [Google Scholar]
- Chang WP, Chan CC, Wang JD. 60-Co contamination in recycled steel resulting in elevated civilian radiation doses: causes and challenges. Health Phys. 1997;73:465–472. doi: 10.1097/00004032-199709000-00004. [DOI] [PubMed] [Google Scholar]
- Chareyron B. Radiological hazards from uranium mining. In: Broder BJ, Hasche-Berger A, editors. Uranium, Mining andHydrogeology. Springer; 2008. pp. 451–458. [Google Scholar]
- Charfen CO, Sneed PK, Wara WN, Larson D, Philips T, Prados M, Weaver K, Malec M, Acord P, Lamborn K, Lamb S, Ham B, Gutin P. High activity iodine-125 interstitial implant for gliomas. Int J Radiat Oncol Biol Phys. 1992;24:583–591. doi: 10.1016/0360-3016(92)90702-j. [DOI] [PubMed] [Google Scholar]
- Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat. Environ Biophys. 2008;47:275–283. doi: 10.1007/s00411-007-0147-7. [DOI] [PubMed] [Google Scholar]
- Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska E, Marciniak M, Janiak M. Single low doses of x-rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat Res. 2004;161:335–341. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- Chen WL, Luan YC, Shieh MC, Chen ST, Kung HT, Soong KL, Yeh YC, Chou TS, Mong SH, Wu JT, Sun CP, Deng WP, Wu MF, Shen ML. Is chronic radiation an effective prophylaxis against cancer? J Amer Physicians Surgeons. 2004;9:6–10. [Google Scholar]
- Choi NC, Timothy AR, Kaufman SD, Carey RW, Aisenberg AC. Low dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin’s lymphoma. Cancer. 1979;43:1636–1642. doi: 10.1002/1097-0142(197905)43:5<1636::aid-cncr2820430512>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Lung cancer rate vs. mean radon level in U.S. counties of various characteristics. Health Phys. 1997;72:114–119. doi: 10.1097/00004032-199701000-00016. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Test of the linear-no-threshold theory of radiation carcingenesis for inhaled radon decay products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cotter TG. Apoptosis and cancer: the genesis of a research field. Nature Reviews/Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- Cowan GA, Adler HH. The variability of the natural abundance of 235U. Geochimicaet Cosmochimica Acta. 1976;40:1487–1490. [Google Scholar]
- Cowan GA. A natural fission reactor. Sci Amer. 1976:235–236. [Google Scholar]
- Cuttler JM, Pollycove M. Nuclear Energy and health. Dose-Response. 2009;7:52–89. doi: 10.2203/dose-response.08-024.Cuttler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler JM. Low-dose irradiation therapy to cure gas gangrene infections. Int J Low Radiat. 2004;1:318–328. [Google Scholar]
- Cuttler JM. Book review: Roentgen treatment of infections by JF Kelly and DA Dowell. Can Nucl Soc Bull. 2008;29:43–44. [Google Scholar]
- Dauer LT, Brooks AL, Hoel DG, Morgan WF, Stram D, Tran P. Review and evaluation of updated research on the health effects associated with low-dose ionizing radiation. Radiat Prot Dosim. 2010;140:103–136. doi: 10.1093/rpd/ncq141. [DOI] [PubMed] [Google Scholar]
- Elmore E, Lao X-Y, Kapadia R, Redpath JL. Threshold-type dose response for induction of neoplastic transformation by 1 GeV/nucleon iron atoms. Radiat Res. 2009;171:674–770. doi: 10.1667/RR1673.1. [DOI] [PubMed] [Google Scholar]
- Erickson BE. The therapeutic use of radon: a biomedical treatment in Europe; an alternative remedy in the United States. Dose-Response. 2007;5:48–62. doi: 10.2203/dose-response.06-007.Erickson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenbach A, Kovacs J, Franke A, Jorgens K, Ammer K. Radon therapy for the treatment of rheumatic diseases—review and meta-analysis of controlled clinical trials. RheumatolInt. 2005;25:205–210. doi: 10.1007/s00296-003-0419-8. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Relative implications of protective responses versus damage induction at low dose and low-dose-rate exposure using microdose approach. Radiat Prot Dosim. 2003;104:337–346. doi: 10.1093/oxfordjournals.rpd.a006197. [DOI] [PubMed] [Google Scholar]
- Fornalski KW, Dobrzynski L. Ionising radiation and the health of nuclear industry workers. Int J Low Radiat. 2009;6:57–78. [Google Scholar]
- Fornalski KW, Dobrzynski L. The healthy worker effect and nuclear industry workers. Dose-Response. 2010;8:125–147. doi: 10.2203/dose-response.09-019.Fornalski. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornalski KW, Dobrzynski L. Pooled Bayesion analysis of twenty-eight studies on radon induced lung cancer. Health Phys. 2011;101:265–273. doi: 10.1097/HP.0b013e31821115bf. [DOI] [PubMed] [Google Scholar]
- Frigerio NA, Eckerman RF, Stowe RS. Carcinogenic hazard from low-level, low-rate radiation. Part 1, Report ANL/ES-26. Argonne, IL: Argonne National Laboratory; 1973. [Google Scholar]
- Garisto F, Barber DH, Chen E, Inglot A, Morrison CA. Alpha, beta and gamma dose rates in water in contact with used CANDU fuel. Nuclear Waste Management Organization; Toronto, Canada: 2009. p. 41. NWMO TR-2009-27. [Google Scholar]
- Gregoire O, Cleland MR. Novel approach to analyzing the carcinogenic effect of ionizing radiation. Int J Radiat Biol. 2006;82:13–19. doi: 10.1080/09553000600567624. [DOI] [PubMed] [Google Scholar]
- Gridley DS, Williams JR, Slater JM. Low-dose/low-dose-rate radiation: a feasible strategy to improve cancer radiotherapy? Review article. Cancer Therapy. 2005;3:105–130. [Google Scholar]
- Gutierrez J. 2012. Personal Communication.[ www.nighthawkminerals.com], Pritchett, Colorado 81064. [Google Scholar]
- Hoeijmakers JH. DNA damage, aging and cancer. New Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Sakomato K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26:177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Hwang SL, Guo HR, Hsieh WA, Hwang JS, Lee SD, Tang JL, Chen CC, Chang TC, Wang JD, Chang TC. Cancer risks in a population with prolonged low dose-rate g-radiation exposure in radiocontaminated buildings, 1983–2002. Int J Radiat Biol. 2006;82:849–858. doi: 10.1080/09553000601085980. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Further study of prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole-life irradiation. Radiat Res. 2005;163:418–423. doi: 10.1667/rr3316. [DOI] [PubMed] [Google Scholar]
- Ina Y, Tanooka H, Yamada Tand Sakai K. Suppression of thymic lymphoma induction by lifelong, low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163:153–158. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hosoi Y, Yamada S, Ono T, Sakamoto K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X-irradiation. Radiat Res. 1996;146:582–585. [PubMed] [Google Scholar]
- Jaworowski Z. Observations on the Chernobyl disaster and LNT. Dose-Response. 2010;8:148–171. doi: 10.2203/dose-response.09-029.Jaworowski. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirim-Markus IB. Radiation exposure normalization taking account of specific effects at low doses and dose-rates. Atomic Energy. 2002;93:836–844. [Google Scholar]
- Kelley JF. Roentgen Treatment of Infection. The Year Book Publishers; Chicago: 1942. [Google Scholar]
- Koana T, Takasima Y, Okada O, Ikehata M, Miyakoshi J, Sakai K. A threshold exists in the dose-response relationship for somatic mutation frequency induced by X-irradiation of Drosophila. Radiat Res. 2004;161:391–396. doi: 10.1667/rr3152. [DOI] [PubMed] [Google Scholar]
- Kojiro K. The healthy worker effect in a long-term follow-up population. Japanese J Cancer Clinics. 1999;45:1307–1310. [Google Scholar]
- Lacoste-Collin L, Jozan S, Cances-Lauwers V, Pipy B, Gasset G, Caratero C, Courtade-Saidi M. Effect of continuous irradiation with a very low dose of gamma rays on lifespan and the immune system in SJL mice prone to B-cell lymphoma. Radiat Res. 2007;168:725–732. doi: 10.1667/RR1007.1. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Thresholds and transitions for activation of cellular radioprotective mechanisms-correlations between HRS/IRR and the ‘inverse’ dose-rate effect. Int J Radiat Biol. 2007;83:479–489. doi: 10.1080/09553000701370902. [DOI] [PubMed] [Google Scholar]
- Levine CA, Seaborg GT. The occurrence of plutonium in nature. J Amer Chem Soc. 1951;73:3278–3283. [Google Scholar]
- Lewis P. Personal communication. Free Enterprise Radon Health Mine; Boulder, MT: 2011. [Google Scholar]
- Luckey TD. Documented optimum and threshold for ionizing radiation. Int. J. Nuclear Law. 2007;1:378–409. [Google Scholar]
- Luckey TD. Radiation hormesis overview. RSO Magazine. 2008a;8:22–39. [Google Scholar]
- Luckey TD. The health effects of low-dose ionizing radiation. J Amer Physicians Surgeons. 2008b;13:39–42. [Google Scholar]
- Mahur AK, Kumar R, Sengupta D, Prasad R. Radon exhalation rate in Chatrapur beach sand samples of high background radiation area and estimation of its radiological implications. Indian J Phys. 2009;83:1011–1018. [Google Scholar]
- McLeary M. Radium Hill mine and low-level radioactive waste repository. Government of South Australia; 2004. Report Book 2004/9. [Google Scholar]
- Meshik AP. Record of cycling operation of the natural nuclear reactor in the Oklo area of Gabon. Phys Rev Lett. 2004;93:182–302. doi: 10.1103/PhysRevLett.93.182302. [DOI] [PubMed] [Google Scholar]
- Micke O, Seegenschmiedt MH. Consensus guidelines for radiation therapy of benign diseases: a multicenter approach in Germany. Int J Radiat Oncol Biol Phys. 2002;52:496–513. doi: 10.1016/s0360-3016(01)01814-4. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Jackson J, Morrison DP, Carlisle SM. Low doses of radiation increased the latency of spontaneous lymphomas and spinal osteo-sarcomas in cancer-prone, radiation-sensitive Trp 53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Gragtmans NJ, Morrison DP. Beta-radiation-induced resistance to MNNG initiation of papilloma but not carcinoma formation in mouse skin. Radiat Res. 1999;121:180–186. [PubMed] [Google Scholar]
- Mitchel RE, Burchart P, Wyatt H. Fractionated, low-dose-rate ionizing radiation exposure and chronic ulcerative dermatitis in normal and Trp53 heterozygous C57BL/6 mice. Radiat Res. 2007;168:716–724. doi: 10.1667/RR1124.1. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Burchart P, Wyatt H. A lower dose threshold for the in vivo protective adaptive response to radiation tumorigenesis in chronically exposed normal and Trp53 heterozygous C57BL/g mice. Radiat Res. 2008;170:765–775. doi: 10.1667/RR1414.1. [DOI] [PubMed] [Google Scholar]
- Mitchel RE. The dose window for radiation-induced protective and adaptive responses. Dose-Response. 2010;8:192–208. doi: 10.2203/dose-response.09-039.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunobu F, Yamaoka K, Hanamoto K, kojima S, Hosaki Y, Ashidak K, Sugita K, Tanizaki Y. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res. 2003;44:95–99. doi: 10.1269/jrr.44.95. [DOI] [PubMed] [Google Scholar]
- Mortazavi SMJ, Karam PA. Apparent lack of cancer susceptibility among residents of the high background area of Ramsar, Iran: can we relax our standards? In: McLaughin JP, editor. The Natural Radiation Environment VII. Elsevier; Amsterdam: 2005. pp. 1141–1147. [Google Scholar]
- Mortazavi SMJ, Rahmani MR, Rahnama A, Saeed-Pour A, Nouri E, Hosseini N, Aghaiee M. The stimulatory effects of topical application of radioactive lantern mantle powder on wound healing. Dose Response. 2009;7:149–159. doi: 10.2203/dose-response.08-022.Mortazavi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. Artificial transmutation of the gene. Science. 1927;116:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- Niewald M, Seegenschmiedt MH, Micke O, Garber S, the GCGBD of the DEGRO (German Society for Radiation Oncology) Randomized multicenter trial on the effect of radiotherapy for plantar fasciitis (painful heal spur) using very low doses-a study protocol. Radiat Oncol. 2008;3:327–332. doi: 10.1186/1748-717X-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Immunological mechanism of the low-dose radiation-induced suppression of cancer metastases in a mouse model. Dose-Response. 2010;8:209–226. doi: 10.2203/dose-response.09-016.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Magae J, Kawakami Y, Koana T. Reduction in mutation frequency by very low dose gamma irradiation of Drosophila melanogaster germ lines. Radiat Res. 2009;171:1–8. doi: 10.1667/RR1288.1. [DOI] [PubMed] [Google Scholar]
- Ootsuyama A, Tanooka H. Threshold-like dose of local β irradiation repeated throughout lifespan of mice for induction of skin and bone tumors. Radiat Res. 1991;125:98–101. [PubMed] [Google Scholar]
- Pandey R, Shankar BS, Sharma D, Salnis B. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol. 2005;81:801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- Raabe OG. Concerning the health effects of internally deposited radionuclides. Health Phys. 2010;98:515–536. doi: 10.1097/HP.0b013e3181c20e25. [DOI] [PubMed] [Google Scholar]
- Ramachandran TV. Environmental thoron (220Rn) : A review. Iran J Radiat Res. 2010;8:129–147. [Google Scholar]
- Rao NS, Sengupta D, Guin R, Saha SK. Natural radioactivity measurements in beach sand along southern coast of Orissa, eastern India. Environ Earth Sci. 2009;59:593–601. [Google Scholar]
- Ridenour LN. Modern physics for the engineer. Vol. 1. McGraw-Hill; 1961. p. 201. [Google Scholar]
- Rockwell T, Muckerheide J. Testimony to the NRC’s Advisory Committee on Nuclear Waste and Materials, Radiation, Science and Health. 2008. Apr 8,
- Rodel F, Keilholz L, Herrmann M, Sauer R, Hildebrandt G. Radiobiological mechanisms in inflammatory diseases of low dose radiation therapy. Int J Radiat Biol. 2007;83:357–366. doi: 10.1080/09553000701317358. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, Oda T, Iwasak T, Fujita K, Yamada T, Tanooka H. Suppression of carcinogenic processes in mice by chronic low dose-rate gamma-irradiation. Int J Low Radiat. 2003;1:142–146. [Google Scholar]
- Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity in Biology, Toxicology and Medicine. 2004;2:293–316. doi: 10.1080/15401420490900254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL, Lundgren D. Pulmonary carcinogenesis in the F344 and Wistar rat following inhalation of 239PuO2. Radiat Res. 1995;144:206–214. [PubMed] [Google Scholar]
- Sanders CL. Prevention of cigarette smoke induced lung cancer by low LET ionizing radiation. Nuclear Engineering and Technology (Korea) 2008;40:539–550. [Google Scholar]
- Sanders CL. Radiation Hormesis and the Linear-No-Threshold Assumption. Springer-Verlag; Germany: 2010. p. 214. [Google Scholar]
- Sanders CL, Scott BR. Smoking and Hormesis as Confounding Factors in Radiation Pulmonary Carcinogenesis. Dose-Response. 2008;6:53–79. doi: 10.2203/dose-response.06-003.Sanders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Di Palma J. Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose-Response. 2006;5:230–255. doi: 10.2203/dose-response.06-002.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Stochastic thresholds: A novel explanation of nonlinear dose-response relationships for stochastic radiobiological effects. Dose-Response. 2005;3:547–567. doi: 10.2203/dose-response.003.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegenschmiedt MH, Katalinic A, Makoski H, Haase W, Gademann G, Hassenstein E. Radiation therapy for benign diseases. Patterns of care study in Germany. Int J Radiat Oncol Biol Phys. 2000;47:195–202. doi: 10.1016/s0360-3016(99)00537-4. [DOI] [PubMed] [Google Scholar]
- Sengiyumva G. Tanzania: Uranium reserves need proper handling. 2010. Feb 1, Tanzania Daily News.
- Shadley JD, Wolff JK. Very low doses of x-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis. 1987;2:95–96. doi: 10.1093/mutage/2.2.95. [DOI] [PubMed] [Google Scholar]
- Shen RN, Lu L, Kaiser HE, Broxmeyer HE. Murine AIDS cured by low dosage total body irradiation. Adv Exp Med Biol. 1997;407:451–458. doi: 10.1007/978-1-4899-1813-0_66. [DOI] [PubMed] [Google Scholar]
- Shen RN, Hornback NB, Lu L, Li L, Chen T, Brahmi A, Broxmeyer HE. Low dose total irradiation: a potent anti-retroviral agent in vivo. Int J Radiat Oncol Biol Phys. 1989;16:165–170. doi: 10.1016/0360-3016(89)90024-2. [DOI] [PubMed] [Google Scholar]
- Stirling CH, Andersen MB, Potter EK, Halliday AN. Low-temperature isotopic fractionation of uranium. Earth and Planetary Science Letters. 2007;264:208–225. [Google Scholar]
- Takatori M, Hattori S, Yagi M. Clinical significance of low-dose radiation therapy: radiation hormesis. Int J Low Radiat. 2010;7:511–519. [Google Scholar]
- Takatori M. Personal Communication 2011.
- Tapio S, Jacob V. Radioadaptive response revisited. Radiat Environ Biophys. 2007;46:1–12. doi: 10.1007/s00411-006-0078-8. [DOI] [PubMed] [Google Scholar]
- Taylor LS. Some non-scientific influences on radiation protection standards and practice. Health Phys. 1980;32:851–874. [PubMed] [Google Scholar]
- Thompson RE, Nelson DF, Popkin JH, Popkin Z. Case-control study of lung cancer risk from residential radon exposure in Worcester County, Massachusetts. Health Phys. 2008;94:228–241. doi: 10.1097/01.HP.0000288561.53790.5f. [DOI] [PubMed] [Google Scholar]
- Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, Drozhko EG. Multifactorial analysis of lung cancer dose-response relationships for workers at the Mayak nuclear enterprise. Health Phys. 1997;73:899–905. doi: 10.1097/00004032-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Ulsh BA. Checking the foundation: recent radiobiology and the linear no-threshold theory. Health Phys. 2010;99:747–758. doi: 10.1097/HP.0b013e3181e32477. [DOI] [PubMed] [Google Scholar]
- Von Pannewitz G. Die Rontgentherapie der Arthritis de formans. Ergebnisse der medizmischen Strahlenforschung. 1933;6:62–126. [Google Scholar]
- Vrijheid M, Cardis E, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, Yoshimura T, Ahn Y-O, Ashmore P, Auvinen A, Bae J-M, Engels H, Gulis G, Habib RR, Hosoda Y, Kurtinaitis J, Malker H, Moser M, Rodriguez-Artalejo F, Rogel A, Tardy H, Telle-Lamberton M, Turai I, Usel M, Veress K. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: design, epidemiological methods and descriptive results. Radiat Res. 2007;167:361–379. doi: 10.1667/RR0554.1. [DOI] [PubMed] [Google Scholar]
- Williams JA, Williams JR, Yuan X, Dillehay LE. Protracted exposure radiosensitization of human malignant glioma. Radiat Oncol Invest. 1998;6:255–263. doi: 10.1002/(SICI)1520-6823(1998)6:6<255::AID-ROI2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wolff S. Aspects of the adaptive response to very low doses of radiation and other agents. Mutat Res. 1996;358:135–142. doi: 10.1016/s0027-5107(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto O, Seyama T, Itoh H, Fujimoto Oral administration of tritiated water (HTO) in the mouse. III. Low dose-rate irradiation and threshold dose-rate for radiation risk. Int J Radiat Biol. 1998;73:535–541. doi: 10.1080/095530098142086. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Mitsunobu F, Hanamoto K, Mori S, Tanizaki Y, Sugita K. Study on biologic effects of radon and thermal therapy on osteoarthritis. J Pain. 2004;5:20–25. doi: 10.1016/j.jpain.2003.09.005. [DOI] [PubMed] [Google Scholar]