Abstract
Chronic exposure of mammals to low dose-rates of ionizing radiation affects proliferating cell systems as a function of both dose-rate and the total dose accumulated. The lower the dose-rate the higher needs to be the total dose for a deterministic effect, i.e., tissue reaction to appear. Stem cells provide for proliferating, maturing and functional cells. Stem cells usually are particularly radiosensitive and damage to them may propagate to cause failure of functional cells. The paper revisits 1) medical histories with emphasis on the hemopoietic system of the victims of ten accidental chronic radiation exposures, 2) published hematological findings of long-term chronically gamma-irradiated rodents, and 3) such findings in dogs chronically exposed in large life-span studies. The data are consistent with the hypothesis that hemopoietic stem and early progenitor cells have the capacity to tolerate and adapt to being repetitively hit by energy deposition events. The data are compatible with the “injured stem cell hypothesis”, stating that radiation–injured stem cells, depending on dose-rate, may continue to deliver clones of functional cells that maintain homeostasis of hemopoiesis throughout life. Further studies perhaps on separated hemopoietic stem cells may unravel the molecular-biology mechanisms causing radiation tolerance and adaptation.
Keywords: chronic irradiation, hemopoietic tolerance, stem cell adaptation
INTRODUCTION
The patho-physiology in acutely irradiated mammals was first summarized in the seminal monograph by Victor P. Bond, John O. Archambeau and T.M. Fliedner, published in 1965; its title is “Mammalian Radiation Lethality – A Disturbance in Cellular Kinetics” (Bond et al. 1965). This monograph also presents the then available and still today relevant knowledge regarding the pathogenesis of the hemopoietic syndromes that develop after acute radiation exposure to varying doses.
The current contribution addresses the effects of chronic radiation exposure on hemopoiesis, by referring to some previously published representative data on low dose-rate induced responses in the hemopoietic system. This data was obtained mainly in rats and dogs and in reports on a number of radiation accidents (for summary: Fliedner et al. 2002a). It emerges that, in line with early observations (Lamerton et al. 1960), the relationship between daily low-dose level exposures and clinical signs and symptoms in the exposed organisms is determined by the damage accumulating especially in the rapidly turning over cell renewal systems – such as the hemopoietic tissue. This means that the tissue effect of hemopoietic failure depends on both total absorbed dose, in short dose, and on the dose-rate or frequency of repetitive exposures.
During continuous exposures of tissues individual and defined micro-masses in the exposed tissues experience at certain time intervals energy deposition events (hits) from charged particle tracks. The dose values, i.e., sizes, of these hits depend on the size of the micromass and the energy spectrum provided by the given radiation quality (ICRU, 1983). The longer the time interval between consecutive hits in a given individual micromass, such as a cell, the longer is the time for the hit cell to respond to the previous hit without disturbance from a second hit. Thus, dose-rate effects depend on both, the spectrum of hit sizes and of the time intervals between consecutive hits. Indeed, experimental evidence with low-LET radiation exposures speaks in favor of low dose-rates being less efficient per total dose to cause tissue effects than high dose-rates or single exposures. This also complies with theoretical analyses (Scott 1980, Scott et al. 1988; Kutkov et al. 2011). The more damage from individual hits is allowed to accumulate, the lower is the total dose needed for the tissue effect to occur.
The analysis of the revisited data focuses on the responses of hemopoietic stem cells regarding their potential of tolerance and adaptation in the course of being chronically exposed to different dose-rates. Stem cells are pluripotential or committed and divide upon demand in a cascade of signaling in order to provide for functioning cells through repetitive cell divisions of differentiating cells and cell maturation. In mammalian hemopoiesis, the time of cell differentiation and maturation to deliver fully functional cells is species specific and ranges over several days up to about one week (Bond et al.1965). Even if hemopoietic stem cells are generally rather radiosensitive, they are not necessarily the most radiosensitive cells. Thus, especially some progenitor cells in the GI-tract (Potten and Hendry 1995) and testes (Oakberg 1971) can be far more radiosensitive than hemopoietic stem cells. Differences amongst hemopoietic progenitor cells with some being rather radioresistant have also been seen (Baird et al. 1990; Bierkens et al. 1991; Fliedner et al. 2002c). For instance, marrow repopulating ability was more radioresistant to gamma-radiation than were spleen colony-forming cells (Fliedner et al. 2002c). However, despite the ranges of individual stem cell responses to radiation, the hemopoietic cell system in mammals is generally more radiosensitive than the GI cell system or skin. These graded system differences in radiosensitivities are the reason for the hemopoietic failure to occur at doses lower than the doses that cause the GI-tract failure or acute skin damage. At very low dose-rates, the total dose that is needed to bring about the tissue effect of hemopoietic failure reaches very high values, so that at low dose-rates hemopoiesis continues to fully function clinically up to very high total doses, as is explained also theoretically (Scott 1980; Scott et al. 1988, Kutkov et al. 2011). Thus, both total dose and dose-rates need consideration in evaluating radiation effects in red bone marrow. Limited data are available on the effects of varying dose-rates for a given accumulated dose (Fritz et al. 1982, 1986). The mechanisms of the considerable tolerance to hemopoietic failure at low dose-rates are largely unknown but likely linked to stem cell responses.
The analysis of revisited data in view of current knowledge allows suggesting further studies on stem cell responses to low dose-rates. The outcome also questions the justification of current radiation protection recommendations for workers as for the general public.
MATERIAL, METHODS AND OUTCOME
Human Accidental Chronic Radiation Exposure
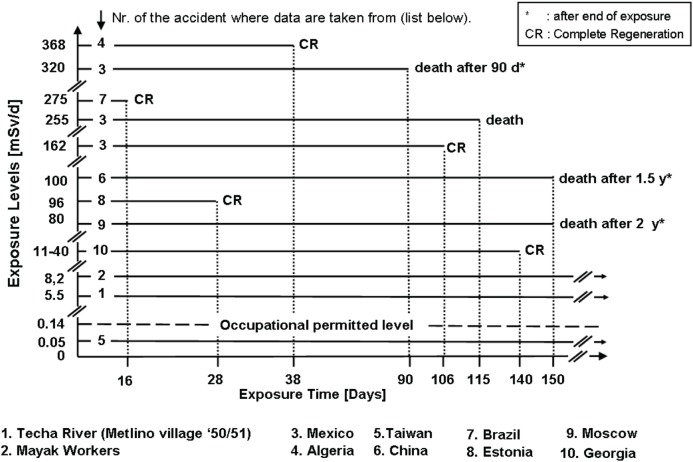
Table 1 lists ten accidental events of chronic radiation exposure and the incidences of death following a given duration of exposure. Figure 1 shows corresponding dose levels and clinical outcomes of some of the exposed individuals. The given dose levels in Figure 1 are listed in mSv, as presented in the original literature, and are here synonymous with RBE-weighted dose, i..e., with gray-equivalent, Gy-Eq (Bolch et al. 2009; Kutkov et al. 2011). Note that the majority of exposures were external whole body gamma-irradiation, but exposures at the Mayak plant and at the Techa River were both external and internal with mixed radiation qualities and varying dose rates from internal exposure. This data set and the individual clinical symptoms and findings have been and are helpful for understanding the pathogenesis and clinical courses of all individual victims (Fliedner et al. 2002a,b).
TABLE 1.
Selected radiation accidents and deaths, involving chronic exposure of humans to ionizing radiation.
| Accident | Victims | No. of Deaths | Duration of Exposure |
|---|---|---|---|
| Techa (since ‘49) | > 28,000 | ? | Lifetime |
| Mayak (since ‘48) | > 1,800 | ? | several years |
| Mexico (1962) | 5 | 4 | 24 – 115 d |
| Algeria (1978) | 7 | 0 | hrs – 38 d |
| Taiwan (since ‘82) | 10,200 | ? | 1 – 19 y |
| China (1985) | 3 | 2 | 150 d |
| Brazil (1987) | 249 | 4 | 16 d |
| Estonia (1994) | 10 | 1 | hrs – 28 d |
| Moscow (1995) | 1 | 1 | ∼ 150 d |
| Georgia (1997) | 11 | 0 | 60 – 140 d |
FIGURE 1.
Effective dose-rate (daily dose levels) in ten selected radiation accidents. Exposure times are in days as indicated by the horizontal dotted lines. Below a dose-rate of 8.2 mSv/d beyond 150 days there was no lethality. Even after a dose-rate of 368 mSv/d for 38 days complete recovery could be observed. This display shows the potential wide range of individual responses with the tendency towards reconstitution with decreasing dose-rates and exposures times.
Especially revealing are the data on the exposed Techa River population as well as the disease histories of some of the Mayak patients who suffered protracted radiation exposure from external and internal radiation sources over weeks and months working in a radiation environment (Okladnikova and Pedsternikova 2002, Akleyev et al. 2002). Into these groups of chronically low- dose-rate exposed people belong also the victims of accidental exposure in certain buildings in Taiwan due to the fact that the steel used for house construction contained radioactive Co-60 that led to continuous whole body gamma exposure of the inhabitants of these houses. Exposure in these groups, however, was not severe enough to cause clinical complaints.
Clinical problems were recognized and described for the Mexican accident (Martinez et al. 1966), the Algerian accident (Cosset et al. 2002), the Chinese accident (Wang et al. 1996, Wang et al 2002), the Brazilian accident (Valverde et al. 2002), as well as for the accidents in Estonia (Vôsumaa 2002), Moscow (Baranov 2002) and Georgia (IAEA, 2000). Figure 1 shows the extent of daily exposures to continuous low dose-rates and their outcome in selected victims as representatives of all listed accidents.
The above data are consistent with the blood-forming tissue being the critical site of relatively high radiosensitivity, as it is apparent clinically and also leads to some practical clinical consequences regarding diagnosis and therapy (IAEA 2005). For instance, after people leaving the radiation field that causes a daily exposure to dose ranges between 80 and 100 mSv over a period of a few months, the attending physician will be able to observe signs of an initial recovery in the hemopoietic system of seemingly healthy individuals. However, at later times and after leaving the radiation field, as indicated in Figure 1, victims developed hematological signs and symptoms of bone marrow failure with the risk of lethal pancytopenia due to myelo-proliferative disorder (MPD), or perhaps cancer, if they survived. Noteworthy, in all these cases there was evidence for a temporary hemopoietic recovery after leaving the radiation field. In the accidents that occurred in China and Moscow, with continuous whole body gamma exposures to dose-rates of 80 – 100 mSv/day for about half a year, leukemia developed within 1.5 - 9 years after exposure.
In the Algerian accident, continuous whole-body gamma radiation exposure was at a dose-rate of 368 mSv/day from an (unrecognized) sealed radiation source; the exposure lasted for 38 days in four persons. All victims developed severe pancytopenia from which they recovered spontaneously.
In the Mexican accident, five people were continuously whole body exposed for about 2 1/2 months to Co-60 gamma rays at dose-rates between 160 and 320 mSv/day. These victims were not aware that the steel capsule in their kitchen shelf contained a radiation source. Four of these five people died from hemopoietic failure 1 – 3.5 months after exposure. Only one person survived and showed a spontaneous hemopoietic recovery after exposure to 162 mSv/day. It should be stressed that there were significant differences in the upper and lower dose estimates in these individuals.
Such case histories may lead to the following hypotheses: in all accidents with continuous whole body radiation exposures (chronic, protracted, or intermittent), the degree of tolerance or failure of the hemopoietic system appears to be crucial for the clinical outcome regarding morbidity and mortality, either during or within the first few years after exposure. If the dose-rate level is below about 10 mSv/day, human beings can survive for several years without any organ failure. One may argue that the survivors eventually may develop cancer or other non-malignant diseases with the consequence of life shortening. If the dose-rate level exceeds 10 mSv/day, the chance of developing bone marrow failure within a relatively short period of time, i.e., from weeks to years, increases depending on exposure conditions of dose-rate and total dose and appears individually determined. Initial recovery of hemopoietic function was a feature in all at lower dose-rates. Still, intensive studies of the individual impairments have not yet revealed fully the patho-mechanisms underlying the hemopoietic failure. The crucial question focuses on the individual responses of hemopoietic stem cells to the various dose-rates over different periods of exposure time. The capacity of stem cells for reestablishing homeostasis in blood formation appears to span a remarkable range of damage severity.
Experimental Animal Studies
Studies with Rats
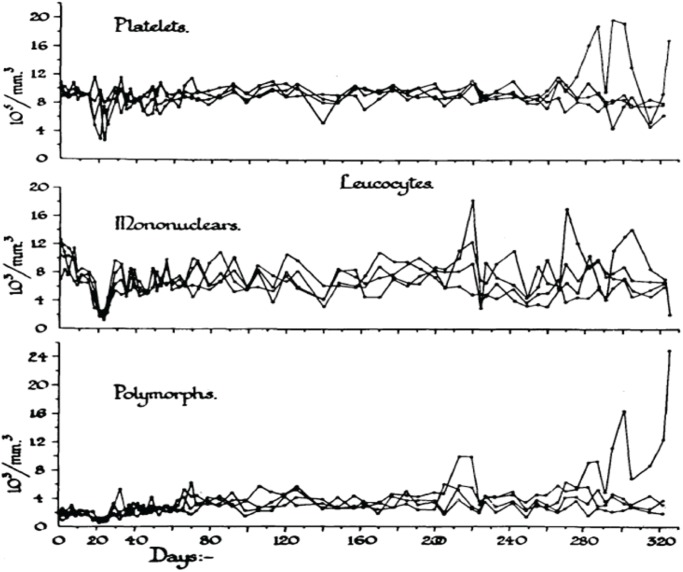
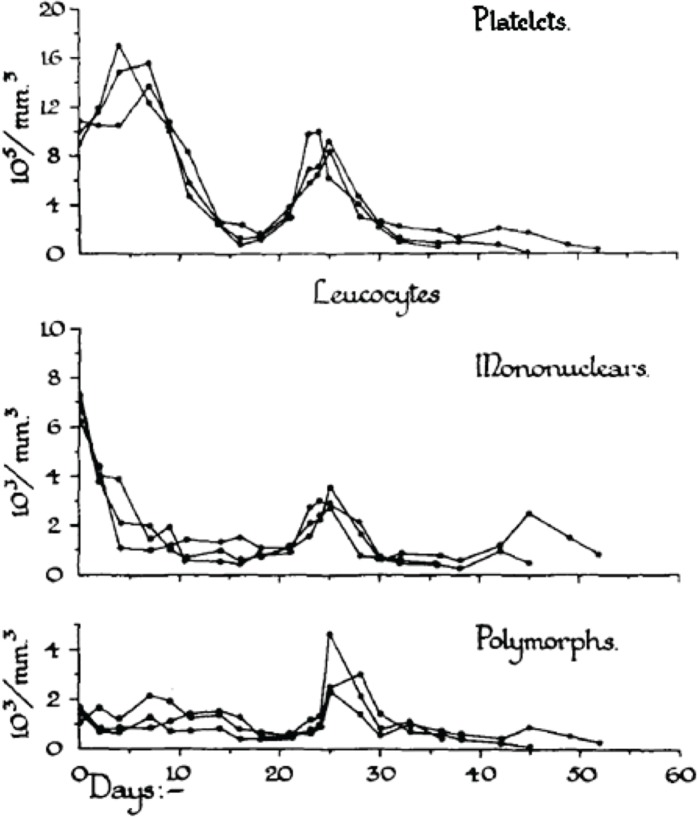
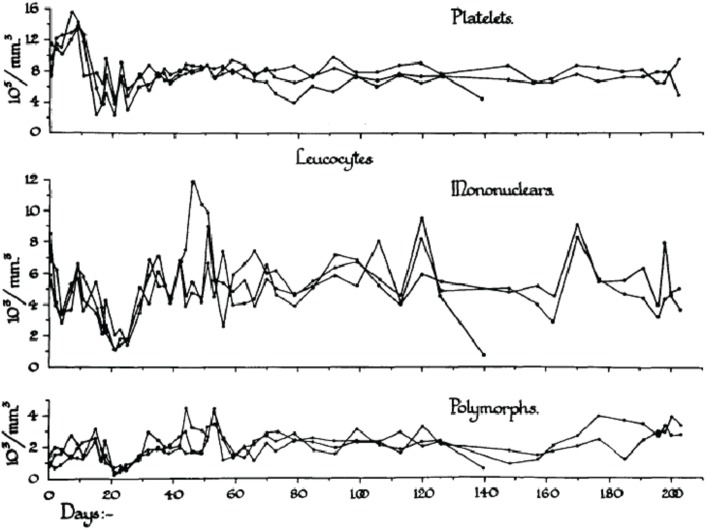
In order to analyze the tolerance of hemopoietic cell renewal systems systematically, extended animal studies are required. A number of such large studies have been conducted on hemopoietic responses to chronic whole body radiation exposures especially in rodents (Lamerton et al. 1960; Testa et al. 1973; Chu-Tse and Lajtha 1975; Gidali et al. 1979; Baird et al. 1990). Since such studies with low-dose exposed animals have considerable difficulties currently being funded, re-analyses of old data promises to answer renewed questions on hemopoietic tolerance during chronic low dose-rate irradiation. In the 1950s, Lamerton and his group in London (Lamerton et al. 1960) were the first to systematically examine the effects of protracted irradiation of the blood forming organs of the rat. Figures 2A – 2D are taken from Lamerton et al. 1960 and show hematological responses in rats following continuous whole body gamma irradiation using dose-rates of 1.76 Gy/day (Figure 2D), 0.84 Gy/day (Figure 2C), 0.50 Gy/day (Figure 2B) and 0.16 Gy/day (Figure 2A). The figures express the peripheral blood counts of platelets, mononuclear cells, and polymorph cells as a function of time of exposure at the four dose-rates. One can easily see that the exposures to 0.16 Gy/day and 0.50 Gy/day do not necessarily produce a lethal outcome. After initial turbulences of cell counts, the hemopoietic system in rodents is apparently capable to tolerate continuous irradiation at a certain level for some time individually before the hemopoietic system collapses. With the assumption of radiation-induced cell loss especially in the stem cell compartment at dose-rates of 0.16 and 0.50 Gy/day, as mirrored by the initial peripheral blood counts, it appears that the organism tries to maintain homeostasis through increased cell production that compensates for radiation-induced cell loss.
FIGURE 2A.
Response to continuous gamma-radiation of rats at 0.16 Gy/day. The data express peripheral blood counts of platelets, mononuclear cells, and polymorph cells. Reproduced with permission from (Lamerton et al. 1960).
FIGURE 2D.
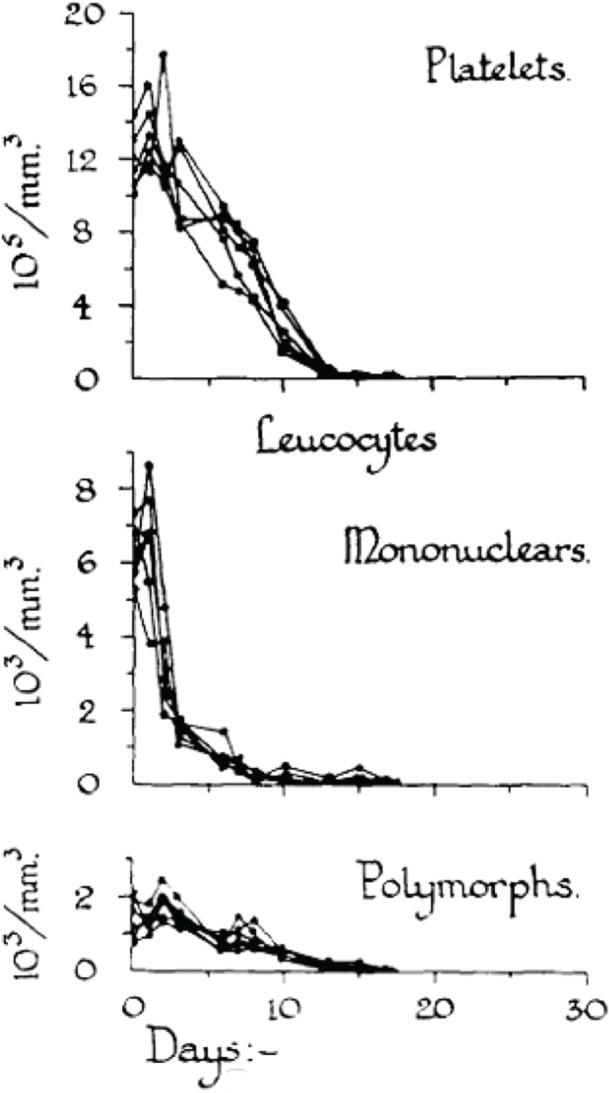
Response to continuous gamma-radiation of rats at 1.76 Gy/day. The data express peripheral blood counts of platelets, mononuclear cells, and polymorph cells. Reproduced with permission from (Lamerton et al. 1960).
FIGURE 2C.
Response to continuous gamma-radiation of rats at 0.84 Gy/day. The data express peripheral blood counts of platelets, mononuclear cells, and polymorph cells. Reproduced with permission from (Lamerton et al. 1960).
FIGURE 2B.
Response to continuous gamma-radiation of rats at 0.50 Gy/day. The data express peripheral blood counts of platelets, mononuclear cells, and polymorph cells. Reproduced with permission from (Lamerton et al. 1960).
Obviously, the hemopoietic system fails fully within less than 20 days at 1.76 Gy/day and within about 50 days at 0.84 Gy/day. However, at this dose-rate, an initial short-lasting rise in peripheral blood cell counts turns to a decline to a minimum at approximately 20 days, followed by a small increase in all cell counts to a maximum at about 25 days, after which the system irreversibly tends to fail over the next 25 days. At 0.50 Gy/day, the initial response is a short lasting rise within the first days of exposure. This is followed by a decline in cell counts to a minimum again at about 20 days. Thereafter, the system appears capable to reestablish homeostasis with some cell count undulations during the 200 days of observation. The early temporary rise is hardly seen at 0.16 Gy/day where the initial drop in cell counts reaches a minimum at about 20 days followed by recovery to a cell count plateau of homeostasis, again with some cell count oscillations during the 300 days of observation. It is the hypothesis that the remarkable capacity of recovery at 0.50 Gy/day and 0.16 Gy/day is, nevertheless, accompanied by some functional deficit that may appear in the cell count oscillations. This presumed deficit may be indicative of cell kinetic adaptations in the pool of stem cells, all of which must carry damage that possibly is stochastically distributed in the stem cell pool giving rise to injured stem cell clones.
It has been postulated that the early temporary increase of viable leukocytes, which is most pronounced at 0.80 Gy/day and least obvious at 0.16 Gy/day, is compatible with the assumption of less injured stem cells being able to maintain cell production and deliver mature platelets and leukocytes. This partly abortive rise is assumed to derive from injured stem cells which disappear in the long run. Another cause of the early temporary leucocyte increase may be a release of these cells upon an increased distension of sinusoidal bone marrow segments associated with parenchymal loss (Fliedner et al. 2002b,c). After 1.76 Gy/day, there is no temporary rise but a straight forward decline of the platelets, the mononuclear cells and the polymorphs indicating that the stem cell compartment has been decimated at that dose-rate.
In his original presentation in 1960, Dr. Lamerton pointed out: “Under continuous irradiation the response of the renewal tissue of the body may be essentially different from that under acute exposure. It is reasonable to believe, that all the renewal tissues of the body have some capacity for maintenance of cell populations under continuous irradiation, but that they will vary greatly in the time taken for the establishment of steady states and in the dose-rates that can be tolerated” (Lamerton et al. 1960). Since then one has learned that the crucial cells that determine hemopoietic responses to radiation are the blood forming stem cells, whose individual patterns of tolerance at chronic, especially low dose-rate, irradiations is still little understood.
Studies with Dogs
Even more revealing are the studies with dogs. These were whole-body gamma-irradiated (from a Co-60 source) continuously throughout life at relatively low dose-rates (Fritz et al. 1982, 1986; Seed and Meyers 1993). The dose-rates to the whole body over the entire life span were 3 mGy/day, 7.5 mGy/day, 18.8 mGy/day, 37.5 mGy/day, 75 mGy/day and 127.5 mGy/day. Higher dose-rates of 262.5 mGy/day, 375 mGy/day and 640 mGy/day resulted in the death of all animals within 100 days. This is the largest systematic hematological chronic irradiation study known, where dogs were chronically exposed to radiation for their entire lifetime, i.e., until death occurred in the radiation field.
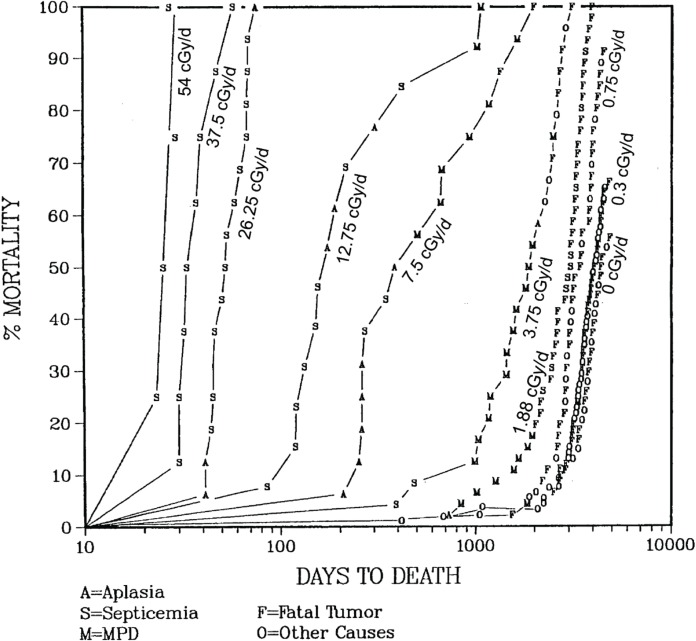
Figure 3 shows the mortality curves for the above mentioned dose-rate groups (the figure lists dose- rates cGy/day complying with the original publication). Irrespective of the detailed findings, these data, in general, show a trend in responses in that a rather sudden tissue response leading to death occurred after dose-rate dependent time intervals. Thus, at dose rates higher than 18.8 mGy/day, death was nearly always due to hemopoietic insufficiency in the individual groups of dogs. The higher the dose-rates, the shorter were the time intervals, and thus the lower were the total doses, until the issue effects occurred. Moreover, the range of total doses that accumulated prior to onset of the tissue effect was relatively broad at dose-rates of 37.5, 18.8 and 7.5 mGy/day. The relationship between dose-rate and exposure time, i.e. accumulated dose, at which the hemopoietic tissue effect developed conforms to theoretical analyses (Scott 1980; Kutkov et al, 2011).
FIGURE 3.
Mortality curves of dogs after whole-body chronic gamma-irradiation at er dose-rates incGy/day, adapted from (Fritz 2002).
Within the high dose-rate groups (540 mGy/day, 375 mGy/day and 262.5 mGy/day) irradiation resulted in a lethal outcome for all animals within a time span below 100 days of exposure. All observed deaths were caused by hemopoietic failure. At these dose-rates, there is apparently no chance for effective adaptation of the hemopoietic system over time to accumulating radiation induced damage and cell loss.
In the middle dose-rate groups (127.5 mGy/day, 75 mGy/day and 37.5 mGy/day) there is a remarkable span in the survival times. While for example in the 127.5 mGy/day group the first death occurs after about 80 days of irradiation, the last death is observed after about 1000 days. The observed causes of death within the same dose group shift from hemopoietic insufficiency with septicemia and aplasia to myeloproliferative disorders (MPD) and fatal tumors. Apparently there are significant differences between individual dogs in the processes of tolerance and failure of the hemopoietic cell system during chronic radiation exposure.
In the dose-rate group 18.8 mGy/day still some dogs died from MPD, but below this dose-rate the relative numbers of deaths from fatal tumors increases to the level seen in control dogs. Figure 3 lists the incidence of fatal tumors in these dose-rate groups, as described in the original literature.
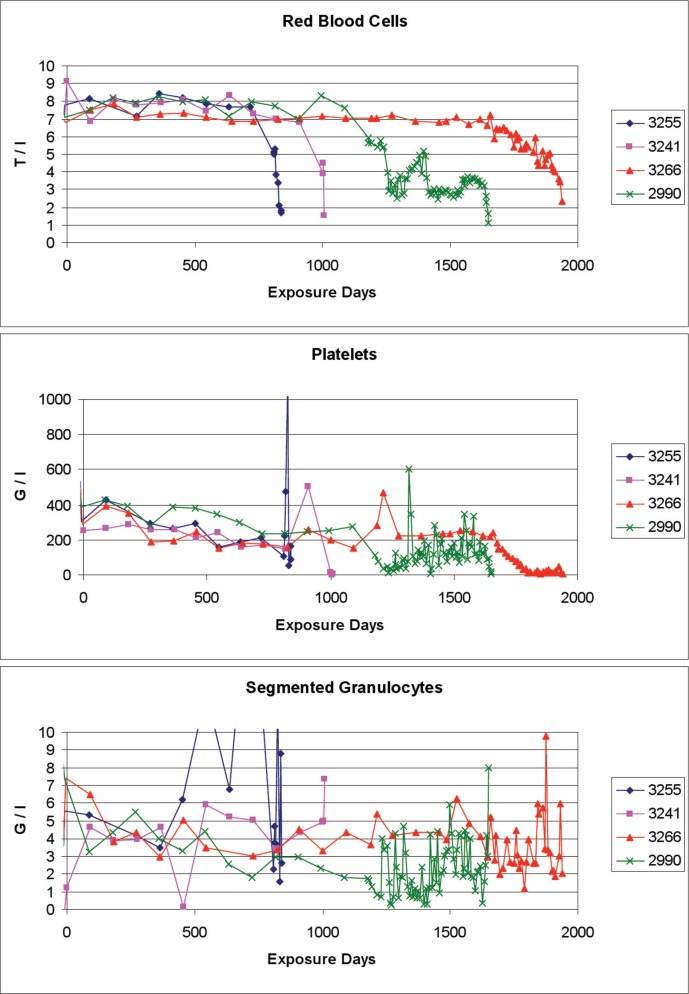
Figure 4 shows examples of the blood cell concentrations of 4 dogs during exposure to 18.8 mGy/day with MPD being the cause of death. Over time spans that vary strongly between the individual animals, the red blood cell concentrations appear to be nearly normal until a rather sudden failure appears. The platelet concentrations decline slowly until about 800 days of exposure, when disturbances set in at various times as signals of the beginning of proliferative disorders with the eventual hemopoietic collapse more than 500 days later. Regarding the segmented granulocyte concentrations, the values fluctuate rather widely already during the early days of exposure with some stabilization lasting from about 200 to 400 days, when fluctuations aggravate again in 2 dogs, and at later times the other 2 dogs follow this pattern. Obviously these fluctuations are prodromal to the collapse of the hemopoietic system about 500 days later. These data indicate for the clinician that the examination of the blood counts alone would probably not necessarily point to the existence and the severity of chronic radiation exposure of the subject.
FIGURE 4.
Example blood cell counts of 4 dogs of the cohort of whole-body chronically gamma-irradiated dogs, at a dose-rate of 18.8 mGy/day. Data of numbered dogs were selected from (Beagle Dog Tissue Archive 2012).
In the group of 92 dogs exposed to 3 mGy/day, there were no significant changes in the concentration of red cells, platelets, and segmented granulocytes in the individual dogs in a clinically relevant way, throughout the study (Fritz et al. 1982, 1986, Fritz 2002). This indicates that under clinical conditions the examination of the blood counts alone would not point to a chronic radiation exposure of the subject. In fact, in this group some dogs survived up to 5000 days, i.e., about 13 years - a full life span -, within the radiation field. The cause of death in these dogs was similar to the control, dominated by fatal tumor.
However, viewing the whole cohort of this group, radiation effects aspire, in that the red cell counts tended to decline slowly with exposure times beyond about 1000 days, the granulocyte counts in a number of dogs became elevated temporarily at between about 1500 to 2500 days, and the platelet counts in a number of dogs became elevated temporarily at between about 1500 to 3500 days.
In summary, the above canine studies show - dependent on dose-rate - two major causes of death: hemopoietic failure and tumors. With decreasing low dose-rates, hemopoietic failure becomes infrequent and finally disappears at ∼ 3 mGy/day. Then, tumors become the predominant fate at a near full life span, similar to the control unirradiated dogs.
It appears to be a particular challenge to explain the reason of, and the pathophysiological cell system effects that result in, the observed wide range of survival times during chronic exposure. The responses of the dogs were individually unpredictable at low dose-rates. Some of these dogs succumbed earlier than others rather abruptly with hemopoietic failure. This seems to indicate that individual genetic control causes individually varying radiosensitivities in terms of capacity for tolerance or failure of the blood forming system.
Simulations of hemopoietic processes in radiation fields using mathematical models of blood cell turnover
Simulation results showed that reductions in peripheral blood cell concentrations as they are seen in the dog studies above at dose-rates of more than 3 mGy/day can be explained by continuous radiation induced excess cell losses in the stem (and precursor) cell pools of suitable mathematical compartment models of hemopoiesis (Graessle 2002). This stem cell loss is combined with a compensational increase of cell production by the remaining stem cells. Still, some questions for this type of modelling chronic radiation effects remain unanswered. For example, during about 100 days after onset of irradiation with more than 3 mGy/day, the concentrations of segmented neutrophil cells and platelets having fallen initially stabilized commensurate with homeostasis. As a consequence, the radiation effect on the stem cells had to be modelled not only as a function of dose-rate but also as a function of exposure time, with the dose-rates per group of dogs being constant throughout the studies. In other words, the function of exposure time becomes a function of accumulated dose. In biological terms this means that some characteristic radiation effects at the cellular level are changing during the exposure. Possible causes could be the adaptation of the hemopoietic cell to repetitive low dose exposures with injured stem cells acquiring the potential for maintaining hemopoietic homeostasis, at least for some period of time. This was considered in the approach to simulate human patient data of the Techa River cohort (Akushevich et al. 2011). Moreover, the remarkable maintenance of peripheral blood cell concentrations within normal ranges – yet with a tendency to a slow steady decline of erythrocytes and a concomitant increase of segmented neutrophil cells - over an entire life span of dogs exposed to 3 mGy/day could be the consequence of adaptation of the hemopoietic system to sustain repetitive damage, with stem cells being the pace makers.
The simulation study of Graessle (Graessle 2002) also considered a stochastic approach of the variation of survival times between individuals. Stochastic simulations suggested that widely varying survival times, as derived from the dog studies, can result from small randomly appearing variations in biological parameters, such as in radiation sensitivity or in potential of selfreplication. Such variations do not necessarily have to include a whole stem cell population of an individual, but could also be caused by variations in subpopulations or fluctuations between clones of stem cells (Abkowitz et al. 1996, Laukkanen et al. 2005), probably including clones of radiation injured stem cells.
Implications for research
There are still wide gaps in understanding the pathological and patho-physiological mechanisms that govern tolerance and failure of tissues under chronic low-level radiation exposure. The critical cell system to suffer first because of its radiosensitivity is the hemopoietic system. The number of individuals that were involved in accidental chronic low-level exposures is limited, so are the observations of low dose-rate exposed experimental animals. In view of new radiobiological data, experimental tools, and techniques the need of re-evaluation of the available data seems obvious. This approach would complement mathematical models and theoretical analyses using an advanced hazard function for varying cell types and systems (Scott 1980) leading to recognize a defined relationship between dose-rate and the total dose in terms of exposure time for a tissue effect to appear (Kutkov et al. 2011). The theoretically derived data so far correspond well with the experimental results of the above dog studies.
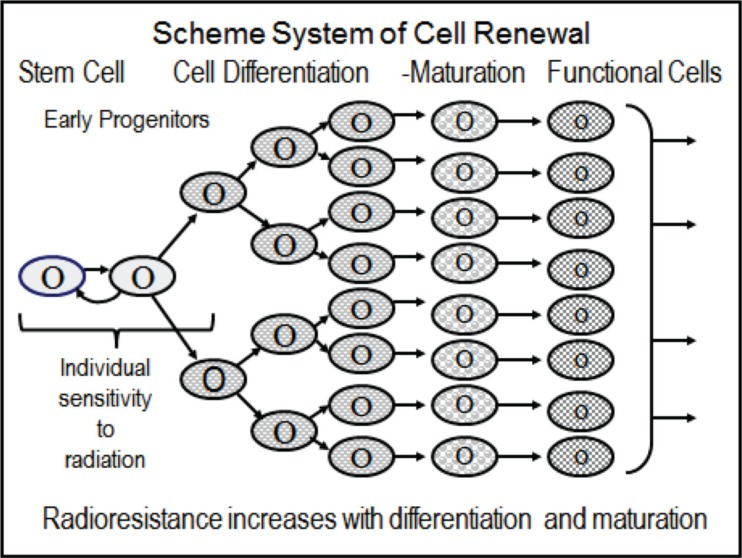
Pioneering in this field were the early observations presented in the 1960s by Lamerton and his group (Lamerton et al. 1960). Their results, today, focus on the role of the stem cell pool in triggering clinical signs and symptoms following low-level chronic radiation exposure. As schematically seen in Figure 5, a proliferating cell system requires one intact cell type, the stem cell, and early progenitor cells for maintaining cell replication and homeostasis of the system. Following irradiation, be it acute or chronic, all cells may be affected with the stem cells and early progenitor cells being amongst the most radiosensitive of all. At relatively low doses or dose-rates, then, hundreds of stem and progenitor cell clones may emerge carrying individual damages, as one would expect from an “injured cell hypothesis.” The consequence would be a large number of cell clones feeding into a wide variety of proliferating and perhaps abnormal cell populations. To explore them may lead to far-reaching results with broad translational effects also on research and practice in oncology.
FIGURE 5.
Scheme of stem cells maintaining production of functional cells in tissues. Note that differentiating, maturing and functional cells are less radiosensitive than progenitor cells including stem cells; these again, may possess considerable differences in individual sensitivities to ionizing radiation.
At comparatively low dose-rates, used in the dog studies (Fritz et al. 1982, 1986, Fritz 2002. Seed and Meyers 1993) the hemopoietic system exhibited clearly a remarkable and unexpected potential for repair and adaptation of the mammalian organism. Obviously, at low dose-rates, the blood-forming stem cells show a great capacity to tolerate, and adapt to, radiation injury by reconstituting an initially disturbed homeostatic cell production rate that balances the radiation induced cell losses, depending on the accumulated dose. Indeed, it is remarkable that exposed dogs surviving at least 3500 days at a dose- rate of 3 mGy/day accumulated doses of more than 10 Gy without suffering cell proliferation failure and functional breakdown. The molecular-biological mechanisms causing stem cells to have a relatively high level tolerance and to adapt to chronic low-level damage for a given time still appear to be far from being understood.
In order to relate cell responses to dose and dose-rate, microdosimetry promises advantages for distinguishing between direct and non-targeted effects on the low-level exposed system (ICRU 1983, ICRU 2011, Feinendegen et al. 2011). For instance, whole body gamma-irradiation from Co-60, as used in the dog experiments discussed above, causes each micromass of 1 ng, i.e., the average mass of a mammalian cell, to absorb per energy deposition event on average 0.3 mGy (ICRU 1983) with the production of about 45 reactive oxygen species (ROS) in each hit micro-mass (Feinendegen et al. 2011). In other words, a whole body dose of 0.3 mGy will cause each cell (ng) to be hit stochastically on average by one event of 0.3 mGy. A whole body dose of 3 mGy then brings on average 10 energy deposition events from Co-60 gamma radiation per cell (ng). It follows that a dose-rate of 3 mGy per day means that every cell (ng) in the body is stochastically hit by an event on average every 2.4 hours, i.e., the average time interval between two consecutive hits per given cell (ng) is 2.4 hours. This also means that on average during each 2.4 hours about 10 % of all cells (ng) in the exposed tissue become hit by an event, - or all cells (ng) are hit ten times a day. The dog experiments discussed above use this dose-rate leading to accumulated doses of more than 10 Gy over a life time of more than 3500 days, without hemopoietic failure. In analogy, each cell (ng) of the dogs that were exposed for 3500 days to 3 mGy/day was hit 30,000 times by an energy deposition event each also causing on average individual bursts of some 45 ROS. This attests a large capacity of the relatively radiosensitive hemopoietic system, especially stem and early progenitor cells, to tolerate repetitive damage throughout life. Up-regulation of defences, repair and damage removal, also in terms of adaptive protection, may all be involved. Regarding induction of leukaemia, data in dogs are not available. But data in adult humans were analysed and attributed again a most efficient defense system likely to be responsible for the extremely low probability of ∼10−14 for a lethal leukemia to develop per energy deposition event in a hemopoietic stem cell (Feinendegen et al. 1995).
At higher dose-rates, there appear to be individual limits of accumulated doses at which stem cell failure with hemopoietic break-down occurs, leading to death. This could be the consequence of a genetic predisposition of stem cells toward a relatively increased radiosensitivity during repetitive exposure over time. Be it as it may, selected stem and early progenitor cells escaping lethality would deliver clones during repetitively occurring insults from chronic exposure and determine the individually observed degree of radiosensitivity and, thus, life expectancy.
The degree of spatial and temporal heterogeneity of energy deposition events in the exposed body at a dose-rate of 3 mGy/day from Co-60 gamma radiation prompts the question of contribution of non-targeted effects in addition to targeted effects on short- and/or long term function of stem and early progenitor cells (Morgan and Sowa 2009). One of the non-targeted effects derives from bystander phenomena – both potentially damaging and protecting (Mothersill and Seymour 2006). These may be triggered theoretically by any hit cell (ng) in the exposed body and affect stem cells. To what degree stem and early progenitor cells are involved in bystander phenomena and how they respond is unknown but obviously would, if known, help clarifying the capacity and mechanisms of the hemopoietic system to tolerate and adapt to low dose-rates. Another question in this context addresses genomic instability (Kadhim et al. 2006). This phenomenon usually is discussed in the context of oncogenesis in the descendants of irradiated precursor cells. However, genomic instability may perhaps develop in progenitor cells also in the course of time at low dose-rates and switch gene expression in descendant cells to a state of up-regulated protection. The latter may enable stem and early progenitor cells to acquire tolerance and become adapted to repetitive energy depositions with their bursts of ROS.
The questions of direct and non-targeted responses of stem cells at chronic low-dose irradiation may be answered in the near future. The new techniques of isolating stem cells may open the avenue to study these cells and their responses to injury separately from those of multi-cellular systems.
New data from such studies may lead also to practical consequences in the therapy of radiation syndromes and also for choosing optimal radiation- and chemotherapy, for instance, in oncology.
IMPLICATIONS FOR CLINICAL PRACTICE
The current data analyses predict that an individual may spend days, weeks or even months in a radiation field without becoming alarmed. If a clinician finds hematological changes that are not readily explained, he should examine for the possibility of a radiation exposure being the reason for the hematological abnormalities. This scenario demands special expertise and guidance for diagnosis and therapy also considering stem cell transplantation (IAEA 2005; Fliedner et al. 2002a, 2009, Azzam et al. 2010; Ende and Azzam 2011).
IMPLICATIONS FOR RADIATION PROTECTION
The data presented and discussed above also touch the question of dose limits in radiation protection in different scenarios of chronic exposures. Current dose limits are fixed numbers without much attention to dose-rate. The concept of dose-rate should be built into the exposure limits. A special case is that of exponentially decreasing dose-rates over time from internal radionuclide exposures, such as following accidental radionuclide intake or planned in nuclear medicine. Thus, certain marrow progenitor cells (GM-CFC) respond to exponentially decreasing dose-rates specifically and show corresponding dose-rate profiles which also should be considered (Goddu et al. 1998, 2000).
There is a great discrepancy between recommended dose rate limits, for instance, of 1 mGy/year for the general public, on the one hand and, on the other hand, the observed dose rate of 3 mGy/day at which the hemopoietic system keeps providing homeostasis and full function in the service to the whole body. Even if the two endpoints, tissue effects regarding blood formation, and stochastic effects regarding tumor development, need separate assessments, the by three orders of magnitude lower recommended dose-rate limit for the general public compared with the experimental dose-rate in dogs, at which the hemopoietic system still maintains full function without apparent radiation-induced increase in tumor incidence, questions the justification of the radioprotection recommendations. Usually, radioprotection recommendations are based on risk coefficients derived from linear extrapolation of tumor risk observed at high doses to low doses. This, however, appears incompatible with the current data in dogs showing an at least three orders of magnitude lower risk of hemopoietic failure without an apparent increase in tumor risk per unit accumulated dose from chronic exposure. This assessment, in fact, supports the notion that the linear-no-threshold hypothesis is invalid and in need of adjustment (Feinendegen et al. 2011, Tanooka 2011).
REFERENCES
- Abkowitz JL, Catlin SN, Guttorp P. Evidence that hematopoiesis may be a stochastic process in vivo. Nat Med. 1996;2(2):190–197. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- Akleyev A, Kossenko MM, Startsev NV. Techa River population: long-term medical follow-up. Brit J Radiol suppl. 2002;26:32–39. [Google Scholar]
- Akushevich IV, Veremeyeva GA, Dimov GP, Ukraintseva SV, Arbeev KG, Akleyev AV, Yashin AI. Modeling hematopoietic system response caused by chronic exposure to ionizing radiation. Radiat Environ Biophys. 2011;50(2):299–311. doi: 10.1007/s00411-011-0351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, Yang Z, Li M, Kim S, Kovalenko OA, Khorshidi M, Ende N. The effect of human chord blood therapy on the intestinal tract of lethally irradiated mice: possible use for mass casualties. Int J Radiat Biol. 2010;86:467–475. doi: 10.3109/09553000903567987. [DOI] [PubMed] [Google Scholar]
- Baird MC, Hendry JH, Testa NG. Radiosensitivity increases with differentiation status of murine hemopoietic progenitor cells selected during enriched bone marrow subpopulations and recombinant growth factors. Radiat Res. 1990;123:292–298. [PubMed] [Google Scholar]
- Baranov AE. The Russian truck driver radiation accident and other Russian experiences. Brit J Radiol suppl. 2002;26:75–79. [Google Scholar]
- Beagle Dog Tissue Archive 2012. Woloschak Lab http://janus.northwestern.edu/dog_tissues/data.php.
- Bierkens JG, Hendry HJ, Testa NG. Recovery of the proliferative and functional integrity of mouse bone marrow in long-term cultures established after whole-body irradiation at different doses and dose rates. Exp Haematol. 1991;19:81–86. [PubMed] [Google Scholar]
- Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry - standardization of nomenclature. J Nucl Med. 2009;50:477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- Bond VP, Fliedner TM, Archambeau JO, editors. Mammalian Radiation Lethality: A Disturbance in Cellular Kinetics. Academic Press; New York, London: 1965. [Google Scholar]
- Chu-Tse W, Lajtha IJ. Haemopoietic stem-cell kinetics during continuous irradiation. Int J Radiat Biol. 1975;27:41–50. doi: 10.1080/09553007514550041. [DOI] [PubMed] [Google Scholar]
- Cosset JM, Perdereau B, Helfre S, Brixy F, Gongora R, Jammet H. The 1978 Algerian accident: history and 23-year follow-up. Brit J Radiol suppl. 2002;26:40–43. [Google Scholar]
- Ende N, Azzam EI. Consideration for the treatment of mass casualties based on pathology of the fatalities of Hiroshima and Nagasaki. Int J Radiat Biol. 2011;87:443–444. doi: 10.3109/09553002.2011.538129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinendegen LE, Loken MK, Booz J, Muehlensiepen H, Sondhaus CA, Bond VP. Cellular mechanisms of protection and repair induced by radiation exposure and their consequences for cell system responses. Stem Cells. 1995;13(suppl 1):7–20. [PubMed] [Google Scholar]
- Feinendegen LE, Brooks AL, Morgan WF. Biological consequences and health risks of low-level exposure to ionizing radiation: commentary on the workshop. Health Phys. 2011;100:247–59. doi: 10.1097/HP.0b013e31820a83ae. [DOI] [PubMed] [Google Scholar]
- Fliedner TM, Feinendegen LE, Hopewell JW, editors. Chronic irradiation: tolerance and failure in complex biological systems. Brit J Radiol. 2002a;(suppl 26) [Google Scholar]
- Fliedner TM, Graessle D, Paulsen C, Reimers K, Weiss M. The haematopoietic system: determinants of response to chronic ionizing radiation exposure. Brit J Radiol suppl. 2002b;26:247–257. [Google Scholar]
- Fliedner TM, Graessle D, Paulsen C, Reimers K. Structure and function of bone marrow hemopoiesis: mechanisms of response to ionizing radiation exposure. Cancer Biother Radiopharm. 2002c;17:404–426. doi: 10.1089/108497802760363204. [DOI] [PubMed] [Google Scholar]
- Fliedner TM, Chao NJ, Bader JL, Boettger A, Case C, Jr, Chute J, Confer DL, Ganser A, Gorin LC, Gourmelon P, Graessle DH, Krawisz R, Meineke V, Niederwieser D, Port M, Powles R, Sirohi B, Weinstock DM, Wiley A, Coleman CN. Stem cells, multiorgan failure in radiation emergency medical preparedness: A U.S./European consultation workshop. Stem Cells. 2009;27:1205–1211. doi: 10.1002/stem.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz TE, Tolle DV, Doyle DE, Seed TM, Cullen SM. Hematologic responses of beagles exposed continuously to low doses of 60Co-gamma radiation. In: Baum SJ, Ledney GD, Thierfelder S, editors. Experimental hematology today. Karger; Basel: 1982. 1982. pp. 229–240. [Google Scholar]
- Fritz TE, Seed TM, Tolle DV, Lombard LS. Late effects of protracted whole body irradiation by 60Co gamma rays. In: Thompson RC, Mahaffey IA, editors. Life-span radiation effects studies in animals: what can they tell us? Office of Scientific and Technical Information United States Department of Energy; Washington DC: 1986. pp. 116–141. [Google Scholar]
- Fritz TE. The influence of dose, dose rate and radiation quality on the effect of protracted whole body irradiation of beagles. Brit J Radiol suppl. 2002;26:103–111. [Google Scholar]
- Gidali J, Bojtori I, Feher I. Kinetic basis for compensated hempoiesis during continuous irradiation with low doses. Radiat Res. 1979;77:285–291. [PubMed] [Google Scholar]
- Goddu SM, Howell RW, Giuliani DC, Rao DV. Biological dosimetry of bone marrow for incorporated yttrium-90. J Nucl Med. 1998;39:547–552. [PubMed] [Google Scholar]
- Goddu SM, Bishayee A, Bouchet LG, Bolch WE, Rao DV, Howell RW. Marrow toxicity of 33P- versus 32P-orthohosphate: Implications for therapy of bone pain and bone metastases. J Nucl Med. 2000;41:941–951. [PubMed] [Google Scholar]
- Graessle DH. Mathematical modeling of the blood platelet renewal system as an approach to analyzing the effects of chronic irradiation on haematopoiesis. Brit J Radiol suppl. 2002;26:202–207. [Google Scholar]
- IAEA (International Atomic Energy Agency) The Radiological Accident in Lilo. IAEA; Vienna: 2000. [Google Scholar]
- IAEA (International Atomic Energy Agency) Generic Procedures for Medical Response during a Nuclear or Radiological Emergency. IAEA; Vienna: 2005. [Google Scholar]
- ICRU (International Commission on Radiation Units and Measurements) Microdosimetry. Report 36. ICRU; Bethesda, MD, USA: 1983. [Google Scholar]
- ICRU (International Commission on Radiation Units and Measurements) Quantification and Reporting of Low-Dose and other Heterogeneous Exposures. Report 86. ICRU; Bethesda, MD, USA: 2011. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Hill MA, Moore SR. Genomic instability and the role of radiation quality. Radiat Prot Dosimetry. 2006;122:221–227. doi: 10.1093/rpd/ncl445. [DOI] [PubMed] [Google Scholar]
- Kutkov V, Buglova F, McKenna T. Severe deterministic effects of external exposure and intake of radioactive material: basis for emergency response criteria. J Radiol Prot. 2011;3:237–253. doi: 10.1088/0952-4746/31/2/003. [DOI] [PubMed] [Google Scholar]
- Lamerton LF, Pontifex AH, Blackett NH, Adams K. Effects of protracted irradiation on the blood-forming organs of the rat: I. Continuous exposure. Brit J Radiol. 1960;33:287–301. doi: 10.1259/0007-1285-33-389-287. [DOI] [PubMed] [Google Scholar]
- Laukkanen MO, Kuramoto K, Calmels B, Takatoku M, von Kalle C, Donahue RE, Dunbar CE. Low-dose total body irradiation causes clonal fluctuation of primate hematopoietic stem and progenitor cells. Blood. 2005;105(3):1010–1015. doi: 10.1182/blood-2004-04-1498. [DOI] [PubMed] [Google Scholar]
- Martinez RG, Cassab GH, Ganem GG, Guttman EK, Lieberman ML, Vater LB. Observationes sobre la exposicion accidental de una familia a una fuente de cobalto 60. Revista Medica. 1966;(suppl 1):5–60. [Google Scholar]
- Morgan WF, Sowa MB. Non-targeted effects of ionizing radiation: Implications for risk assessment and the radiation dose response profile. Health Phys. 2009;97:426–432. doi: 10.1097/HP.0b013e3181ab98c7. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects and the DNA paradigm: an “out of field” perspective. Mutat Res. 2006;59:5–10. doi: 10.1016/j.mrfmmm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169:515–532. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- Okladnikova ND, Pedsternikova VS. Deterministic effects of occupational exposure to chronic radiation. Brit J Radiol. 2002;(suppl 26):26–31. [Google Scholar]
- Potten CS, Hendry JJ. Radiation and Gut. Elsevier; Amsterdam: 1995. [Google Scholar]
- Scott BR. Proposed estimates of the probability of inducing pulmonary injury sufficient to cause death from radiation pneumonitis and pulmonary fibrosis after briefly inhaling a mixture of insoluble β-emitting particles. Health Phys. 1980;38:635–642. doi: 10.1097/00004032-198004000-00011. [DOI] [PubMed] [Google Scholar]
- Scott BR, Hahn FF, McClellan RO, Seiler FA. Risk estimates for radiation-induced bone marrow syndrome lethality in humans. Risk Anal. 1998;8:393–402. doi: 10.1111/j.1539-6924.1988.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Seed TM, Meyers SM. Chronic radiation-induced alterations in hematopoietic repair during preclinical phases of aplastic anemia and myeloproliferation disease: assessing unscheduled DNA synthesis capacity. Cancer Res. 1993;53:4518–4527. [PubMed] [Google Scholar]
- Tanooka H. Meta-analysis on non-tumour doses for radiation-induced cancer on the basis of dose-rate. Int J Radiat Biol. 2011;87:645–652. doi: 10.3109/09553002.2010.545862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa NG, Hendry JH, Lajtha IG. The response of mouse haemopoietic colony formers to acute or continuous gamma irradiation. Biomedicine. 1973;19:183–186. [PubMed] [Google Scholar]
- Valverde NJ, Oliveira AR, Curado MP. Protracted irradiation and the acute radiation syndrome in the Goiânia accident. Brit J Radiol. 2002;(suppl 26):63–70. [Google Scholar]
- Vôsumaa E. The Estonian accident. Brit J Radiol. 2002;(suppl 26):71–74. [Google Scholar]
- Wang G, Mong S, Huang S, Chen Z, Cheng X, Ye G. Medical observation of a case of sub-acute radiation sickness transformed into acute myelogeneous leukemia. Chin J Radiation Med Prot. 1996;16:49–50. [Google Scholar]
- Wang JC, Lin YP, Hwang S, Hsieh WA, Chen JC, Tang JL, Hwang JS, Lu WJ, Chang WP. Late haematological changes in a population exposed to chronic low dose-rate radiation during childhood. Brit J Radiol. 2002;(suppl 26):44–49. [Google Scholar]