Abstract
Herein, we report about two Caucasian patients with the histopathological diagnosis of Merkel cell carcinoma suffering from extensive lymph node metastases. The extent of the disease was diagnosed by Ga-68-DotaTATE-PET-CT. Both patients had rapid disease progression, one of them despite a three months course of sunitinibe followed by four chemotherapy cycles of cisplatin and etoposide. Both patients were sent for peptide receptor radiotherapy with 90Y-DotaTATE or 177Lu-DotaTATE in combination with capecitabine. Additional external beam radiotherapy of the cervical and inguinal lymph nodes was given to the patient with progressive disease despite chemotherapy. Temporary partial response in both patients was achieved. Despite extensive therapeutic efforts, fatal outcome could not be prevented 10 and 14 months after first clinical symptoms.
Keywords: Merkel cell carcinoma (MCC), PET-CT, 68Ga-DotaTATE, 90Y-DotaTATE, 177Lu-DotaTATE, Peptide Receptor Radiotherapy (PRRT), Theranostic
Merkel cell carcinoma (MCC) is a rare, highly malignant neuroendocrine tumour of the skin which was first described by Toker in 1972 [1]. The most widely accepted origin of MCC is the Merkel cell functioning as cutaneous mechanoreceptor. The Merkel cell arises from the epidermis by an Atoh1-dependent mechanism [2,3]. In adults, Merkel cells undergo slow turnover and are replaced by cells originating from epidermal stem cells [3]. Other authors favour that MCC has its origin in immature, totipotent stem cells acquiring neuroendocrine characteristics during malignant transformation [4]. MCC occurs predominantly in Caucasians over 65 years of age with no definite gender predilection [5]. As MCC is often asymptomatic in the early course of the disease diagnosis may be delayed until the detection of regional lymphatic nodal spread or distant metastases. Due to the rarity of the disease imaging findings have been reported only in case series [6-8]. Imaging studies have a limited role in diagnosis of the clinically evident primary skin lesions, and the definite diagnosis of MCC is exclusively pathologic. However, imaging may be helpful in the assessment of the depth of the invasion and it is crucial in the evaluation of regional and distant metastatic disease. Thus, imaging has an impact on staging, surgical guidance, further oncological management and follow-up [5,8]. Due to its neuroendocrine tumour characteristics locoregional recurrence and distant metastases can be evaluated with somatostatin receptor scintigraphy [8,9] or Ga-68-Dota-labelled peptides [10]. FDG-PET / FDG-PET-CT has been reported to allow staging and detection of recurrent disease but was also reported to be false negative in one patient with a weakly proliferative nodal MCC (Ki-67 < 10%) [11-13]. FDG-PET-CT led to significant changes in disease staging and management in 46% of patients compared with clinical examination alone [14]. Treatment consists of surgery and palliative radiation therapy. Furthermore, various chemotherapy regimens are available without consensus about the most adequate therapeutic strategy. In an advanced stage, prognosis is usually poor with an average interval of eight months between diagnosis and death [15].
The first patient was a 76-year-old Caucasian women with MCC originating from the right pharyngeal tonsil. 68Ga-DotaTATE PET-CT (Figure 1) demonstrated uptake in the primary tumour and extensive cervical and right axillary lymph node metastases and histopathologically confirmed metastatic involvement of the head of the pancreas. In addition, the patient had a known meningioma. Three months passed between initial symptoms with enlarged cervical lymph nodes and histopathological diagnosis (Figure 2). Two courses of peptide receptor radiotherapy with cumulative 10 GBq 90Y-DotaTATE in combination with capecitabine led to a disease stabilisation with a good quality of life. Despite all therapeutic efforts, disease progression occurred six months after histopathological diagnosis of the MCC being fatal within further four weeks.
Figure 1.
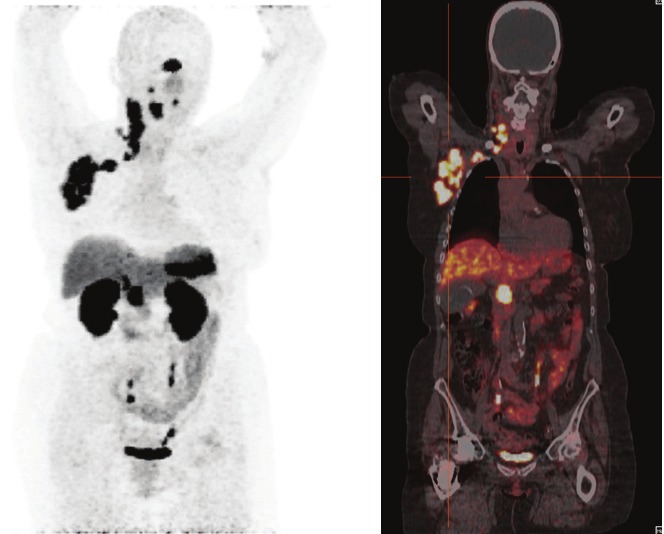
68Ga-DotaTATE PET-CT of a 76-year-old women demonstrating extensive pathologic foci in the right pharyngeal tonsil, and right cervical and right axillary lymph node metastases, and histopathologically confirmed metastatic involvement of the head of the pancreas. In addition, intense uptake in a meningeoma can be noted.
Figure 2.
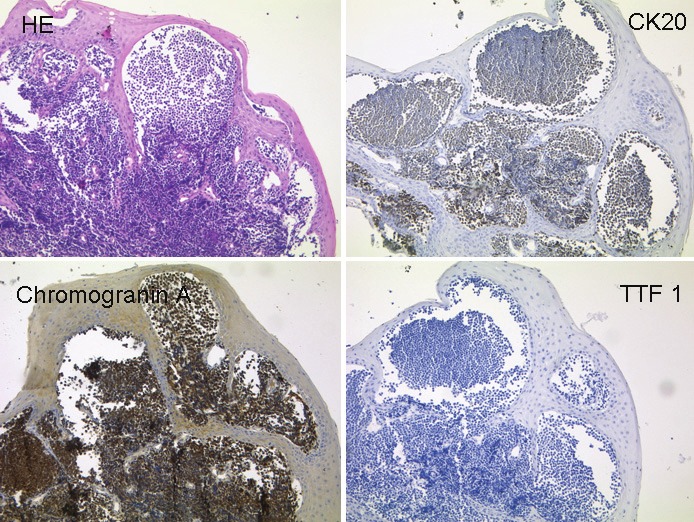
The histological section of a resected specimen of the right pharyngeal tonsil shows infiltrates of a smallblue cell invasive tumour (A, HE). Tumour cells express the epithelial marker Cytokeratin 20 (B), the neuroendocrinemarker Chromogranin A (C) but not the neuroendocrine marker TTF1 (D) (all images x200). The tumour cells show thetypical immunohistochemical pattern of a Merkel cell tumour.
The second patient was a 67-year-old Caucasian man with cervical, abdominal and inguinal lymph node masses from MCC. Disease progression occurred despite a three months course of sunitinib followed by four chemotherapy cycles of cisplatin and etoposide. Thus, two courses of peptide receptor radiotherapy with cumulative 14 GBq 177Lu-DotaTATE were given, the first cycle in addition with capecitabine. Additional external beam radiation of the cervical and inguinal lymph nodes resulted in temporary disease regression (Figure 3A and B), most prominent in the left cervical and left inguinal region that had received external beam radiation in addition the peptide receptor radiotherapy. The patient died 11 months after histopathological diagnosis of MCC. 68Ga-DotaTATE PET-CT images were made after completion of chemotherapy and before the first PRRT cycle with capecitabine and three months later after additional external beam radiation of cervical and inguinal lymph nodes demonstrating partial response.
Figure 3.
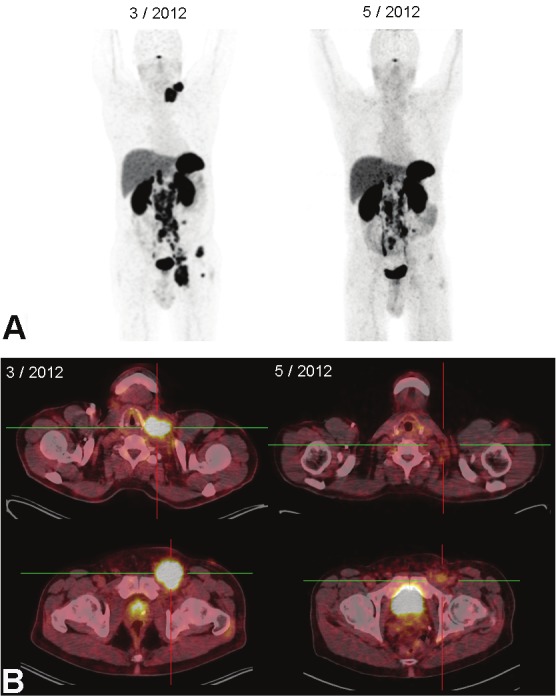
68Ga-DotaTATE PET-CT of a 67-year-old man demonstrating extensive disease with left cervical, abdominal and left inguinal lymph nodes before PRRT (left images) and after the first cycle with 7.2 GBq 177Lu-DotaTATE with capecitabine and external beam radiation of cervical and inguinal lymph nodes (50,4 Gy) (right images).
To our knowledge, this is the first report about two patients with MCC in whom the extent of the disease was diagnosed by 68Ga-DotaTATE PET-CT resulting in subsequent peptide receptor radiotherapy with DotaTATE, radiolabeled with 90Y- or 177Lu, in combination with capecitabine, exemplifying a “theranostic” strategy. Despite extensive therapeutic efforts, fatal outcome could not be prevented 10 and 14 months after first clinical symptoms with enlarged lymph nodes.
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110. [PubMed] [Google Scholar]
- 2.Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978;42:2311–2321. doi: 10.1002/1097-0142(197811)42:5<2311::aid-cncr2820420531>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, Szpalski C, Achouri Y, Bloch W, Hassan BA, Blanpain C. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoefler H, Kerl H, Rauch HJ, Denk H. New immunocytochemical observations with diagnostic significance in cutaneous neuroendocrine carcinoma. Am J Dermatopathol. 1984;6:525–530. doi: 10.1097/00000372-198412000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JH, Rumball E. Merkel cell tumour: clinical behaviour and treatment. Br J Surg. 1991;78:138–142. doi: 10.1002/bjs.1800780205. [DOI] [PubMed] [Google Scholar]
- 6.Eftekhari F, Wallace S, Silva EG, Lenzi R. Merkel cell carcinoma of the skin: imaging and clinical features in 93 cases. Br J Radiol. 1996;69:226–233. doi: 10.1259/0007-1285-69-819-226. [DOI] [PubMed] [Google Scholar]
- 7.Gollub MJ, Gruen DR, Dershaw DD. Merkel cell carcinoma: CT findings in 12 patients. AJR Am J Roentgenol. 1996;167:617–620. doi: 10.2214/ajr.167.3.8751663. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen BD, McCullough AE. Imaging of Merkel cell carcinoma. Radiographics. 2002;22:367–376. doi: 10.1148/radiographics.22.2.g02mr14367. [DOI] [PubMed] [Google Scholar]
- 9.Kwekkeboom DJ, Hoff AM, Lamberts SW, Oei HY, Krenning EP. Somatostatin analogue scintigraphy. A simple and sensitive method for the in vivo visualization of Merkel cell tumors and their metastases. Arch Dermatol. 1992;128:818–821. doi: 10.1001/archderm.128.6.818. [DOI] [PubMed] [Google Scholar]
- 10.Schneider C, Schlaak M, Bludau M, Markiefka B, Schmidt M. Ga-68-Dotatate-PET-CT positive metastatic lymph node in a 69-year-old woman with Merkel Cell Carcinoma. Clin Nucl Med. 2012;37:1108–1111. doi: 10.1097/RLU.0b013e318266d3b3. [DOI] [PubMed] [Google Scholar]
- 11.Belhocine T, Pierard GE, Frühling J, Letesson G, Bolle S, Hustinx R, Dargent JL, Flamen P, Rigo P. Clinical added-value of 18FDG PET in neuroendocrine-merkel cell carcinoma. Oncol Rep. 2006;16:347–352. [PubMed] [Google Scholar]
- 12.Concannon R, Larcos GS, Veness M. The impact of (18)F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: results from Westmead Hospital, Sydney, Australia. J Am Acad Dermatol. 2010 Jan;62:76–84. doi: 10.1016/j.jaad.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Peloschek P, Novotny C, Mueller-Mang C, Weber M, Sailer J, Dawid M, Czerny C, Dudczak R, Kletter K, Becherer A. Diagnostic imaging in Merkel cell carcinoma: lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur J Radiol. 2010;73:317–323. doi: 10.1016/j.ejrad.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Maury G, Dereure O, Du-Thanh A, Mariano-Goulart D, Guillot B. Interest of (18)F-FDG PET-CT scanning for staging and management of merkel cell carcinoma: a retrospective study of 15 patients. J Eur Acad Dermatol Venereol. 2011;25:1420–1427. doi: 10.1111/j.1468-3083.2011.03994.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith DF, Messina JL, Perrott R, Berman CG, Reintgen DS, Cruse CW, Glass FL, Fenske NA, DeConti RC, Trotti A 3rd. Clinical approach to neuroendocrine carcinoma of the skin (Merkel cell carcinoma) Cancer Control. 2000;7:72–83. [PubMed] [Google Scholar]


