Abstract
Objective
Phenylketonuria (PKU) is a hereditary metabolic disorder that often results in neuropsychological impairment, even in individuals treated early and continuously. This study was conducted to examine processing speed, variability in processing speed, and the relationship between processing speed variables and executive abilities in children with early- and continuously-treated PKU.
Method
Participants were 42 children with PKU and 81 typically-developing children from 7 to 18 years of age. Children completed three computerized reaction time (RT) tasks (simple reaction time, go/no-go, stimulus-response compatibility) and seven tasks assessing executive abilities (working memory, inhibitory control, strategic processing).
Results
Performance of children with PKU was significantly slower and more variable than that of controls across the three tasks administered. When age was considered, it was shown that processing speed improved with age to a comparable degree for both groups. Variability in processing speed, however, decreased more with age for the PKU than control group, reflecting the fact that variability in younger, but not older, children with PKU was greater than that of controls. With regard to executive abilities, processing speed and variability contributed to performance on most, but not all, executive tasks; and after controlling for processing speed and variability, executive impairments were still identified in working memory and inhibitory control (not strategic processing).
Conclusions
These findings indicate that information processing is slower and less efficient in children with PKU. In addition, processing speed and variability contribute to some, but not all, of the impairments in executive abilities observed in children with PKU.
Keywords: Phenylketonuria, reaction time, processing speed, executive, variability
Phenylketonuria (PKU) is a hereditary disorder characterized by the inefficient metabolism of the amino acid phenylalanine (Phe) due to abnormalities in the phenylalanine hydroxylase enzyme. Untreated PKU results in neurological abnormalities (Moyle, Fox, Bynevelt, Arthur, & Burnett, 2007) and intellectual disability (de Groot, Hoeksma, Blau, Reijngoud, & van Spronsen, 2010; Paine, 1957). Even with early and continuous dietary treatment to limit Phe intake, PKU is associated with slightly lower than expected intelligence (for a review, Brumm & Grant, 2010) and deficits in specific aspects of cognition such as executive abilities and processing speed (for reviews, Albrecht, Garbade, & Burgard, 2009; Christ, Huijbregts, de Sonneville, & White, 2010; DeRoche & Welsh, 2008; Janzen & Nguyen, 2010).
Two primary neural mechanisms are hypothesized to underlie brain dysfunction and subsequent neuropsychological impairment in individuals with PKU: dopamine deficiency and white matter abnormalities. Turning first to dopamine, this essential neurotransmitter is synthesized from the amino acid tyrosine (Tyr), of which Phe is a precursor (for an overview, de Groot et al., 2010). Because Phe is not properly metabolized, Tyr production is limited, which in turn limits dopamine synthesis. In addition, Tyr and Phe are among the large neutral amino acids (LNAAs) that competitively bind to the large neutral amino acid type 1 (LAT1)-transporter for passage across the blood-brain barrier. Compared with other LNAAs, Phe binds more strongly with the LAT1-transporter. As a result, high blood Phe impedes the transport of available Tyr across the blood-brain barrier, which further limits dopamine synthesis (de Groot et al., 2010).
Dopamine deficiency has long been viewed as the primary neural mechanism underlying brain dysfunction and impaired cognition in individuals with PKU. In recent years, however, increasing evidence has emerged suggesting that white matter abnormalities also play a role. Neuroimaging studies have identified white matter abnormalities even in individuals with early-and continuously-treated PKU (Anderson et al., 2004, 2007; Anderson & Leuzzi, 2010; White et al., 2010), with the prevalence and severity of these abnormalities increasing at higher Phe levels (Anderson et al., 2004; Anderson & Leuzzi, 2010).
Of particular relevance to the current investigation, it is probable that the white matter abnormalities associated with PKU result in slowed information processing due to disruptions in the speed with which neural signals are transmitted. Indeed, slowed information processing is a common finding in studies of individuals with PKU (Anderson et al., 2007; Channon, Mockler, & Lee, 2005; Feldmann, Denecke, Grenzebach, & Weglage, 2005; Moyle, Fox, Arthur, Bynevelt, & Burnett, 2007; Moyle et al., 2007b; for a meta-analysis, Albrecht et al., 2009), and PKU-related white matter abnormalities are associated with slowed performance across a range of cognitive tasks (Anderson et al., 2004; Anderson et al., 2007). It is also possible that white matter abnormalities and slowed information processing contribute to the impairments in executive abilities observed in individuals with PKU due to inefficiencies in the interconnections between prefrontal cortex and posterior brain regions (Anderson & Leuzzi, 2010). As pointed out by Salthouse, slowed processing may result in “impairments of higher order processes such as abstraction, elaboration, or integration, because not all of the relevant information will be available in a usable form when it is needed” (Salthouse, 1996, p. 406).
In the current study, processing speed and variability in processing speed were examined, as well as the relationship of each with executive abilities, in children with early- and continuously-treated PKU. In typically-developing children, information processing speed increases with age (Conners, Epstein, Angold, & Klaric, 2003; Nettelbeck & Burns, 2010), and faster processing has been associated with developmental improvements in executive abilities including working memory (Salthouse & Babcock, 1991; Salthouse, 1992; Zanto, Toy, & Gazzaley, 2010), inhibitory control (Bugg, DeLosh, Davalos, & Davis, 2007), and strategic processing (Imbo & Vandierendonck, 2007). To our knowledge, however, research has not been conducted with a focus of either variability in processing speed or the relationship between processing speed and executive abilities in children with PKU.
To address these issues, RT and variability in RT were evaluated using three speeded tasks (simple reaction time, go/no-go, stimulus-response compatibility) in children with PKU and typically-developing control children. RTs from three tasks were used to ensure that processing speed findings were not due to the particularities of a single task. Executive performance on tasks assessing working memory, strategic processing, and inhibitory control was also examined in relation to RT and variability in RT. By assessing children across a broad age range (i.e., 7 to 18 years of age), it was possible to explore possible differential effects of age on RT, variability in RT, and relationships with executive abilities across our PKU and control groups.
We also investigated the relationships between Phe levels, processing speed variables, and executive variables. Some researchers have identified significant negative relationships between Phe and cognition (e.g., Weglage et al., 1996; Diamond et al., 1997), whereas others have not (Mazzocco et al., 1994; Anderson et al., 2004). Reflecting this inconsistency, previous findings from our laboratory (e.g., White et al., 2002; Christ et al., 2006) have been mixed in this regard, possibly due to the restricted range of Phe in our samples of early- and continuously-treated children with PKU. Given that the current study was also conducted with early- and continuously-treated children, we expected similar mixed results. That is not to say that we consider control of Phe to be unimportant. Rather, we expected that there might simply be too little variability in Phe to detect statistically significant correlations in a consistent manner.
Method
Participants
A total of 42 children (23 girls, 19 boys) with PKU were recruited through the Division of Medical Genetics at St. Louis Children’s Hospital in Missouri and the Metabolic Clinic at the Child Development and Rehabilitation Center at Doernbecher Children’s Hospital in Portland, Oregon. All children were diagnosed with PKU soon after birth and were treated early and continuously using dietary treatment to limit Phe intake. Blood Phe was not available for three children due to missing values in medical records. Blood Phe obtained closest to the time of participation in the study (typically the same day) was available for 39 children and ranged from 121 to 1574 μmol/L (M = 546, SD = 331). Average blood Phe obtained within the year prior to participation in the study was available for 38 children (for one child only one Phe level was obtained within the year prior to study) and ranged from 176 to 1124 μmol/L (M = 495, SD = 264).
The performance of children with PKU was compared with that of 81 typically-developing control children (42 girls, 39 boys) recruited from the St. Louis and Portland communities. The only exception was that two children in the control group failed to complete a verbal fluency task. No child had a history of major medical (e.g., head injury with loss of consciousness, diabetes) or learning disorder (e.g., dyslexia) unrelated to PKU, and no child was taking sapropterin dihydrochloride or medication for ADHD at the time of study.
Years of age for children in the PKU (M = 11.8, SD = 3.5) and control (M = 12.3, SD = 3.2) groups ranged from 7 to 18. Years of education ranged from 1 to 13 for the PKU group (M = 6.0, SD = 3.4) and 1 to 14 for the control group (M = 6.3, SD = 3.3). There was no significant difference in age or education between the groups (p > .05 in both instances). General intellectual ability was estimated using the Vocabulary and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (Psychological Corporation, 1999). Estimated IQ for the PKU group ranged from 83 to 139 (M = 106.0, SD = 10.9), whereas estimated IQ for the control group ranged from 82 to 143 (M = 114.6, SD = 13.9). The IQ of the control group was significantly higher than that of the PKU group [t(121) = 3.51, p < .001].
To determine whether IQ should be controlled in conducting statistical analyses, we examined the relationships between Vocabulary scores and processing speed variables. (Due to the nonverbal nature of Matrix Reasoning and processing speed tasks and likely colinearity, relationships between Matrix Reasoning scores and processing speed variables were not examined). There were no significant correlations between Vocabulary scores and processing speed variables for either the control (rs ranged from −.16 to .09) or PKU (rs ranged from −.30 to −.10) groups. Given the lack of statically significant correlations, IQ was not controlled in statistical analyses.
Procedure
Approval for this study was obtained from institutional review boards on the protection of human subjects at Washington University and Oregon Health & Sciences University. Written informed consent was obtained for all participants prior to enrollment and completion of study procedures. A battery of tasks lasting 1.5 hours was administered in a standard order during a single session. All children were tested by a research coordinator in a quiet room, free of distractions, at either Washington University or Oregon Health & Sciences University.
Processing Speed
Processing speed was assessed using variables selected from three speeded tasks: simple RT, go/no-go, and stimulus-response compatibility. Data from the go/no-go task have been reported elsewhere (Araujo et al., 2009; Christ et al., 2006), but response monitoring and inhibitory control were the foci of previous studies. In the current study, we focused on basic processing speed by examining data from the control conditions of each speeded task. All tasks were presented on a computer monitor, and responses to stimuli were made via manual key press. For each task, intra-individual RT mean and RT standard deviation (SD) served as dependent variables to permit examination of average processing speed and variability in processing speed, respectively.
Simple RT Task
For the simple RT task, children were instructed to respond to a centrally-positioned cross by pressing a response key as rapidly as possible using their dominant hand. To discourage anticipatory responding, the inter-trial interval varied randomly from 700 to 2500 ms. If children responded in less than 100 ms after stimulus presentation (anticipatory error), a brief tone followed by the message “Too quick” were presented. If children failed to respond within 1500 ms (omission error), a brief tone followed by the message “Too slow” were presented. Following 10 practice trials, children completed 40 experimental trials. Response speed and accuracy were recorded for each trial. RT mean and RT SD for correct trials were included in processing speed analyses.
Go/No-Go Task
On each trial of this task, one of four shapes (square, triangle, diamond, circle) appeared at the center of the monitor, with an inter-trial interval of 2,000 ms. At the beginning of the task, one shape was designated as the non-target, with the designated shape counterbalanced across children. Children were instructed to press a centrally-positioned response key as quickly as possible when any shape other than the non-target shape appeared (go trials; processing speed condition) and to withhold their response when the non-target shape appeared (no-go trials; inhibitory control condition). Non-targets were randomly presented on 25% of the trials. If children responded in less than 100 ms after presentation of a target or non-target (anticipatory error), a brief tone and the message “Early response” were presented. If children failed to respond within 1,500 ms of presentation of a target (omission error), a tone and the message “Too slow” were presented. If children responded to a non-target (commission error), a brief tone and the message “No response needed” were presented. Children completed 20 practice trials followed by 200 experimental trials. Response speed and accuracy were recorded for each trial. RT mean and RT SD for correct go trials were included in processing speed analyses.
Stimulus-Response Compatibility Task
At the beginning of each trial of this task, an array of three horizontally-aligned circles appeared on the monitor. After 300 ms, the middle circle brightened (i.e., filled with color) for 500 ms. After 300 ms, one of the peripheral circles brightened. Two experimental conditions were administered: response compatible (processing speed condition) and response incompatible (inhibitory control condition). In the compatible condition, children were instructed to press a left response key when the left circle brightened and a right response key when the right circle brightened. In the incompatible condition, children were instructed to press the left response key when the right circle brightened and the right response key when the left circle brightened. The circles remained on the screen for 3,000 ms or until children completed a response. After a response was made, or following 3,000 ms, the circles disappeared and there was a blank inter-trial interval of 2,000 ms. Children completed practice blocks of 10 compatible trials followed by 10 incompatible trials and were given feedback regarding the accuracy of each response (“Correct” or “Wrong Response” appeared on the monitor). Following practice, children completed 96 experimental trials (with no feedback regarding accuracy) during which the two conditions were presented in alternating blocks of 16 trials each. At the beginning of each block, children were instructed to press a key on either the same or opposite side as the peripheral circle that brightened. On each trial, left and right circles were equally likely to brighten. Response speed and accuracy were recorded for each trial. RT mean and RT SD for correct compatible trials were included in processing speed analyses.
Executive Abilities
Tasks assessing working memory, inhibitory control, and strategic processing were administered to evaluate the relationship between processing speed and executive abilities.
Working Memory
Digit span task
The digit span subtest from the Children’s Memory Scale (Cohen, 1997) was administered to assess simple storage and manipulation in working memory. In the digit span forward condition, children repeated series of orally-presented digits in the order of presentation (e.g., 8-2-6-9). In the digit span backward condition, children repeated series of digits in reverse order (e.g., 9-6-2-8). Raw scores for digit span forward and backward were included in analyses.
2-back task
Two conditions were presented: letter and location. In both conditions, children observed one of eight letters (C, F, H, J, N, P, Q, S) positioned at one of eight locations along an imaginary circle that was eccentric to central fixation (+). In the location condition, children were asked to press a target button when any letter appeared in the same location as two trials ago (regardless of letter identity) and to press a non-target button otherwise. In the letter condition, children were asked to press a target button when a letter appeared that was identical to the letter presented two trials ago (regardless of location) and to press a non-target button otherwise. In both conditions, stimuli remained on the screen for 2,500 ms, with an inter-trial interval of 1,000 ms. Children heard a “beep” following correct responses and a “bloop” following incorrect responses. Children completed a practice block of 24 trials followed by 96 experimental trials. The condition presented first was counterbalanced across children. Response speed and accuracy were recorded for each trial. For purposes of the current study, number of commission errors (i.e., incorrect responses for non-target trials) for each condition was included in analyses.
Recognition span task
Two conditions were presented: shape and location. Children observed as a series of 12 shapes (e.g., star, oval, cross) appeared one at a time in one of 12 locations on a 3 × 4 grid. Depending on the condition, children were asked to remember either the shapes or the grid locations in the order presented. After each series was presented, children observed a screen with all possible shapes and locations presented and were asked to point to the items in the order of presentation. The maximum number of items recalled in correct serial order for each condition was included in analyses to assess working memory.
Inhibitory Control
Go/no-go task
This task was described in detail earlier. To assess inhibitory control, the number of incorrect responses occurring during the no-go condition (i.e., commission errors) was included in analyses.
Strategic Processing
California Verbal Learning Test – Children’s Version (CVLT-C)
During the CVLT-C (Delis, Kramer, Kaplan, & Ober, 1994), children were instructed to recall a list of 15 words from 3 semantic categories (i.e., toys, clothing, fruits) that were presented orally over 5 repeated learning trials. To assess strategic processing, the number of words reported within semantic clusters on the fifth learning trial was included in analyses.
Verbal fluency
We also examined performance on the food/drink condition of the Verbal Fluency subtest from the NEPSY (Korkman, Kirk, & Kemp, 1998) to assess strategic processing. An in-depth description of results from this task in children with PKU is presented elsewhere (Banerjee, Grange, Steiner, & White, 2010). Briefly, children were instructed to name as many words that fall into the category of food or drink as quickly as possible for 60 seconds. For purposes of the current study, the total number of words correctly reported was included in analyses.
Results
Effects of Group on Processing Speed
To examine the effects of group on processing speed and variability in processing speed (see Table 1), we conducted two repeated measures analyses of variance (ANOVA), with RT mean and RT SD serving as dependent variables in separate analyses. In both analyses, task (simple RT, go/no-go, stimulus-response compatibility) served as the within-subjects factor, and group (PKU, control) served as the between-subjects factor.
Table 1.
Means and standard deviations for processing speed variables (ms).
| Variable | Control
|
PKU
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean
|
Standard Deviation
|
Mean
|
Standard Deviation
|
|||||
| M | SD | M | SD | M | SD | M | SD | |
|
|
|
|
|
|
||||
| Simple Reaction Time | 383 | 80 | 94 | 48 | 435* | 103 | 138* | 81 |
| Go condition of Go/No-Go | 587 | 134 | 145 | 57 | 642* | 138 | 183* | 77 |
| Compatible condition of Stimulus- Response Compatibility | 479 | 175 | 155 | 127 | 565* | 162 | 199 | 130 |
Note:
indicates significantly slower or more variable performance for the PKU than control group.
For RT mean, results revealed a significant main effect of task [F(2, 242) = 157.05, p < .001, ηp2 = .57]. Paired sample t-tests revealed faster performance on the simple RT task than either the go/no-go [t(122) = −23.21, p < .001] or stimulus-response compatibility [t(122) = −8.96, p < .001] tasks and faster performance on the stimulus-response compatibility task than the go/no-go task [t(122) = −8.14, p < .001]. There was also a significant main effect of group [F(1, 121) = 8.52, p < .005, ηp2 = .07], with slower performance for the PKU than control group. The interaction between task and group was not significant, indicating that processing speed was generally slower for the PKU than control group across the tasks administered.
For RT SD, results again revealed a significant main effect of task [F(2, 242) = 27.09, p < .001, ηp2 = .18]. Paired sample t-tests revealed less variable performance on the simple RT task than either the stimulus-response compatibility [t(122) = −6.20, p < .001] or go/no-go [t(122) = −9.56, p < .001] tasks. There was also a significant main effect of group [F(1, 121) = 9.06, p < .005, ηp2 = .07], with more variable performance for the PKU than control group. The interaction between task and group was not significant, indicating that variability in processing speed was generally greater for the PKU than control group across the tasks administered.
Clinical Significance of Group Differences in Processing Speed
The clinical significance of differences in processing speed and variability in processing speed was also examined. Because there was no interaction between task and group, composite z scores were computed for RT mean and RT SD across the three tasks administered. The mean and SD of the RT mean and RT SD of the control group were first calculated for each task. Next, z scores based on these values were computed for each child on each task. Finally, z scores for each child on each task were averaged to obtain RT mean and RT SD composite z scores.
T-tests revealed significant between-group differences in RT mean [t(121) = −3.00, p < .005] and RT SD [t(121) = −3.46, p < .001] composite z scores. Scores for the PKU group [RT mean = .52; RT SD = .63] were significantly higher than those of the control group, indicating slower and more variable performance. Thus, processing speed and variability in processing speed were at least one-half SD poorer for the PKU than control group.
Relationships between Age and Processing Speed
In previous studies we identified differential effects of age on strategic processing (White et al., 2001) and working memory (White et al., 2002) across PKU and control groups, suggesting greater impairment as a function of increasing age in children with PKU. To examine the relationships between age and processing speed in the current study, separate hierarchical linear regression analyses were conducted using the composite z scores for RT mean and RT SD noted earlier. In both regressions, age was entered in the first step, group was entered in the second step, and the interaction between age and group was entered in the final step. Analyses were also conducted to examine possible quadratic effects of age, but in no analysis did age2 account for significant variance beyond that attributable to age; as such, age2 was removed from the results reported here.
For RT mean, age accounted for a significant proportion of the variance in the composite z score [R2 = .37, F(1, 121) = 70.11, p < .001], with faster performance as a function of increasing age. As expected based on findings from the ANOVA examining group effects, group accounted for additional variance beyond that attributable to age [ΔR2 = .05, ΔF(1, 120) = 9.82, p < .005], with slower performance for the PKU than control group. The interaction between age and group did not account for a significant proportion of the variance beyond that attributable to age and group.
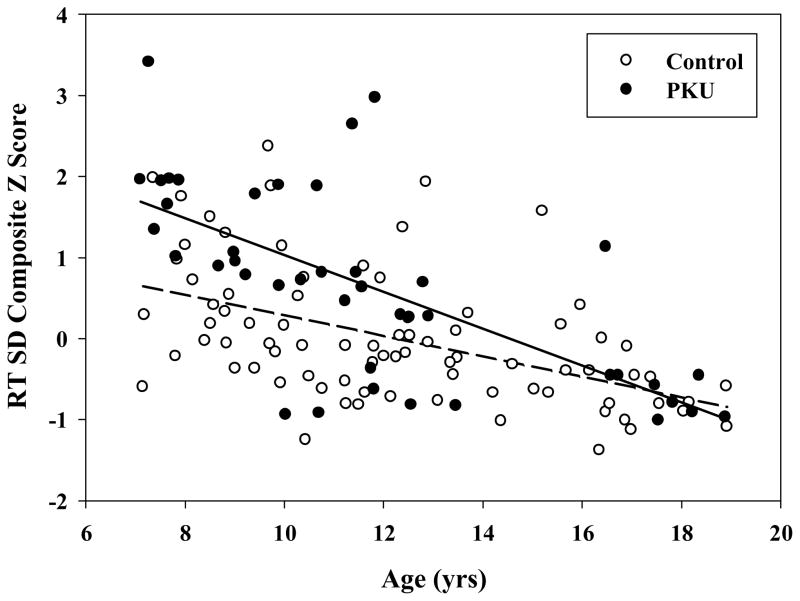
For RT SD, age again accounted for a significant proportion of the variance in the composite z score [R2 = .32, F(1, 121) = 56.40, p < .001], with less variable performance as a function of increasing age. As expected based on findings from the ANOVA examining group effects, group accounted for additional variance beyond that attributable to age [ΔR2 = .07, ΔF(1, 120) = 13.08, p < .001], with more variable performance for the PKU than control group. The interaction between age and group was also significant [ΔR2 = .03, ΔF(1, 119) = 5.26, p < .05]. As shown in Figure 1, although variability decreased as age increased for both the PKU [R2 = .46, F(1, 40) = 33.61, p < .001] and control [R2 = .24, F(1, 79) = 25.43, p < .001] groups, at younger ages variability in performance was greater for the PKU than control group. In terms of clinical significance, variability in processing speed was approximately 1 SD poorer for the PKU than control group at the youngest ages, although variability was comparable at the oldest ages.
Figure 1.
Effect of age on RT SD z-score composite for PKU and control groups.
Relationships between Phe Levels and Processing Speed
We next examined the relationships between Phe levels and processing speed and variability in processing speed in children with PKU. Four separate hierarchical linear regression analyses were conducted using the composite z scores for RT mean and RT SD as dependent variables and Phe closest to time of testing and average Phe over the past year as independent variables. Because Phe closest to time of testing [r = .52, p < .001] and average Phe over the past year [r = .63, p < .001] were significantly correlated with age, age was entered in the first step of all analyses. Phe level was entered in the second step, and the interaction between age and Phe level was entered in the final step.
For RT mean in the analysis examining the possible contribution of Phe closest to time of testing, as expected, age accounted for a significant proportion of the variance in the composite z score [R2 = .57, F(1, 37) = 49.25, p < .001], with faster performance as a function of increasing age. Neither Phe closest to time of testing nor the interaction between age and Phe closest to time of testing accounted for additional variance. Similarly, in the analysis examining the possible contribution of average Phe over the past year, age accounted for a significant proportion of the variance in the composite z score [R2 = .55, F(1, 36) = 43.97, p < .001], but neither average Phe over the past year nor the interaction between age and average Phe over the past year accounted for additional variance.
For RT SD, in the analysis examining the possible contribution of Phe closest to time of testing, again as expected, age accounted for a significant proportion of the variance in the composite z score [R2 = .49, F(1, 37) = 35.33, p < .001], with less variable performance as a function of increasing age. Neither Phe closest to time of testing nor the interaction between age and Phe closest to time of testing accounted for additional variance. Similarly, in the analysis examining the possible contribution of average Phe over the past year, age accounted for a significant proportion of the variance in the composite z score [R2 = .48, F(1, 36) = 32.98, p < .001]. In contrast with findings from other analyses, however, average Phe over the past year accounted for additional variance beyond that attributable to age [ΔR2 = .09, ΔF(1, 35) = 7.43, p < .01], indicating that higher Phe levels are associated with greater variability in processing speed. The interaction between age and average Phe over the past year did not account for additional variance.
Relationships between Processing Speed and Executive Abilities
We examined the relationship between processing speed and four measures of working memory (i.e., digit span forward, digit span backward, the averaged number of commission errors in the spatial and letter conditions of the 2-back task, and the averaged maximum number of items recalled in correct serial order in the shape and location conditions of the recognition span task). Conditions of the 2-back and recognition span tasks were combined because there was no significant interaction between group and condition for either task in repeated measures ANOVAs for each task. We also examined the relationship between processing speed and one measure of inhibitory control (i.e., number of commission errors in the no-go condition of the go/no-go task) and two measures of strategic processing (i.e., number of semantically clustered words on the fifth learning trial of the CVLT-C and number of words correctly reported on the food/drink verbal fluency task).
Separate hierarchical linear regression analyses were conducted for each measure. In all analyses, age was entered in the first step, composite z score for either RT mean or RT SD was entered in the second step (separate analyses were conducted for each of these processing speed variables), group was entered in the third step, the interaction between age and composite z score for either RT mean or RT SD was entered in the fourth step, the interaction between age and group was entered in the fifth step, the interaction between composite z score for either RT mean or RT SD and group was entered in the sixth step, and the interaction between age, composite z score for either RT mean or RT SD, and group was entered in the seventh step. In no analysis did interactions between age and group, between composite z score for either RT mean or RT SD and group, or between age, composite z score for either RT mean or RT SD, and group account for significant variance beyond that attributable to other variables entered in the models. As such, results from these interactions are not discussed further.
Working Memory
Digit span task
Age accounted for a significant proportion of the variance in digit span forward [R2 = .21, F(1, 121) = 32.35, p < .001] and backward [R2 = .32, F(1, 121) = 56.33, p < .001], with improved performance as a function of increasing age in both instances. For digit span forward, other variables in the models did not account for additional variance. For digit span backward, however, additional variance was explained by composite z score for both RT mean [ΔR2 = .04, ΔF(1, 120) = 6.80, p < .01] and RT SD [ΔR2 = .05, ΔF(1, 120) = 9.18, p < .01], indicating that working memory was poorer as processing speed slowed and variability in processing speed increased. There was also a trend [ΔR2 = .02, ΔF(1, 119) = 3.60, p < .06] suggesting that variance in digit span backward was also attributable to group, with poorer performance for the PKU than control group. No other variables in the models explained additional variance. These findings show that processing speed and variability play roles in digit span backward but not forward, and that the PKU group’s performance on simple working memory tasks was similar to that of controls after taking differences in processing speed and variability into account.
2-back task
Age accounted for a significant proportion of the variance in the averaged number of commission errors in the spatial and letter conditions of the 2-back task [R2 = .21, F(1, 121) = 32.50, p < .001], with fewer errors as age increased. Composite z score for RT mean [ΔR2 = .04, ΔF(1, 120) = 7.00, p < .01] and RT SD [ΔR2 = .10, ΔF(1, 120) = 16.52, p < .001] accounted for additional variance; similar to findings from digit span backward, working memory was poorer as processing speed slowed and variability in processing speed increased. Group accounted for additional variance beyond that attributable to age and composite z score for RT mean [ΔR2 = .03, ΔF(1, 119) = 4.11, p < .05], with more errors for the PKU than control group. The interaction between age and composite z score for RT mean [ΔR2 = .04, ΔF(1, 118) = 7.35, p < .01] also explained additional variance, indicating that there was a significant differential effect of processing speed on number of incorrect responses depending upon age. No other variables in the models explained additional variance. These findings again demonstrate that processing speed and variability play roles in working memory. In addition, performance on this more complex working memory task was compromised in children with PKU, even after accounting for between-group differences in processing speed and variability.
Recognition span task
Age accounted for a significant proportion of the variance in the averaged maximum number of items correctly recalled in the shape and location conditions of the recognition span task [R2 = .24, F(1, 121) = 38.18, p < .001], with improved performance as age increased. Composite z score for RT mean [ΔR2 = .14, ΔF(1, 120) = 26.73, p < .001] and RT SD [ΔR2 = .11, ΔF(1, 120) = 20.65, p < .001] accounted for additional variance, again demonstrating that working memory was poorer as processing speed slowed and variability in processing speed increased. Group accounted for additional variance beyond that attributable to age and composite z score for RT mean [ΔR2 = .04, Δ F(1, 119) = 8.59, p < .01] and RT SD [ΔR2 = .04, Δ F(1, 119) = 8.21, p < .01], with poorer performance for the PKU than control group. No other variables in the models explained additional variance. Again, these findings indicate that processing speed and variability play roles in working memory. In addition, performance on this complex working memory task was compromised in children with PKU, even after accounting for between-group differences in processing speed.
Inhibitory Control
Go/no-go task
Age accounted for a significant proportion of the variance in number of commission errors in the no-go condition of the go/no-go task [R2 = .05, F(1, 121) = 6.47, p < .02], with fewer errors as age increased. Composite z score for RT mean and RT SD did not account for additional variance beyond that attributable to age. Group, however, accounted for additional variance beyond that attributable to age and RT mean [ΔR2 = .07, ΔF(1, 119) = 9.40, p < .01] and RT SD [ΔR2 = .03, ΔF(1, 119) = 4.40, p < .04], with more errors for the PKU than control group. No other variables in the models explained additional variance. These findings show that inhibitory control was compromised in children with PKU.
Strategic Processing
CVLT-C
Age accounted for a significant proportion of the variance in the number of semantically clustered words on the fifth learning trial of the CVLT-C [R2 = .24, F(1, 121) = 37.17, p < .001], with more words clustered as age increased. Composite z score for RT mean [ΔR2 = .03, ΔF(1, 120) = 4.03, p < .05] and RT SD [ΔR2 = .05, ΔF(1, 120) = 8.78, p < .01] accounted for additional variance, indicating that strategic processing was poorer as processing speed slowed and variability in processing speed increased. No other variables in the models explained additional variance. These findings demonstrate that processing speed and variability play roles in strategic processing, and that the PKU group’s strategic processing was similar to that of controls after taking differences in processing speed and variability into account.
Verbal fluency task
Age accounted for a significant proportion of the variance in number of words correctly reported on the food/drink verbal fluency task [R2 = .37, F(1, 119) = 71.21, p < .001], with more words generated as age increased. Composite z scores for RT mean [ΔR2 = .04, ΔF(1, 118) = 7.74, p < .01] and RT SD [ΔR2 = .03, ΔF(1, 118) = 4.88, p < .03] accounted for additional variance, again indicating that strategic processing was poorer as processing speed slowed and variability in processing speed increased. No other variables in the models explained additional variance. Similar to findings from the CVLT-C, these results show that processing speed and variability play roles in strategic processing, and that the PKU group’s strategic processing was similar to that of controls after taking differences in processing speed into account.
Relationships between Phe Levels and Executive Abilities
We next examined the relationships between Phe levels and executive abilities in children with PKU using hierarchical linear regression analyses. Separate analyses were conducted with each executive variable serving as the dependent variable. As noted earlier, because Phe closest to time of testing and average Phe over the past year increased significantly as a function of age, age was entered in the first step of all analyses. Phe level (either Phe closest to time of testing or average Phe over the past year) was entered in the second step, and the interaction between age and Phe level was entered in the final step.
With a single exception (number of commission errors in the no-go condition of the go/no-go task), age accounted for a significant proportion of the variance in executive performance (significant R2s ranged from .16 to .58, p < .05 in all instances). Beyond the variance attributable to age, Phe closest to time of testing accounted for a significant proportion of the variance in the averaged number of errors in the spatial and letter conditions of the 2-back task [ΔR2 = .13, ΔF(1, 36) = 8.41, p < .01] and the number of commission errors in the no-go condition of the go/no-go task [ΔR2 = .18, ΔF(1, 36) = 8.32, p < .01]. After removing the contribution of age, number of errors increased as Phe increased in both instances. Turning to average Phe over the past year, this variable accounted for a significant proportion of the variance in the averaged number of errors in the spatial and letter conditions of the 2-back task [ΔR2 = .13, ΔF(1, 35) = 9.00, p < .005] and the number of semantically clustered words on the fifth learning trial of the CVLT-C [ΔR2 = .20, ΔF(1, 35) = 11.02, p < .005]. After removing the contribution of age, number of errors increased on the 2-back task and number of words semantically clustered decreased on the CVLT-C as Phe increased. The interaction between age and either Phe closest to time of testing or average Phe over the past year did not account for additional variance in performance on any executive task.
Discussion
The current study was conducted to explore both processing speed (i.e., RT mean) and variability in processing speed (i.e., RT SD) in children with early- and continuously-treated PKU. It was hypothesized that, in comparison with typically-developing control children, processing speed would be both slower and more variable in children with PKU. To test these hypotheses, we examined the basic processing speed components of three tasks: simple reaction time, go/no-go, and stimulus-response compatibility.
Results supported our hypotheses. Specifically, compared with controls, the performance of children with PKU was slower and more variable across the three speeded tasks. The processing speed finding is consistent with previous research (e.g., Albrecht et al., 2009; Anderson et al., 2007; Channon et al., 2004), whereas identification of greater variability in processing speed adds a new dimension to PKU research. Because variability in processing speed is considered a measure of efficiency (Simmonds et al., 2007), together these findings indicate that performance on cognitive tasks in children with PKU is compromised in terms of both speed and efficiency.
With regard to clinical significance, processing speed and variability in processing speed were approximately one-half standard deviation slower for children with PKU than controls. When age was considered, however, it became clear that variability in processing speed was compromised in younger children with PKU but approximated that of controls by late adolescence. Although longitudinal study is necessary to reach definitive conclusions, these results suggest that consistency in performance on cognitive tasks may improve as children with PKU age, although their responses continue to be slower than those of typically-developing children. Additional research is also needed to examine the possibility that younger children with PKU use a different approach in completing speeded tasks, which could result in greater variability.
Because processing speed is associated with performance on executive tasks (Salthouse, 1996) and executive abilities are compromised in children with PKU (for a review, Christ et al., 2010), the relationship between speed and executive abilities was also explored. To do so, variables from four working memory tasks, one inhibitory control task, and two strategic processing tasks were considered. Not surprisingly, performance on executive tasks generally improved with age. After taking the effects of age into account, processing speed or variability in processing speed contributed to performance on five of the seven executive tasks; the only exceptions were digit span forward and go/no-go (number of commission errors), which are arguably less demanding and complex than the other tasks administered. Overall, these findings support the notion that processing speed plays a significant role in executive abilities.
Of particular relevance to the current investigation, after controlling for age and processing speed, it was apparent that children with PKU performed more poorly than controls on the two most demanding working memory tasks (with a trend for poorer performance on a third) and on the inhibitory control task. In terms of variability in processing speed, children with PKU performed more poorly than controls on one of the four working memory tasks and on the inhibitory control task. In other words, primary impairments in working memory and inhibitory control were evident even after carefully controlling for processing speed and variability in processing speed. As such, compromised executive abilities in children with PKU cannot be attributed solely to impairments in processing speed. That said, strategic processing in children with PKU appeared comparable to that of controls after taking age and speed and variability into account.
In contrast with our findings, in a previous study of children with PKU Anderson et al. (2007) reported that processing speed and executive abilities were unrelated, suggesting that impairment in executive abilities is independent of slowed processing speed. Findings from our study, however, indicate that this is not always the case. There are a number of possible reasons for the discrepancy between studies. Anderson et al. used principal components to examine the relationships between processing speed and executive factor scores, whereas we used hierarchical linear regression to examine relationships between processing speed, variability in processing speed, and performance on executive tasks. In addition, the studies employed different tasks to assess these relationships. For example, Anderson et al. used tasks requiring more complex visuomotor abilities to assess processing speed (i.e., Coding, Symbol Search, Contingency Naming Test), whereas we used RT conditions requiring simple detection of targets. Our simpler tasks may have provided more ideal measures of processing speed because they were less affected by influences from other cognitive domains. Finally, our sample size was larger than in the Anderson et al. study, which provided greater power to detect significant relationships. Overall, differences between our study and that of Anderson et al. may have been related to differences in analytic approach, task demands, or sample size.
We also examined the relationships between Phe levels and age, processing speed, and executive abilities. Consistent with previous research (e.g., Walter & White, 2004; Feillet et al., 2010), Phe closest to the time of testing and average Phe over the year preceding testing both increased as a function of age in children with PKU. With regard to relationships between Phe levels and processing speed and executive abilities, we had anticipated some inconsistencies in our findings due to the restricted range of Phe levels in our early- and continuously-treated sample of children with PKU, which makes it difficult to detect significant correlations. Nonetheless, we found that after controlling for the contribution of age, higher Phe levels were associated with greater variability in processing speed, more errors on 2-back and go/no-go tasks, and less semantic clustering on the CVLT-C. In other words, as would be expected, higher Phe levels were associated with poorer performance.
We then pondered the possible neurobiological mechanisms (specifically dopamine disregulation and white matter abnormalities) underlying the impairments in processing speed and executive abilities we identified in children with PKU. Although speculative, our overall pattern of results regarding cognition and Phe levels suggests that dopamine disregulation may play a substantial role. That is not to say that white matter abnormalities played no role in our findings. Because we did not evaluate the relationships between cognition and white matter abnormalities, however, it is not possible to reach a definitive conclusion in this regard. Future research is clearly needed to elucidate the separate, combined, or synergistic contributions of dopamine disregulation and white matter abnormalities to neuropsychological function in individuals with PKU.
It should be mentioned that the current study had a number of limitations. Although our sample size was relatively large in comparison with many studies of PKU, a larger sample would have increased statistical power, and some of our null findings may have reached statistical significance with a larger sample. Our sample was also limited in terms of the number of children represented at each year of age, which prohibited examination of possible differences in the approaches taken to complete our various tasks within discrete age bands. In addition, we chose not to control for IQ differences between our PKU and control group due to the likelihood that executive abilities contribute to IQ (particularly as assessed by the Matrix Reasoning subtest). As such, controlling for IQ would have potentially eliminated important disorder-specific effects on executive abilities.
In closing, findings from the current study clearly indicate that processing speed and variability in processing speed are compromised in children with PKU. In addition, although primary impairments in executive abilities are present in children with PKU, the contribution of processing speed to such impairments requires ongoing investigation. Neuroimaging and biochemical studies will be of great interest to further elucidate the neural underpinnings of both processing speed and executive impairments. Ongoing investigation of these issues will enhance our understanding of PKU and guide the development of interventions to prevent and/or treat neuropsychological impairment in individuals with PKU.
Acknowledgments
This research was supported by a National Institute of Child Health and Human Development grant (R01HD044901). The authors wish to thank Laurie Sprietsma and Kathleen Huntington for their contributions to the study.
References
- Albrecht J, Garbade SF, Burgard P. Neuropsychological speed tests and blood phenylalanine levels in patients with phenylketonuria. A meta-analysis. Neuroscience & Behavioral Reviews. 2009;33:414–421. doi: 10.1016/j.neubiorev.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Anastasoaie V, Kurzius L, Forbes P, Waisbren S. Variability of blood phenylalanine and its relationship to children with PKU. Molecular Genetics and Metabolism. 2008;93:221–268. doi: 10.1016/j.ymgme.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Anderson AE, Avins L. Lowering brain phenylalanine levels by giving other large neutral amino acids. A new experimental therapeutic approach to phenylketonuria. Archives of Neurology. 1976;33:684–686. doi: 10.1001/archneur.1976.00500100018008. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Leuzzi V. White matter pathology in phenylketonuria. Molecular Genetics and Metabolism. 2010;99:S3–S9. doi: 10.1016/j.ymgme.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Developmental Neuropsychology. 2007;32:645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Wood SJ, Francis DE, Coleman L, Warwick L, Casanelia S, Anderson VA, Boneh A. Neuropsychological functioning in children with early-treated phenylketonuria: impact of white matter abnormalities. Developmental Medicine & Child Neurology. 2004;46:230–238. doi: 10.1017/s0012162204000386. [DOI] [PubMed] [Google Scholar]
- Antshel KM. ADHD, learning, and academic performance in phenylketonuria. Molecular Genetics and Metabolism. 2010;99:S52–S58. doi: 10.1016/j.ymgme.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Araujo GC, Christ SE, Steiner RD, Grange DK, Nardos B, McKinstry RC, White DA. Response monitoring in children with phenylketonuria. Neuropsychology. 2009;23:130–134. doi: 10.1037/a0013488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi B, Seddigh A, Tehrani-Doost M, Alaghband-Rad J, Ashrafi MR. Executive dysfunction in treated phenylketonuric patients. European Child & Adolescent Psychiatry. 2009;18:360–368. doi: 10.1007/s00787-009-0738-8. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Grange DK, Steiner RD, White DA. Executive strategic processing during verbal fluency performance in children with phenylketonuria. Child Neuropsychology. 2011;17:105–117. doi: 10.1080/09297049.2010.525502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm VL, Grant ML. The role of intelligence in phenylketonuria: A review of research and management. Molecular Genetics and Metabolism. 2010;99:S18–S21. doi: 10.1016/j.ymgme.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Bugg JM, DeLosh EL, Davalos DB, Davis HP. Age differences in Stroop interference: contributions of general slowing and task-specific deficits. Aging, Neuropsychology, and Cognition. 2007;14:155–167. doi: 10.1080/138255891007065. [DOI] [PubMed] [Google Scholar]
- Channon S, German E, Cassina C, Lee P. Executive functioning, memory, and learning in phenylketonuria. Neuropsychology. 2004;18:613–620. doi: 10.1037/0894-4105.18.4.613. [DOI] [PubMed] [Google Scholar]
- Channon S, Mockler C, Lee P. Executive functioning and speed of processing in phenylketonuria. Neuropsychology. 2005;19(5):679–686. doi: 10.1037/0894-4105.19.5.679. [DOI] [PubMed] [Google Scholar]
- Christ SE, Huijbregts SCJ, de Sonneville LMJ, White DA. Executive function in early-treated phenylketonuria: Profile and underlying mechanisms. Molecular Genetics and Metabolism. 2010;99:S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Christ SE, Steiner RD, Grange DK, Abrams RA, White DA. Inhibitory control in children with phenylketonuria. Developmental Neuropsychology. 2006;30:845–864. doi: 10.1207/s15326942dn3003_5. [DOI] [PubMed] [Google Scholar]
- Cohen MJ. Children’s Memory Scale Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. Journal of Abnormal Child Psychology. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- Dawson C, Murphy E, Maritz C, Chan H, Ellerton C, Carpenter RHS, Lachmann RH. Dietary treatment of phenylketonuria: the effect of phenylalanine on reaction time. Journal of Inherited Metabolic Disease. 2011;34:449–454. doi: 10.1007/s10545-010-9276-2. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Children’s Version Manual. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated Phenylketonuria: intelligence and executive function. Developmental Neuropsychology. 2008;33:474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62:1–207. [PubMed] [Google Scholar]
- Dyer CA. Pathophysiology of phenylketonuria. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:104–112. [Google Scholar]
- Feillet F, MacDonald A, Hartung D, Burton B. Outcomes beyond phenylalanine: An international perspective. Molecular Genetics and Metabolism. 2010;99:S79–S85. doi: 10.1016/j.ymgme.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Feldmann R, Denecke M, Grenzebach M, Weglage J. Frontal lobe dependent functions in treated phenylketonuria: Blood phenylalanine concentrations and long-term deficits in adolescents and young adults. Journal of Inherited Metabolic Disease. 2005;28:445–455. doi: 10.1007/s10545-005-0445-7. [DOI] [PubMed] [Google Scholar]
- Gorus E, De Raedt R, Mets T. Diversity, dispersion, and inconsistency of reaction time measures: effects of age and task complexity. Aging Clinical and Experimental Research. 2006;18:407–417. doi: 10.1007/BF03324837. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: Review of hypotheses. Molecular Genetics and Metabolism. 2010;99:S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Imbo I, Vandierendonck A. The development of strategy use in elementary school children: working memory and individual differences. Journal of Experimental Child Psychology. 2007;96:284–309. doi: 10.1016/j.jecp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Janzen D, Nguyen M. Beyond executive function: Non-executive abilities in individuals with PKU. Molecular Genetics and Metabolism. 2010;99:S47–S51. doi: 10.1016/j.ymgme.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Keys BA, White DA. Exploring the relationship between age, executive abilities, and psychomotor speed. Journal of the International Neuropsychological Society. 2000;6:76–82. doi: 10.1017/s1355617700611098. [DOI] [PubMed] [Google Scholar]
- Martynyuk AE, van Spronsen FJ, Van der Zee EA. Animal models of brain dysfunction in phenylketonuria. Molecular Genetics and Metabolism. 2010;99:S100–S105. doi: 10.1016/j.ymgme.2009.10.181. [DOI] [PubMed] [Google Scholar]
- Mazzocco MMM, Nord AM, Van Doorninck W, Greene CL, Kovar CG, Pennington BF. Cognitive development among children with early-treated phenylketonuria. Developmental Neuropsychology. 1994;10:133–151. [Google Scholar]
- Moyle JJ, Fox AM, Arthur M, Bynevelt M, Burnett JR. Meta-analysis of neuropsychological symptoms of adolesents and adults with PKU. Neuropsychology Review. 2007;17:91–101. doi: 10.1007/s11065-007-9021-2. [DOI] [PubMed] [Google Scholar]
- Moyle JJ, Fox AM, Bynevelt M, Arthur M, Burnett JR. A neuropsychological profile of off-diet adults with phenylketonuria. Journal of Clinical and Experimental Neuropsychology. 2007;29:436–441. doi: 10.1080/13803390600745829. [DOI] [PubMed] [Google Scholar]
- Myerson J, Robertson S, Hale S. Aging and intraindividual variability in performance: analyses of response time distributions. Journal of the Experimental Analysis of Behavior. 2007;88:319–337. doi: 10.1901/jeab.2007.88-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettelbeck T, Burns NR. Processing speed, working memory, and reasoning ability from childhood to old age. Personality and Individual Differences. 2010;48:379–384. [Google Scholar]
- Paine R. The variability in manifestations of untreated patients with phenylketonuria (phenylpyruvic aciduria) Pediatrics. 1957;20:290–302. [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Robbins T. Chemical neuromodulation of frontal-executive functions in humans and other animals. Brain Research. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychologica. 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. International Journal of Adolescent Medicine and Health. 2004;16:41–45. doi: 10.1515/ijamh.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- Weglage J, Pietsch M, Funders B, Koch HG, Ullrich K. Deficits in selective and sustained attention processes in early treated children with phenylketonuria – result of impaired frontal lobe functions? European Journal of Pediatrics. 1996;155:200–204. doi: 10.1007/BF01953938. [DOI] [PubMed] [Google Scholar]
- White DA, Connor LT, Nardos B, Shimony JS, Archer R, Snyder AZ, McKinstry RC. Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: a DTI study of the corpus callosum. Molecular Genetics and Metabolism. 2010;99:S41–S46. doi: 10.1016/j.ymgme.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Age-related working memory impairments in children with prefrontal dysfunction associated with phenylketonuria. Journal of the International Neuropsychological Society. 2002;8:1–11. [PubMed] [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]