Abstract
A series of new bacteriochlorins was synthesized using 132-oxobacteriopyropheophorbide- a (derived from bacteriochlorophyll-a) as a starting material, which on reacting with o-phenylenediamine and 1,10-diaminonaphthalene afforded highly conjugated annulated bacteriochlorins with fused quinoxaline, benzimidazole and perimidine rings respectively. The absorption spectra of these novel bacteriochlorins demonstrated remarkably red-shifted intense Qy absorption bands observed in the range of 816–850 nm with high molar extinction coefficients (89,900–136,800). Treatment of 132-oxo-bacteriopyropheophorbide a methyl ester with diazomethane resulted in the formation of bacterioverdins containing a fused six member methoxy substituted cyclohexenone (verdin) as an isomeric mixture. The pure isomers which exhibit long wavelength absorptions in the near-IR region (865–890 nm) are highly stable at room temperature with high reactivity with O2 at the triplet photoexcited state, favorable redox potential and could be potential candidates for use as photosensitizers in photodynamic therapy (PDT).
Introduction
Studies on porphyrins and related compounds as photosensitizers in the field of photodynamic therapy (PDT) suggest that long wavelength absorbing chromophores may have advantages in destroying deeply seated tumors.1,2 Porphyrin-based compounds with absorptions in the far red/near-IR region of the electromagnetic spectrum may have various applications as optical materials and sensors3 or for PDT.4 The availability of cheap LED diode lasers in the range of 800–900 nm will make PDT more economical and practical.5 One approach used in the synthesis of long-wavelength absorbing compounds exploits the strategy of extending the π- conjugated macrosystem of the porphyrin chromophore.6 This has been achieved by introducing fused aromatic rings directly attached to the porphyrin,7 or by building extended cyclic pyrrolic macrocycles.8,9 Another strategy that has been largely adopted, utilizes partial reduction of the porphyrin system to produce chlorins or bacteriochlorins.4 Introduction of electron-withdrawing substituents in such systems at an appropriate position(s) produces significant bathochromic shifts in their long-wavelength absorptions.10 In some instances introduction of fused exocyclic ring(s), directly attached to the reduced porphyrinic skeleton, was found to be beneficial in shifting Qy absorptions into the near-IR region.11 Among tetrapyrrolic systems, bacteriochlorins are of particular interest, because they exhibit intense Qy bands in the long wavelength region.12 Earlier, Dolphin and coworkers13 and Pandey et al.14 synthesized bacteriochlorins which possessed two fused rings on the opposite pyrrolic units and had long wavelength absorptions in the range of 760–790 nm. Unfortunately, further studies on these chromophores have not been reported.
The synthesis of stable bacteriochlorins has been a challenging aspect of photosensitizer development in the field of PDT. A decade ago, Robinson et al.15,16,17 reported the synthesis of a series of bacteriopurpurins, which had previously been erroneously reported as being so unstable that their characterization was not possible18. In this instance, it was found that the presence of two conjugated electron-withdrawing cyclopentenyl rings on the bacteriochlorins produced large bathochromic shifts of their Qy-bands, which absorbed in the range of 846–863 nm.17 A few years ago, Pandey and coworkers19 extended the osmium tetroxide oxidation approach followed by the pinacol-pinacolone reaction in developing certain keto bacteriochlorins derived from chlorins (pyropheophorbide-a, chlorin e6 and purpurinimide). A remarkable difference in photophysical properties and PDT efficacy was observed depending on the position of the keto-group at the position-7 or-8.
Natural bacteriochlorophylls, for example bacteriochlorophyll a 1 and its derivatives, have been shown to possess significant potency as photosensitizers.20 Many of these compounds appear to be unstable during in vivo experiments.21 Bacteriochlorins with fused anhydride and imide rings have been reported to be more stable to photooxidation, while possessing adequate Qy absorptions in the 800–820 nm range.10,22 The utility of these compounds has been extensively investigated by Pandey and coworkers and some of the analogs have shown excellent PDT efficacy, both in vitro and in vivo in various tumor models.
The chemistry involving the enolization of pyropheophorbode was outlined by Pandey and coworkers23 who showed the utility of the resulting product in highly conjugated systems. They followed this approach by first converting the bacteriochlorophyll a 1 to the corresponding α- diketone 8 which was then attached to a variety of fused aromatic moieties. This synthetic design followed the strategy of introducing moieties that extended conjugation on the pyrrolic unit C, while the opposite pyrrolic unit A contained an electron-withdrawing substituent (acetyl group). This arrangement of substituents along the pyrrolic A–C unit axis in bacteriochlorins was expected to provide large bathochromic effects on their Qy absorption bands,10 which could be shifted into required near-IR region (above 800 nm). Here we report our results on the synthesis and spectroscopic, electrochemical and photophysical characterization of a series of novel bacteriochlorins, bearing fused aromatic units.
Results and Discussion
Annulated Aromatic Bacteriochlorins
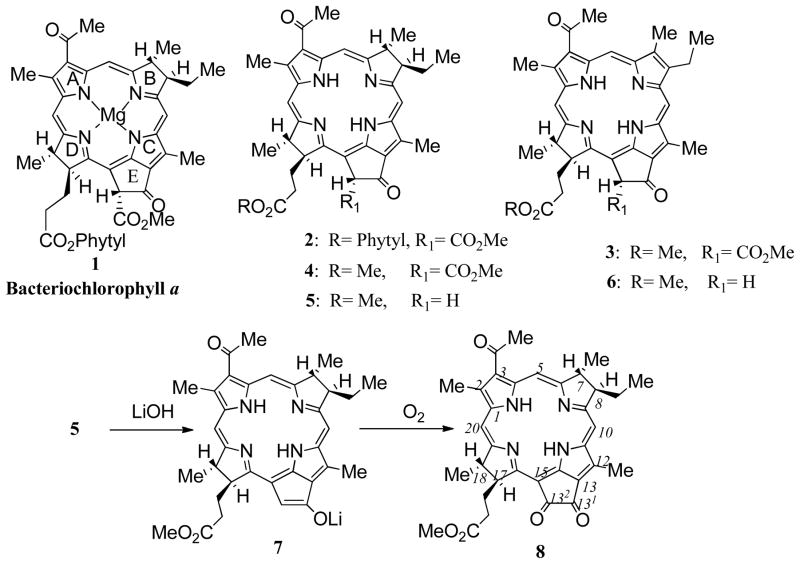
Bacteriochlorophyll a 1 was isolated from Rhodobacter sphaeroides according to a standard procedure.24 A brief treatment with 0.5% aq. HCl afforded bacteriopheophytine a 2. Attempts to transesterify bacteriochlorin 2 using 2% sulfuric acid in methanol were unsuccessful, resulting in the formation of a significant amount (more than 50%) of 3-acetyl-3-devinyl-pheophorbide a 3. Other researchers have also reported difficulties working with bacteriochlorins in the presence of strong acids.25,26 The phytyl ester group was cleaved efficiently using 80% aqueous TFA under an inert atmosphere25 and the resulting bacteriopheophorbide a was esterified using diazomethane to give methyl ester 4 in 89% yield.
A small amount of chlorin 3 (2%) was separated using chromatography on silica. Demethoxycabonylation of bacteriochlorin 4 in collidine afforded bacteriopyropheophorbide a 5 in 95% yield. A minor by-product, isolated chromatographically as a faster moving band, was identified as 3-acetyl-3-devinyl-pyropheophorbide a 6 (3% yield). The synthesis of 132-oxobacteriopyropheophorbide a 8 followed the approach outlined for the auto-oxidation of related pyropheophorbide a and phylloerythrin compounds.23 Hence in situ auto-oxidation of the bacteriopyropheophorbide a 5 using aqueous LiOH in THF for 24 hours followed by an acidic work-up and re-esterification with diazomethane, afforded bacteriochlorin α-diketone 8 in 68% yield.27 Surprisingly, no other oxidized by-products (chlorins or porphyrins) or peripheral oxidation products were isolated from the reaction mixture as was observed in the LiOH-promoted allomerization of pyropheophorbide a.23 As expected, bacteriochlorin 8 demonstrated a moderate bathochromic shift of the Qy and Soret absorption bands, while the Qx band was depressed and has a hypsochromic shift (Figure 1). The electron-withdrawing nature of the cyclopentyldiketone ring E produced a significant hyperchromic effect in the Qy absorption band (768 nm, ε =87,900) when compared to bacteriopyropheophorbide a 5 (754 nm, ε =61,700). The presence of the 132-oxo-functionality was evident in the 1H NMR spectrum of bacteriochlorin 8. Thus, when compared with similar resonances in 5, the signal of the 17-H in 8 was shifted upfield to 5.00 ppm (Δδ = 0.97 ppm). The 132-methylene group resonance, which occurs at 5.33 ppm in 5, was not observed in bacteriochlorin 8 as expected.
Figure 1.
UV/near-IR absorption spectra (in CH2Cl2) of bacteriochlorins: A) 132-oxo bacteriopyropheophorbide a 8; B) quinoxalino-bacteriochlorin 10; C) benzimidazolo-bacteriochlorin 11; D) perimidino-bacteriochlorin 13.
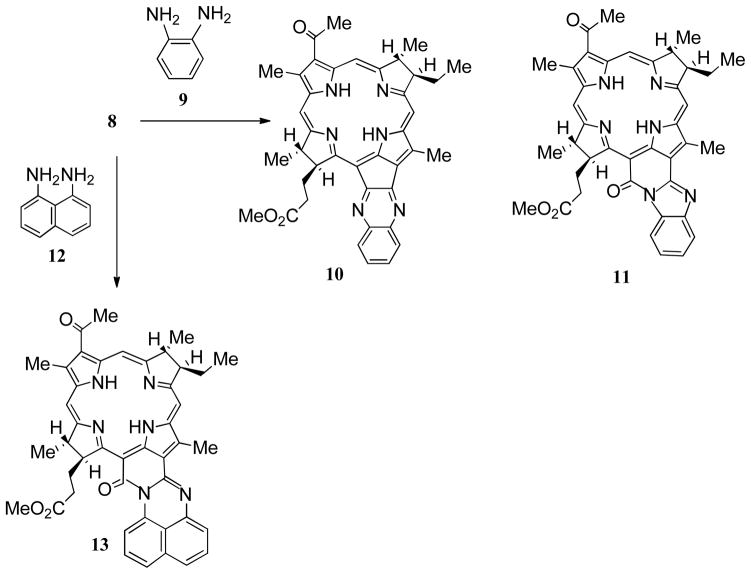
Condensation of bacteriochlorin 8 with 1,2-phenylenediamine 9 in pyridine in the presence of catalytic amounts of TFA27–29 afforded two bacteriochlorins, which were isolated and purified by column and thin-layer chromatography. The minor product identified as quinoxalino-bacteriochlorin 10 (29%) based on HRMS and 1H NMR spectroscopy data. A 1H NMR spectrum of 10 displayed characteristic resonances from the aromatic protons of the quinoxaline fused ring at 7.49 and 7.96 ppm, while a mass ion (M+H) at m/z 653.3 was observed consistent with the structure.It was interesting to observe the effects induced by introduction of fused quinoxaline ring system in its UV/near-IR absorption spectrum (Figure 1). The extension of conjugation at ring E in bacteriochlorin 10 caused a significant bathochromic effect in the Qy absorption band, shifted it to 816 nm. The high extinction coefficient (ε =107,600) indicated a strong hyperchromic effect in the Qy absorption band, due to the annulated polycyclic quinoxaline system in the chromophore 10. The major product (54%) was identified as bacteriochlorin 11, possessing a conjugated benzimidazole ring. The structure of this product was assigned based on mass spectral and 1H NMR data. Thus, the signal of the 17-H atom resonance at 5.43 ppm, indicated the presence of the carbonyl function at the 133-position.27 A similar deshielding effect on the 17-H resonance from the neighboring 133-keto group was reported in a bacteriopurpurin a imide series.22 Signals from the aromatic protons of benzimidazole moiety resonated at d 7.30, 7.86 and 8.06 ppm, which is similar in chemical shift to values reported in a pyropheophorbide a series.28 By UV/near-IR spectroscopy, it is evident that the extended conjugation due to annulated benzimidazole in the bacteriochlorin chromophore 11 produced a large bathochromic shift (Δl=96 nm) in the Qy absorption (850 nm, ε =89,800 M−1cm−1). The intense Qx-band was also shifted to 553 nm (ε =39,200 M−1cm−1). Presumably, bacteriochlorin 11 was formed as an autoxidized by-product (allomer) during the condensation process.28
Using a similar synthetic methodology, condensation of bacteriochlorin 8 with 1,9- diaminonaphtalene 12 in pyridine/TFA gave perimidino-bacteriochlorin 13, which was isolated as a sole product (79%). The annulated polycyclic perimidine moiety caused a remarkable large hyperchromic effect on the Qy absorption band (829 nm, ε =138,800 M−1cm−1), which has extinction coefficient almost twice as large as its Soret band (398 nm, ε =76,900 M−1cm−1) in the electron spectrum (Figure 1D). The exceptionally intense absorption maximum in bacteriochlorin 13 is a unique optical characteristic, which makes this chromophore very attractive for use as a recording material in information-storage media, dopant for nonlinear and luminescent optic materials. In contrast to bacteriochlorins 10 and 11 which are red in solutions, chromophore 13 displays broad absorption over 534–660 nm region, providing its solutions with a blue-green color.
Bacterioverdins
In the search for novel near-IR absorbing sensitizers, we were interested in exploring bacteriochlorins with various six-membered conjugated ring systems. It is known that the presence of a cyclohexenone (verdin) ring in chlorins and porphyrins produces significant bathochromic shifts in the Qy absorption spectra of the molecules, ranging from 50–60 nm for porphyrins30 and from 70–100 nm for chlorins.31 The presence of the reactive a -diketone moiety in bacteriochlorin 8 provided an opportunity to introduce a verdin ring system to the bacteriochlorin chromophore, using a recognized diazomethane ring-enlargement approach.32–33 Utilizing this synthetic methodology, we found that treatment of 132-oxobacteriopyropheophorbide a 8 with excess diazomethane at room temperature resulted in formation of three products (14–16), which were separated and purified chromatographically. Examination of the molecular weight for the compounds showed that each product had the same molecular ion at m/z 609.3, indicating that they were isomeric in nature and all possessing an annulated methoxycyclohexenone (verdin) ring system. As a new subclass of chromophores, we termed these compounds as bacteriovervins.
The structural assignment of bacterioverdins 14–16 was made using detailed 2D/ROESY 1H NMR studies. The fastest moving band on silica was assigned to be the 132-methoxybacterioverdin 14, isolated in 29 %. A 2D/ROESY 1H NMR spectrum of 14 shows that the 131-H exhibits a strong through-space interaction with both signals associated with the 12-methyl and 132-methoxyl groups (Figure 2). By UV/near-IR spectroscopy, bacterioverdin 14 has a Qy absorption band remarkably shifted into the near-IR region (891 nm, ε =51,800 M−1cm−1). When compared to 5, this represents a bathochromic shift of Δλ = 137 nm, while the intensity of the Qy absorption is slightly decreased. The Qx-band was shifted to 550 nm and the Soret (B1 and B2) bands were observed at 368 and 423 nm (Figure 3). To our best of our knowledge, bacterioverdin 14 demonstrates the longest red-shifted Qy absorption of any existing natural or synthetic bacteriochlorin derivative.18
Figure 2.
NOE interactions (selected) observed in 2D/ROESY1H NMR spectra of bacteriochlorins 14–16.
Figure 3.
UV/near-IR absorption spectra (in CH2Cl2) of bacteriochlorins: bacteriopyropheophorbide a 5; B) bacterioverdin 14; C) bacterioverdin 15; D) bacterioisoverdin 16.
The major product of diazomethane ring enlargement reaction, isolated as the second fraction (42% yield), was assigned as the 131-methoxy-bacterioverdin 15. A 1H NMR of this product showed a 17-H resonance at 5.18 ppm, clearly indicating the presence of a carbonyl function at the adjacent 133-position, as had been seen with bacteriochlorins 11 and 13.
Additional evidence of its structure was provided by a 2D/ROESY 1H NMR experiment, which showed distinctive interactions between resonances from the 131-methoxyl group and the 12-methyl substituent, as well as between the 131-methoxyl and the 132-H signals (Figure 2). As with isomer 14, bacterioverdin 15 displays a Qy absorption shifted to the near-IR region (874 nm, ε = 78,500 M−1cm−1), demonstrating a significant bathochromic effect (Δλ = 120 nm).
The third and slowest eluting product of the diazomethane reaction was assigned as bacteriochlorin 16, isolated in 16% yield. The 2D/ROESY1H NMR spectrum clearly shows that the resonance of the 131-methoxyl group exhibits strong through-space interactions with the neighboring 17-H and 132-H atoms (Figure 2). The multiplet attributed to the proton at the 17- position was observed at δ 4.78 ppm, indicating the absence of a neighboring carbonyl functionality at the 133-position in the verdin ring.32 In contrast to bacterioverdins 14 and 15, the ketone functionality in bacteriochlorin 16 is located at the 131-position; thus this product was termed as bacterioisoverdin. Bacterioisoverdin 16 has a Qy absorption at 865 nm (ε = 48,600 M−1 cm−1), while the Qx band occurred at 596 nm. In contrast to bacterioverdins 14 and 15, which are red in solution, this compound has a characteristic deep-green color in solution (Figure 3).
An X-ray crystallographic determination of bacterioverdin 14 provided an unambiguous identification of its structure, in accord with independent 2D/ROESY 1H NMR studies, and presents the first stereochemical parameters for this new class of bacteriochlorin. The compound crystallizes with three independent molecules in the unit cell, which display subtle differences in conformation and orientation of the side chains. Theoretical calculations based on the X-ray coordinates predict a range of 792 to 872 nm for the Qy transition.34 A view of the conformer that yields the best calculated agreement with the experimental value of 891 nm and presumably resembles most closely the conformation in solution is shown in Figure 4. Single crystals of 14 (C36H40N4O5) were grown from CH2Cl2/MeOH. Details and results of the X-ray experiment are included in CIF format in the Supporting Information. An analysis of the structures from the ring enlargement reaction sheds some light on the pathway of ring enlargement occurring within ring E (Scheme 4). Theoretically it is possible that diazomethane reacts at both carbonyl functionalities to produce intermediates 17 and 18. Both intermediates release nitrogen and produce ring enlargement by incorporating the methylene group between the 131- and 132-carbon atoms in the exocyclic ring E to give compound 20, while ring enlargement between the 13- and 131, as well as 15-meso and 132-positions, should produce isomers 19 and 21. The cyclohexanedione derivatives 19–20 should quickly enolize to produce verdins 21–23, which on methylation via diazomethane should afford bacterioverdins 14–16. Interestingly, the product 24 was not found among the isolated reaction products. Apparently, the absence of 24 indicates that the initial diazomethane attack takes place regioselectively at the 131-carbonyl function to form the only intermediate 17, presumably due to the steric hindrance from the neighboring 17- propionic side chain. These data are consistent with earlier reports on the higher reactivity of the carbonyl at the 131-position.29
Figure 4.
X-ray structure of bacterioverdin 14. Peripheral substituents have been omitted for clarity.
Scheme 4.
A possible mechanism for the formation of bacterioverdins (21–23) from 132- oxobacteriopyropheophorbide-a (8)
Electrochemistry
The electrochemical properties of seven bacterichlorins, 8, 10–11 and 13–16, were examined in CH2Cl2 containing 0.1 M TBAP. Cyclic voltammograms are shown in Figure 5 and the redox potentials for each oxidation and reduction are listed in Table 1.
Figure 5.
Cyclic voltammograms of compounds 8, 10–11, and 13–16 in CH2Cl2 containing 0.1 M TBAP at the scan rate of 0.1 V s−1.
Table 1.
Half-wave and Peak Potentials (V vs SCE) of Investigated Compounds (~ 10−3 M) in CH2Cl2 Containing 0.1 M TBAP under RT.
| cpd | oxidation
|
reduction
|
HOMO-LUMO gap | ||||
|---|---|---|---|---|---|---|---|
| Δox 2-1 | 2 nd ox a | 1st ox b | 1st red b | 2nd red b | Δred 1–2 | ||
| 8 | 0.47 | 1.28 | 0.81 (0.07) | −0.69 (0.08) | −1.05 (0.07) | 0.36 | 1.50 |
| 10 | 0.52 | 1.14 | 0.62 (0.08) | −0.81 (0.08) | −1.14 (0.08) | 0.33 | 1.43 |
| 11 | 0.53 | 1.24 | 0.71 (0.06) | −0.63 (0.09) | −0.97 (0.10) | 0.34 | 1.34 |
| 13 | 0.47 | 1.15 | 0.68 (0.08) | −0.69 (0.06) | −1.00 (0.07) | 0.31 | 1.37 |
| 14 | 0.56 | 1.14 | 0.58 (0.06) | −0.72 (0.08) | −1.05 (0.06) | 0.33 | 1.30 |
| 15 | 0.54 | 1.15 | 0.61 (0.06) | −0.70 (0.07) | −1.05 (0.07) | 0.35 | 1.31 |
| 16 | 0.50 | 1.16 | 0.66 (0.08) | −0.63 (0.06) | −0.97 (0.07) | 0.34 | 1.29 |
Epa at a scan sate of 0.1 V s−1;
E1/2 (ΔEp = Epa−Epc at a scan Rate of 0.1 V s−1)
The investigated compounds undergo two reversible reductions at E1/2 = −0.63 to −0.81 V and E1.2 = −0.97 to −1.14 V, respectively, to form the radical anion and dianion. Two one-electron oxidations are also detected at E1/2 = 0.61 to 0.81 V and Ep = 1.14 to 1.28 V to give the radical cation and dication. The first oxidation is reversible, while the second oxidation is quasi-reversible or irreversible due to the instability of the dication generated. However, two reversibleoxidations can be observed at low temperatures for these compounds and examples of cyclic voltammograms at −70 °C are shown in Figure 6 for compounds 10 and 14. As shown in this figure, the second oxidation is located at E1/2 = 1.10 V (10) or E1/2 = 1.17 V (14) and side reactions are not observed after the second oxidation as is the case at room temperature. In addition, the first oxidation of 10 is split into two processes at the low temperature probably due to aggregation of this compound in a certain extent, under these experimental conditions.
Figure 6.
Cyclic voltammograms of compounds 10 and 14 in CH2Cl2 containing 0.1 M TBAP at room temperature and −70 °C.
An almost constant potential difference between two reductions (Δred 1–2 = 0.33 ± 0.03 V) is observed for all compounds (see Table 1), while the potential difference between the first two oxidations is also similar for the examined compounds (Δox 2-1 = 0.51 ± 0.05 V). The electrochemical HOMO–LUMO gap of bacterichlorins in this series is 1.29 to 1.50 V. A similar HOMO–LUMO gap of 1.40 ± 0.05 V has been reported for other bacteriochlorin derivatives in our previous study.35 These values are smaller than the gap of 1.52 ~ 1.60 V for metal-substituted bacteriochlorophyll a36 and also much smaller than the gap of 1.90 ~ 2.10 V often seen for a number of nonplanar porphyrins.37 It is worthy to point out that bacterioverdins 14–16 having a fused cyclohexenenone ring systems exhibit the smallest HOMO–LUMO gap (~1.30 V). These three compounds also exhibit both the easiest reduction and the easiest oxidation as compared to other earlier studied compounds.
Spectroelectrochemistry
The electroreductions and electrooxidation of 8, 10–11 and 13–16 were characterized by UV-vis thin-layer spectroelectrochemistry and the spectral data for the electrogenerated radical cations and radical anions of these bacteriochlorins are listed in Table 2. The spectral changes are reversible upon reversing the applied potentials, consistent with a high stability of the oxidized and reduced forms on the spectroelectrochemical timescale.
Table 2.
The Thin-layer UV-vis Spectral Data of Radical Anions and Cations of Investigated Compounds (~ 10−3 M) in CH2Cl2 Containing 0.1 M TBAP
| cpd | radical anion λ, nm (ε × 10−4 M−1 cm−1)a |
radical cation λ, nm (ε × 10−4 M−1 cm−1)a |
|---|---|---|
| 8 | 364s (5.5) 378 (5.6) 584 (1.7) 768 (1.7) 906 (1.5) | 348 (5.4) 396 (8.7) 518 (0.9) 770 (2.7) 836 (1.2) |
| 10 | 363 (5.2) 403 (6.5) 601 (2.1) 816 (3.9) 908 (1.1) | 365 (7.6) 406 (7.4) 582 (1.3) 730 (1.5) 817 (2.7) |
| 11 | 376 (4.4) 557 (2.1) 615 (2.4) 852 (2.4) 1017 (1.5) | 364 (5.7) 397 (5.7) 434 (6.2) 584 (1.2) 895 (1.8) |
| 13 | 363 (4.2) 539 (2.8) 651 (3.1) 830 (2.3) 1018 (1.4) | 353 (5.3) 413 (8.8) 589 (1.6) 833 (3.4) 885 (1.7) |
| 14 | 365 (1.6) 381s (1.6) 544 (2.5) 644 (2.5) 889 (1.5) | 353 (8.3) 398 (10.0) 434 (9.0) 800 (1.4) 885 (1.8) |
| 15 | 374 (5.1) 398 (5.0) 572 (1.6) 628 (2.3) 1039 (1.1) | 360 (8.0) 398 (8.8) 429 (9.3) 769 (0.7) 896 (1.3) |
| 16 | 369 (6.2) 400 (5.2) 509 (1.4) 761 (1.8) 867 (1.4) | 370 (7.3) 392 (6.3) 641 (1.6) 766 (1.4) 866 (1.9) |
the molar absorptivity (ε ) of radicals is calculated by comparing the relative intensities of initial (neutral species) and the final (radical) spectra, while the ε of the neutral species was obtained by a regular UV-vis measurement in a 1 cm cell; s = shoulder
Examples of the UV-visible spectral changes obtained during the first two oxidations and first two reductions of compound 15 are illustrated in Figure 7. Upon the first reduction of 15 at − 0.90 V (Figure 7a), the 369 nm Soret band and the strong 872 nm visible band decreased in intensity while two weak bands grew in at 628 and 1039 nm. The NIR band at 1039 nm has been described as a marker band for the radical anion of bacteriochlorins and was predicted in MO calculations.35,36 The first two controlled potential reductions for compound 15 are accompanied by reversible spectral changes in the thin-layer cell and these spectra are shown in Figure 7b. As the electron transfer proceeds, the newly generated 628 and 1039 nm bands disappear, accompanied by the appearance of a weak and broad band at 564 nm, which is assigned to the bacteriochlorin dianion.
Figure 7.
UV-visible spectral changes for (a) the first and (b) the second reduction and (c) the first and (d) the second oxidations of compound 15 in a thin-layer cell at indicated potentials in CH2Cl2 containing 0.1 M TBAP.
Three moderate intensity Soret bands are seen at 360, 398, 429 and there is a weak NIR band at 896 nm in the absorption spectrum of the radical anion for 15 (Figure 7c). The NIR band at 896 nm is can be used as a marker band of the bacteriochlorin radical cation, and similar Soret bands were also reported in the radical cation spectrum of other bacteriochlorophyll derivatives.35,36 This is due to the chlorine structure of the electrogenerated radical cation. A further oxidation of the radical cation 15 leads to a disappearance of the 360, 398 and 896 nm bands and the appearance of a stronger band at 448 nm when the controlled potential is switched to 1.30 V (Figure 7d).
Theoretical study
Density functional theoretical (DFT) calculations were performed using the Gaussian 09 program (see Experimental Section) and the structures were optimized at the B3LYP/6−31+G(d,p) level of theory. The calculated HOMO-LUMO gaps are summarized in Table 3. There is a linear correlation between the HOMO-LUMO gaps obtained from electrochemical measurements and theoretical calculations as shown in Figure 8. The slope of the linear plot is 1.3, which indicates that there are no significant contributions of solvation to the redox behavior, due to the highly delocalized HOMO-LUMO on the macrocycle.
Table 3.
HOMO and LUMO Energies Determined from DFT B3LYP/6−31+G(d,p) Level of Theory
| HOMO, eV | LUMO, eV | HOMO-LUMO | |
|---|---|---|---|
| 8 | −5.35 | −3.35 | 2.00 |
| 10 | −5.02 | −3.11 | 1.91 |
| 11 | −5.19 | −3.39 | 1.80 |
| 13 | −5.13 | −3.33 | 1.80 |
| 14 | −5.00 | −3.23 | 1.77 |
| 15 | −4.96 | −3.22 | 1.74 |
| 16 | −5.08 | −3.26 | 1.82 |
Figure 8.
Plot of HOMO-LUMO gaps determined from CV and DFT calculations (B3LYP/6−31+G(d,p)).
Time-dependent DFT calculations using the optimized structure of DFT B3LYP/6−31+G(d,p) revealed the low energy transition with the strong oscillator strength. The strong Qy band in the near-IR region arises from the delocalized π-orbitals of the HOMO-LUMO. The near-IR transition obtained from TD-DFT (hν(DFT)) exhibits a linear correlation with the Qy-transition observed in CH2Cl2 (hν(obs)), as shown in Figure 9. The slope is close to unity (1.15).
Figure 9.
Plot of the transition energies determined from TD-DFT calculations (TDB3LYP/6−31+G(d,p)//B3LYP/6−31+G(d,p)) and electronic absorption spectra.
Photophysical Properties
The dynamics of energy transfer from the triplet excited states of near-IR absorbing chlorines to dioxygen was examined by nanosecond laser flash photolysis measurements. Figure 10 shows a triplet-triplet (T-T) absorption band of 8 observed at 600 nm. Similar T-T absorption bands were observed for 9–12. In the case of 13, however, no T-T absorption band was observed probably due to the electron-transfer quenching of the singlet excited state by the naphthylamine donor moiety of 13. The T-T absorption decay of 8 obeys first-order kinetics (Figure 10a), and this indicates that there is no contribution from the T-T annihilation under the present experimental conditions. The triplet lifetime was determined as 360 μs. The decay of the T-T absorption of 8 in air-saturated PhCN (Figure 10b) was enhanced significantly as compared with what is observed in deaerated PhCN (Figure 10a) because of energy transfer from the triplet excited state of 8 (38*) to O2. The rate constant of energy transfer from 38* to O2 was determined to be 1.5 × 109 M−1 s−1, which is smaller than the reported diffusion rate constant in PhCN.37,38 The rate constants of the triplet decay and energy transfer to O2 for other near-IR absorbing bacteriochlorins were determined similarly and the results are listed in Table 4.
Figure 10.
(a) T-T absorption spectrum of 8 (3.0 × 10−5 M) obtained by the laser flash photolysis in deaerated PhCN at 5.6 μs after laser excitation (562 nm) at 298 K. (b) Decay curves of transient absorbance at 610 nm of 8 (a) in the absence of O2 and (b) in air-saturated PhCN ([O2]) 1.7 × 10−3 M) at 298 K.
Table 4.
Decay Rate Constants of the Triplet-Triplet Absorption under Argon and Air Atmosphere and Rate Constants of Energy Transfer from the Excited States to O2 in PhCN at 298 K
| kobs(argon), s−1 | kobs(air), s−1 | kEN, M−1 s−1 a | |
|---|---|---|---|
| 8 | 1.4 × 104 | 2.5 × 106 | 1.5 × 109 |
| 10 | 2.3 × 104 | 2.5 × 106 | 1.5 × 109 |
| 11 | 1.6 × 104 | 1.9 × 106 | 1.1 × 109 |
| 14 | 4.8 × 104 | 1.5 × 106 | 8.8 × 108 |
| 15 | 4.8 × 104 | 1.4 × 106 | 8.2 × 108 |
| 16 | 1.5 × 104 | 1.1 × 106 | 6.5 × 108 |
kEN = (kobs(argon) − kobs(air)]/[O2]. [O2] under air is 1.7 × 10−3 M.35
Conclusions
The synthesis of a series of novel bacteriochlorins related to bacteriochlorophyll a, possessing annulated heterocyclic systems was achieved. Though the careful synthetic design of introducing conjugated fused ring systems at the pyrrolic unit C, we were successful in preparing near-IR absorbing dyes. Spectroscopic data demonstrated that the outlined modifications resulted in large bathochomic shifts in the Qy-absorption bands, accompanied by a significant hyperchromic effect in several derivatives. Presumably the nature of bathochromic shift could be explained as a result of a decrease in the energy gap between the HOMO and LUMO transitions.38,39
The observed bathochromic effects in the absorption spectra of these derivatives were largest in bacterioverdins 14–16, which possess fused cyclohexenenone ring systems. These bacteriochlorins have Qy-absorptions in the near-IR region (870 – 890 nm), potentially making them attractive candidates as photosensitizers in the field PDT, especially for the treatment of target cells deeply located within tissues. Energy limitations in singlet oxygen generation likely make chromophores with long wavelength absorptions above 900 nm non-practical for PDT.40 Possibly, bacterioverdins 14–16 possess the longest near-IR shifted absorptions, which could be used to achieve a photodynamic effect. Photochemical studies and biological testing of these novel bacteriochlorins are currently under investigation and will be reported elsewhere. The unique optical properties of the series of annulated bacteriochlorins make them attractive for other applications, including information-storage media and optical materials.41 In addition, bacteriochlorin 8 which possesses a reactive a–diketone functionality, could be also useful as a synthetic building block for the preparation of linear porphyrinic arrays and other photosynthetic models.38
Experimental Section
Preparative thin-layer chromatography was performed on 1 mm silica gel plates. Column chromatography was carried out using silica gel 60 (70–230 mesh). Purity of the isolated products after preparative chromatography was monitored using HPLC (RP-18 column) and UV/visible spectroscopy on spectrophotometer. 1H NMR spectra were recorded using deuterochloroform as a solvent. Chemical shifts are reported in ppm downfield from tetramethylsilane as an internal standard. The peak assignments of compounds 14–16 were confirmed by 2D (COSY, NOESY) methods. Fast atom bombardment (FAB) low- and high-resolution mass spectra were obtained on double focusing magnetic sector mass-spectrometer. Nanosecond time-resolved transient absorption measurements were carried out using the laser system. Measurements of nanosecond transient absorption spectrum were performed according to the following procedure. A deaerated solution containing a compound was excited by a by a laser. The photodynamics were monitored by continuous exposure to a xenon lamp (150 W) as a probe light and a photomultiplier as a detector. The solution was oxygenated by nitrogen purging for 15 min prior to measurements. All new compounds decomposed >150 0C.
Theoretical Calculations
Density functional theory (DFT) calculations were performed with Gaussian 09 (Revision A.02)42 The calculations were performed on a 32-processor QuantumCube™ at the B3LYP/lanl2dz level of theory.43 Graphical outputs of the computational results were generated with the GaussView software program (ver. 3.09) developed by Semichem, Inc.44 Electronic excitation energies and intensities were computed by the time-dependent (TD)- DFT calculation at the B3LYP/lanl2dz level.42,45 The size of the integration grid used for all calculation was 10. In each case, 40 excited states were calculated by including all one-electron excitations within an energy window of ±3 hartrees with respect to the HOMO/LUMO energies.
Bacteriopheophorbide a methyl ester (4)
Rb. sphaeroides biomass (200 mL) was suspended in 1-propanol (1.5 L) and stirred at room temperature, in the dark, with constant nitrogen bubbling for 12 hours. The blue-green extract was filtered and aq. 0.5 N HCl (50 mL) was added to the filtrate. The reaction mixture was diluted with aq. 5% NaCl (2 L) and extracted with dicloromethane (3 x 300 mL). The combined extracts were washed with water (3 x 500 mL), dried and evaporated by rotary evaporation. The residue was precipitated from hexanes to give bacteriopheophytine a (2) (590 mg) with purity sufficient to proceed to the next step; UV-vis λmax (Et2O) nm 356, 383, 527, 749; [lit.46 λmax (Et2O) nm (ε × 104) 356 (11.3), 383 (6.27), 525 (2.83), 750 (6.75)]. Compound 2 was dissolved in aq. 80% TFA (100 mL) and stirred in dark at 0°C for 2 hour. The solution was then diluted with ice/water (500 mL) and extracted with dicloromethane (3 x 200 mL). The combined organic extracts were washed with water, treated with diazomethane and evaporated to dryness. The crude residue was chromatographed on silica (eluent: dichloromethane-acetone, gradient 3% – 5% acetone) to give two bands. The minor faster running band was 3-acetyl-3-desvinylpheophorbide a methyl ester (3) isolated as brown-green band (10 mg, 2%), UV-vis λmax (CH2Cl2) nm (ε × 104) 412 (11.9), 509 (1.13), 539 (0.98), 619 (0.91), 678 (4.79); [lit.47 λmax (CH2Cl2) 412, 510, 540, 620, 678 (4.71). HRFABMS C36H38N4O6 [MH]+ calcd 623.2869, obsd 623.2886.
The second major violet band was Bacteriopheophorbide a methyl ester (4) (350 mg, 91%). UV-vis λmax (CH2Cl2) nm (ε × 104) 362 (10.5), 387 (5.19), 532 (2.76), 683 (1.12), 756 (6.27); [lit.48 λmax (CH2Cl2) 362, 387, 503, 530, 628, 680, 755]. HRFABMS C36H40N4O6 [MH]+ calcd 625.3026, obsd 625.3043.
Bacteriopyropheophorbide a methyl ester (5)
Bacteriopheophorbide a methyl ester 4 (350 mg) was dissolved in collidine (20 mL) and refluxed under nitrogen for 2 hours. The reaction mixture was diluted with hexane (500 mL), and the precipitate was filtered off and washed with hexane (100 mL). The residue was chromatographed on silica (eluent: dichloromethane-acetone, gradient 2% – 4% acetone) to separate a minor faster running brown band, identified as 3-acetyl-3-desvinyl-pyropheophorbide a methyl ester (6) (10 mg, 3%), UV-vis λmax (CH2Cl2) nm (ε × 104) 412 (11.9), 509 (1.13), 539 (0.98), 619 (0.91), 678 (4.79); [lit.48 λmax (CHCl3) 412, 510, 540, 620, 677. HRFABMS C34H36N4O4 [MH]+ calcd 565.2814, obsd 565.2803.
Bacteriopyropheophorbide a methyl ester (5) was isolated as the major product. It was recrystallized from dichloromethane/hexane to give violet-black crystals (305 mg, 89 %). UV-vis λmax (CH2Cl2) nm (ε × 104) 360 (10.2), 386 (5.23), 532 (2.69), 682 (1.09), 754 (6.17); [lit.41 λmax (CH2Cl2) 361, 387, 503, 530, 628, 681, 754]. FABMS m/z 567.3 ([MH]+, 100%); HRFABMS C34H38N4O4 [MH]+ calcd 567.2971, obsd 567.2956.
132-Oxo-bacteriopyropheophorbide a methyl ester (8)
Bacteriopyropheophorbide a methyl ester 5 (300 mg) was dissolved in THF (100 mL) and a suspension of lithium hydroxide (0.5 g) in water (4 mL) was added to the solution. The reaction mixture was vigorously stirred overnight at room temperature and then poured into water (0.5 L) containing acetic acid (5 mL). The product was extracted with a dichloromethane/THF mixture (1 : 1). The combined extracts were washed with water, dried over sodium sulfate, briefly treated with excess ethereal diazomethane, and the solvent was evaporated in vacuum. The residue was separated on silica gel (eluent: dichloromethane-acetone, gradient 5%–8% acetone) and product 8 was isolated as yellowish-brown band (215 mg, 68%). Dark-brown solid (from dichloromethane-hexane). UV-vis λmax (CH2Cl2) nm (ε × 104) 346 (7.12), 385 (8.73), 518 (1.89), 676 (0.89), 768 (8.97); 1H NMR δ : 9.05 (s, 1H), 8.51 (s, 1H), 8.45 (s, 1H), 5.06 (dd, 1H), 4.56 (m, 2H), 4.02 (m, 1H), 3.65 (s, 3H), 3.52 (s, 3H), 3.44 (s, 3H), 3.18 (s, 3H), 2.58 (m, H), 2.48 (m, H), 2.24-2.12 (m, 4H), 1.84 (d, 3H), 1.78 (d, 3H), 1.13 (t, 3H), −0.29 and −1.03 (each br s, 2H); FABMS m/z 581.3 ([MH]+, 100%); HRFABMS C34H36N4O5 [MH]+ calcd 581.2739, obsd 581.2744. Anal.: C:70.24, H:6.14, N:9.68; req.: C:70.32, H:6.25, N:9.65.
Condensation of 132-oxo-bacteriopyropheophorbide a methyl ester (8) with 1, 2-phenylene diamine (9)
132-Oxo-bacteriopyropheophorbide a methyl ester 8 (100 mg) was dissolved in pyridine (40 mL) and 1,2- phenylenediamine hydrochloride 9 (200 mg) was added. TFA (0.5 mL) was added and the reaction mixture was heated at reflux for 1 hour, monitoring the progress spectroscopically. The reaction mixture was diluted with water (500 mL) and extracted with dichloromethane. The organic layers were washed with water, dried over sodium sulfate, filtered and evaporated in vacuum. The residue was chromatographed on silica (eluent: dichloromethane-acetone, gradient 2%–5% acetone) to give two bands.
Quinoxalino-bacteriochlorin methyl ester (10) was isolated as a faster moving orange-red band. Yield: 33 mg (29%). Red-brown crystals (from dichloromethane/hexane), UV-vis λmax (CH2Cl2) nm (ε × 104) 357 (7.36), 387 (8.52), 582 (2.87), 759 (1.48), 816 (10.76); 1H NMR δ : 9.24 (s, 2H), 8.51 (s, 2H), 7.96 (m, 2H), 7.49 (m, 2H), 5.03 (dd, 1H), 4.46 (q, 1H), 4.38 (m, 1H), 4.02 (m, 1H), 3.65 (s, 6H), 3.54 (s, 3H), 3.18 (s, 3H), 2.78 (m, H), 2.67 (m, H), 2.44-2.32 (m, 3H), 2.21 (m, 1H), 1.84 (m, 6H), 1.15 (t, 3H), 0.39 and −0.93 (each br s, 2H); 13C NMR δ : 199.2, 174.0, 168.0, 167.0, 166.5, 164.2, 163.7, 154.8, 145.8, 141.3, 141.2, 141.1, 135.4, 134.2, 131.7, 131.0, 130.2, 129.4, 129.2, 128.2, 128.0, 125.0, 105.0, 100.3, 100.1, 97.3, 56.3, 52.4, 51.5, 48.4, 47.5, 33.3, 31.9, 31.4, 29.8, 23.9, 23.5, 13.5, 12.3, 10.8; FABMS m/z 653.3 ([MH]+, 100%); HRFABMS C40H41N6O3 [MH]+ calcd 653.33240, obsd 653.3258.
Benzimidazolo-bacteriochlorin (11) was isolated as the second slower eluting red-brown band. Yield: 59 mg (54%). Dark-purple needles (crystallized from dichloromethane/hexane), UV-vis λmax (CH2Cl2) nm (ε × 104) 374 (8.86), 423 (3.54), 553 (3.92), 773 (0.98), 850 (8.98); 1H NMR δ : 9.26 (s, 2H), 8.71 (s, 1H), 8.59 (s, 1H), 8.06 (m, 1H), 7.86 (m, 1H), 7.30 (m, 2H), 5.43 (dd, 1H), 4.36 (m, 2H), 4.12 (m, 1H), 3.84 (s, 3H), 3.65 (s, 3H), 3.56 (s, 3H), 3.23 (s, 3H), 2.76 (m, 1H), 2.47 (m, 3H), 2.12 (m, 2H), 1.86 (m, 6H), 1.17 (t, 3H), −0.29 and −0.43 (each br s, 2H); 13C NMR δ : 198.5, 173.9, 173.0, 168.9, 167.8, 165.6, 164.2, 148.1, 145.4, 136.9, 135.9, 135.3, 135.1, 132.5, 132.3, 131.3, 130.8, 125.1, 125.0, 119.8, 116.2, 115.9, 102.4, 102.3, 98.5, 97.8, 56.8, 54.9, 51.5, 48.1, 46.9, 33.1, 32.4, 31.1, 29.8, 24.1, 23.4, 13.6, 12.6, 10.8; FABMS m/z 639.3 ([MH]+, 100%); HRFABMS C40H41N6O4 [MH]+ calcd 669.3189, obsd 669.3173.
Perimidino-bacteriochlorin methyl ester (13) 132-Oxo-bacteriopyropheophorbide a methyl ester 8 (50 mg) was dissolved in pyridine (20 mL) and 1,9-diaminonaphthalene hydrochloride (100 mg) was added. TFA (0.2 mL) was added and the reaction mixture was heated at reflux for 1 hour, monitoring the progress spectroscopically. The reaction mixture was diluted with water (300 mL) and extracted with dichloromethane. The combined organic layers were washed with water, dried and evaporated in vacuum. The residue was chromatographed on silica (eluent: dichloromethane-acetone, gradient 2%–5% acetone) to give the title product as dark-green crystals (from dichloromethane/hexane). Yield: 44 mg (79%). UV-vis λmax (CH2Cl2) nm (ε × 104) 357 (6.63), 398 (7.69), 534 (2.13), 660 (1.38), 740 (2.28), 829 (13.68); 1H NMR δ : 9.28 (s, 2H), 8.76 (s, 1H), 8.63 (s, 1H), 8.17 (m, 1H), 7.34 (m, 5H), 5.46 (dd, 1H), 4.38 (m, 1H), 4.19 (m, 1H), 3.82 (s, 3H), 3.66 (s, 3H), 3.65 (s, 3H), 3.22 (s, 3H), 2.74 (m, 1H), 2.46 (m, 3H,), 2.10 (m, 2H), 1.83 (m, 6H), 1.18 (t, 3H), −0.69 and −0.83 (each br s, 2H); 13C NMR δ : 198.6, 173.9, 171.7, 168.7, 166.8, 166.0, 165.6, 146.6, 139.9, 136.0, 135.4, 134.8, 134.4, 134.2, 133.7, 132.7, 132.1, 130.3, 127.7, 127.1, 123.7, 122.7, 121.5, 119.1, 118.3, 115.5, 101.2, 101.1, 99.8, 97.7, 57.0, 54.8, 51.5, 48.0, 47.0, 33.2, 32.2, 31.5, 29.9, 24.2, 23.5, 13.6, 13.3, 10.8; FABMS m/z 719.3 ([MH]+, 100%); HRFABMS C44H43N6O4 [MH]+ calcd 719.3346, obsd 719.3338.
Diazomethane ring-enlargement reaction
132-Oxo-bacteriopyropheophorbide a methyl ester 8 (100 mg) was dissolved in dichloromethane (50 mL) and diazomethane, prepared from 2 g of N-methyl-N-nitroso-p-tolunesulfonamide (Diazald), was added. The reaction mixture was kept at room temperature in a sealed flask in the dark overnight, after which the solvent was evaporated in vacuum. Residue was chromatographed on silica (eluent: dichloromethane-acetone, gradient 3%–5% acetone) to give the three products. The fastest moving orange-red band was 132-methoxy-bacterioverdin methyl ester (14). Dark-red prisms (from dichloromethane/methanol). Yield: 30 mg (29%). UV-vis λmax (CH2Cl2) nm (ε × 104) 360 (9.56), 424 (5.52), 550 (2.52), 799 (2.08), 891 (5.18); 1H NMR δ : 8.93 (s, 1H, 5-H), 8.16 (s, 1H, 20-H), 8.15 (s, 1H, 10-H), 6.89 (s, 1H, 131-H), 4.79 (dd, 1H, 17-H), 4.06 (s, 3H, 132-OCH3), 3.98 (m, 1H, 7-H), 3.86 (q, 1H, 18-H), 3.77 (dd, 1H, 8-H), 3.62 (s, 3H, CO2CH3), 3.44 (s, 3H, 12-CH3), 3.18 (s, 3H, 2-CH3), 3.09 (s, 3H, COCH3), 2.86 (m, H, 171 a-H), 2.56 (m, 1H, 8-CH2, 172 b-H), 2.26 (m, 3H, 8-CH2,171 a′-H,), 2.24 (m, 1H, 172 b′-H), 1.73 (d, 3H, J =8.0 Hz, 7-CH3), 1.64 (d, 3H, J =8.0 Hz, 18-CH3), 1.06 (t, 3H, J =7.6 Hz, 82-CH3), 0.49 and 0.22 (each br s, 2H, 21,23-NH); 13C NMR δ : 198.3, 182.8, 174.5, 174.0, 168.7, 165.7, 162.8, 153.1, 141.4, 140.0, 133.6, 132.0, 130.7, 128.5, 127.6, 126.9, 104.0, 103.4, 101.8, 99.2, 57.6, 55.9, 55.7, 51.4, 46.1, 45.1, 32.9, 32.7, 30.1, 29.7, 28.9, 24.2, 23.4, 13.3, 10.8, 10.4; FABMS m/z 609.3 ([MH]+, 100%); HRFABMS C36H40N4O5 [MH]+ calcd 609.3077, obsd 609.3054.
The second band was identified as 131-methoxybacterioverdin (15) isolated as pink-red crystals. Yield: 45 mg (42%). UV-vis λmax (CH2Cl2) nm (ε × 104) 369 (10.43), 418 (5.12), 552 (3.72), 779 (2.46), 874 (7.85); 1H NMR δ : 9.05 (s, 1H, 5-H), 8.46 (s, 1H, 10-H), 8.37 (s, 1H, 20- H), 5.95 (s, 1H, 132-H), 5.18 (dd, 1H, 17-H), 4.18 (s, 3H, 131-OCH3), 4.16 (m, 1H, 7-H), 4.05 (q, 1H, 18-H), 3.90 (dd, 1H, 8-H), 3.65 (s, 3H, CO2CH3), 3.54 (s, 3H, 12-CH3), 3.50 (s, 3H, 2-CH3), 3.12 (s, 3H, COCH3), 2.76 (m, H, 171 a-H), 2.52 (m, 1H, 8-CH2, 172 b-H), 2.43 (m, 2H, 8-CH2), 2.14 (m, 2H, 171 a′-H, 172 b′-H), 1.76 (d, 3H, J =8.0 Hz, 7-CH3), 1.64 (d, 3H, J =8.0 Hz, 18-CH3), 1.06 (t, 3H, J =7.6 Hz, 82-CH3), 0.36 (br s, 2H, 21,23-NH); 13C NMR δ : 198.5, 189.8, 174.1, 173.6, 167.3, 166.3, 166.2, 165.1, 139.3, 137.1, 134.2, 133.9, 132.2, 129.9, 129.3, 122.1, 103.7, 103.6, 102.3, 101.4, 97.9, 57.2, 55.8, 54.8, 51.4, 47.2, 46.2, 33.0, 32.6, 30.8, 29.5, 24.2, 23.4, 13.5, 12.4, 10.8; FABMS m/z 609.3 ([MH]+, 100%); HRFABMS C36H40N4O5 [MH]+ calcd 609.3077, obsd 609.3066.
The third product was identified 133-methoxybacterioisoverdin (16) isolated as blue-green crystals (from dichloromethane/methanol). Yield: 18 mg (16 %). UV-vis λmax (CH2Cl2) nm (ε × 104) 371 (8.94), 395 (4.69), 596 (2.13), 766 (2.56), 865 (4.86); 1H NMR δ : 8.95 (s, 1H, 5-H), 8.56 (s, 1H, 10-H), 8.36 (s, 1H, 20-H), 6.05 (s, 1H, 131-H), 4.78 (dd, 1H, 17-H), 4.22 (s, 3H, 133- OCH3), 4.18 (m, 1H, 7-H), 4.06 (q, 1H, 18-H), 3.92 (dd, 1H, 8-H), 3.74 (s, 3H, 12-CH3), 3.65 (s, 3H, CO2CH3), 3.51 (s, 3H, 2-CH3), 3.16 (s, 3H, COCH3), 2.56 (m, H, 171 a-H), 2.46 (m, 3H, 8- CH2, 172 b-H), 2.24 (m, 2H, 171 a′-H, 172 b′-H), 1.79 (d, 3H, J =8.0 Hz, 7-CH3), 1.73 (d, 3H, J =8.0 Hz, 18-CH3), 1.09 (t, 3H, J =7.6 Hz, 82-CH3), 0.39 and 0.32 (each br s, 2H, 21,23-NH); 13C NMR δ : 198.4, 185.1, 173.6, 171.0, 169.9, 169.1, 168.9, 163.5, 136.7, 136.2, 134.0, 133.9, 133.8, 131.5, 122.0, 103.1, 102.6, 102.5, 101.4, 96.7, 56.4, 56.1, 54.6, 51.6, 48.5, 47.2, 33.1, 31.9, 31.8, 29.8, 29.6, 23.8, 23.0, 13.4, 12.2, 10.7; FABMS m/z 609.3 ([MH]+, 100%); HRFABMS C36H40N4O5 [MH]+ calcd 609.3077, obsd 609.3078.
Electrochemical Measurements
Cyclic voltammetry was carried out with an EG&G Model 173 potentiostat/galvanostat. A homemade three-electrode cell was used and consisted of a platinum button or glassy carbon working electrode, a platinum wire counter electrode and a saturated calomel reference electrode (SCE). The SCE was separated from the bulk of the solution by a fritted-glass bridge of low porosity which contained the solvent/supporting electrolyte mixture. All potentials are referenced to the SCE. Low temperature measurements were carried out by immersing the cell in a using a dry ice/acetone mixture bath.
Thin-layer UV-visible spectroelectrochemical experiments were performed using a home-built thin-layer cell, which has a transparent platinum net working electrode. Potentials were applied and monitored with an EG&G PAR Model 173 potentiostat. High purity N2 from Trigas was used to deoxygenate the solution and kept over the solution during each electrochemical and spectroelectrochemical experiment.
Tetra-n-butylammonium perchlorate (TBAP, ≥ 99%) were purchased from Fluka Chemical Co. and recrystallized from ethyl alcohol, and dried under vacuum at 40 °C for at least one week prior to use. Dichloromethane (CH2Cl2, 99.8%) was purchased from EMD Chemicals Inc. and used as received.
Photophysical Measurements
Absorption spectra were recorded on a Hewlett-Packard 8453A diode array spectrophotometer. Time-resolved fluorescence and phosphorescence spectra were measured by a Photon Technology International GL-3300 with a Photon Technology International GL-302 nitrogen laser/pumped dye laser system equipped with a four-channel digital delay/pulse generator (Stanford Research System Inc. DG535) and a motor driver (Photon Technology International MD-5020). Excitation wavelengths were from 538 to 551 nm using coumarin 540A (Photon Technology International, Canada) as a dye. Fluorescence lifetimes were determined by a single exponential curve fit. Nanosecond transient absorption measurements were carried out using a Panther OPO pumped a Nd:YAG laser (Continuum, SLII-10, 4–6 ns fwhm) at 430 nm with the power of 3 mJ as an excitation source. Photoinduced events were estimated by using a continuous Xe lamp (150 W) and an InGaAs-PIN photodiode (Hamamatsu 2949) as a probe light and a detector, respectively. The output from the photodiodes and a photomultiplier tube was recorded with a digitizing oscilloscope (Tektronix, TDS3032, 300 MHz). The transient spectra were recorded using fresh solutions in each laser excitation. All experiments were performed at 298 K.
Supplementary Material
Scheme 1.
Scheme 2.
Scheme 3.
Acknowledgments
Support from the NIH (CA127369, R. K. P.), Robert A. Welch Foundation (Grant E-680, K. M. K.) and a Global COE program from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to S.F.), and KOSEF/MEST through WCU projects (R31-2008-000-10010-0), Korea is gratefully acknowledged. We are thankful to Dr. Fajer, Brookhaven National Lab (BNL) for X-ray analysis of one of the bacteriochlorin analogs. The help rendered by Dr. Mark Renner (ZINDO calculations) and Michael Becker for crystallizing compound 14 is also appreciated. X-ray data and were measured at beamline X25 of the National Synchrotron Light Source and supported by Center for Research Resources of the NIH, and the Office of Biological and Environmental Research and Basic Energy Sciences of the US Dept. of Energy, a Grant-in-Aid (No. 20108010 to S.F. and 23750014 to K.O.). We wish to thank Dr. Avinash Phadke (Achillion Pharmaceuticals, Inc.) for valuable discussions, (late) Dr. James L. Alderfer, RPCI, Buffalo for 2D/ROSEY measurements and Dr James G. Pavlovich (UCSB) for help in obtaining mass-spectra and Drs. A. A.Tsygankov and N. Zorin (ISP, Puschino-on-Oka, Russian Federation) for a supply of Rhodobacter sphaeroides biomass. A part of the experimental chemistry/analysis was performed at the former Miravant Medical Technology, Inc. Santa Barbara, CA 93117.
Footnotes
Supporting Information Available. The 1H and 13C NMR spectra of compounds, their transient absorption spectra and crystallographic details for compound 14 in CIF format and an ORTEP diagram of the conformer shown in Fig 4, tables of atom coordinates and absolute energies to document the theoretical calculations, transient absorption spectra and full author list of ref 43 are available free of charge via internet at http://pubs.acs.org.
References
- 1.Ethirajan M, Chen Y, Penny J, Pandey RK. Chem Soc Rev. 2011;40:340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- 2.Dolmans EJGJ, Kadambi A, Hill JS, Waters CA, Robinson BC, Walker JP, Fukumura D, Jain RK. Cancer Res. 2002;62:2151. [PubMed] [Google Scholar]
- 3.Pandey RK, Herman C. Chem Ind (London) 1998:739. [Google Scholar]
- 4.Bonnett R. Chem Soc Rev. 1995:19. and references therein. [Google Scholar]
- 5.Wilson BC. CIBA Found Symp. 1989;52:741. doi: 10.1002/9780470513842.ch5. [DOI] [PubMed] [Google Scholar]
- 6.Jasat A, Dolphin D. Chem Rev. 1997;97:2267. doi: 10.1021/cr950078b. and references therein. [DOI] [PubMed] [Google Scholar]
- 7.Lash TD, Werner TM, Thompson ML, Manley JD. J Org Chem. 2001;66:3152. doi: 10.1021/jo010066s. [DOI] [PubMed] [Google Scholar]
- 8.Sessler JL, Hemmi G, Mody TD, Murai T, Burrell A, Young SW. Acc Chem Res. 1994;27:43. and references therein. [Google Scholar]
- 9.Vogel E, Koch P, Hou X-L, Lex J, Lausman M, Kisters M, Aukauloo M, Richard P, Guilard R. Angew Chem, Int Ed Engl. 1993;32:1600. [Google Scholar]
- 10.Kozyrev AN, Efimov AV, Efremova OA, Perepyolkin, Yu P, Mironov AF. Proc SPIE. 1994;2325:297. [Google Scholar]
- 11.Phadke AS, Robinson BC, Barkigia KM, Fajer J. Tetrahedron. 2000:7661. [Google Scholar]
- 12.Hynninen PH. In: The Chlorophylls. Scheer H, editor. CRC Press; Roca Baton FL: 1991. pp. 146–209.Chen Y, Li G, Pandey RK. Curr Org Chem. 2004;8:1105.Yang E, Kirmaier C, Krayer M, Taniquchi M, Kim HJ, Diers JR, Bocian DF, Lindsey JS, Hollen D. J Phys Chem B. 2011;115:10801. doi: 10.1021/jp205258s.Kim HJ, Lindsey JS. J Org Chem. 2005;70:5475. doi: 10.1021/jo050467y.Tamiaki H, Miyatake T, Tanikaga R, Holzwarth A, Schaffner K. Angew Chem, Int Ed Engl. 1996;35:772.and references therein Sasaki S, Tamiaki H. J Org Chem. 2006;71:2648. doi: 10.1021/jo0523969.
- 13.Yon-Hin P, Wijesekera TP, Dolphin D. Tetrahedron Lett. 1991;32:2875. [Google Scholar]
- 14.(a) Pandey RK, Shiau FY, Ramachandaran K, Dougherty TD, Smith KM. J Chem Soc, Perkin Trans 1. 1992:1377. [Google Scholar]; (b) Ethirajan M, Joshi P, William WH, Jr, Ohkubo K, Fukuzumi S, Pandey RK. Org Lett. 2011;13:1956–1959. doi: 10.1021/ol200314v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson BC, Barkigia KM, Renner MW, Fajer J. J Phys Chem B. 1999;103:7324. [Google Scholar]
- 16.Kay CW, Conti F, Fuhs M, Plato M, Weber S, Bordignon E, Carbonera D, Robinson BC, Renner MW, Fajer J. J Phys Chem B. 2002;106:2769. [Google Scholar]
- 17.Robinson BC. Tetrahedron. 2000;56:6005. [Google Scholar]
- 18.Morgan AR, Skalkos D, Garbo GD, Keck RW, Selman SH. J Med Chem. 1991;34:2126. doi: 10.1021/jm00111a031. [DOI] [PubMed] [Google Scholar]
- 19.Pandey RK, Goswami LN, Chen Y, Grushuk A, Missert JR, Oseroff A, Dougherty TJ. Lasers Surg Med. 2006;38:445. doi: 10.1002/lsm.20352.and references therein Pandey RK, Zheng G. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 6. Academic Press; San Diego: 2000. pp. 157–230.
- 20.Beems EM, Dubbelman TMAR, Lugtenberg J, Van Best JA, Smeets MFMA, Boegheim JP. Photochem Photobiol. 1987;45:639. doi: 10.1111/j.1751-1097.1987.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 21.Henderson BW, Sumlin AB, Owczarczak BL, Dougherty TJ. J Photochem Photobiol B. 1991;10:303. doi: 10.1016/1011-1344(91)80016-b. [DOI] [PubMed] [Google Scholar]
- 22.Kozyrev AN, Zheng G, Dougherty TJ, Smith KM, Pandey RK. Tetrahedron Lett. 1996;37:6431. [Google Scholar]
- 23.(a) Kozyrev AN, Dougherty TJ, Pandey RK. Chem Commun. 1998:481. [Google Scholar]; (b) Joshi P, Ethirajan M, Goswami LN, Srivatsan A, Missert JR, Pandey RK. J Org Chem. 2011;76:8629. doi: 10.1021/jo201688c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mironov AF, Kozyrev AN, Brandis AS. Proc SPIE. 1992. p. 202. [Google Scholar]
- 25.Wasielewski MR, Svec WA. J Org Chem. 1980;45:1969. [Google Scholar]
- 26.Fischer H, Lambrecht R, Mittenzwei H. Z Physiol Chem. 1938;253:1. [Google Scholar]
- 27.(a) Pandey RK, Kozyrev AN, Zheng L. 7147840. US Patent. 2006; (b) Fukuzumi S, Ohkubo K, Zheng X, Chen Y, Pandey RK, Zhan R, Kadish KM. J Phys Chem B. 2008;112:2738–2746. doi: 10.1021/jp0766757. [DOI] [PubMed] [Google Scholar]
- 28.Kozyrev AN, Alderfer JL, Srikrishnan T, Pandey RK. J Chem Soc Perkin Trans 1. 1998:837. [Google Scholar]
- 29.Kozyrev AN, Suresh V, Das S, Senge MO, Shibata T, Alderfer JL, Dougherty TJ, Pandey RK. Tetrahedron. 2000;56:3353. [Google Scholar]
- 30.Pandey RK, Shiau FY, Ramachandaran K, Dougherty TD, Smith KM. J Chem Soc Perkin Trans 1. 1992:1377. [Google Scholar]
- 31.Morgan AR, Rampersaud A, Keck RW, Selman S. Photochem Photobiol. 1987;46:441. doi: 10.1111/j.1751-1097.1987.tb04791.x. [DOI] [PubMed] [Google Scholar]
- 32.Kozyrev AN, Alderfer JL, Dougherty TJ, Pandey RK. Chem Commun. 1998:1083. [Google Scholar]
- 33.Kozyrev AN, Alderfer JL, Dougherty TJ, Pandey RK. Angew Chem Int Ed Engl. 1999;38:126. [Google Scholar]
- 34.ZINDO-S method/HyperChem 7 Program Package.
- 35.Fukuzumi S, Ohkubo K, Chen Y, Pandey RK, Zhan R, Shao J, Kadish KM. J Phys Chem A. 2002;106:5105. [Google Scholar]
- 36.Geskes C, Hartwich G, Scheer H, Maentele W, Heinze J. J Am Chem Soc. 1995;117:7776. [Google Scholar]
- 37.Kadish KM, Caemelbecke VE, Royal G. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 8. Academic Press; San Diego, CA: 2000. pp. 1–97. [Google Scholar]
- 38.(a) Fukuzumi S, Suenobu T, Patz M, Hirasaka T, Itoh S, Fujitsuka M, Ito O. J Am Chem Soc. 1998;120:8060. [Google Scholar]; (b) Fukuzumi S, Ohkubo K, Imahori H, Guldi DM. Chem Eur J. 2003;9:1585. doi: 10.1002/chem.200390182. [DOI] [PubMed] [Google Scholar]; (c) Kawashima Y, Ohkubo K, Fukuzumi S. J Phys Chem A. 2012;116:8942. doi: 10.1021/jp3059036. [DOI] [PubMed] [Google Scholar]
- 39.Noy D, Fiedor L, Hartwich G, Scheer H, Scherz A. J Am Chem Soc. 1998;120:3684. [Google Scholar]
- 40.Frank B, Nonn A. Angew Chem, Int Ed Engl. 1995;34:1795. [Google Scholar]
- 41.Seidal D, Lynch V, Sessler JL. Angew Chem, Int Ed. 2002;41:1422. doi: 10.1002/1521-3773(20020415)41:8<1422::aid-anie1422>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 42.Becke AD. J Chem Phys. 1993;98:5648. [Google Scholar]
- 43.Gaussian 09, Revision A.02. Gaussian, Inc; Wallingford CT: 2009. (Full author list is shown in SI) [Google Scholar]
- 44.Dennington R, II, Keith T, Millam J, Eppinnett K, Hovell WL, Gilliland R. Gaussview. Semichem, Inc; Shawnee Mission, KS: 2003. [Google Scholar]
- 45.Ethirajan M, Joshi P, William WH, Ohkubo K, Fukuzumi S, Pandey RK. Org Lett. 2011;13:1956. doi: 10.1021/ol200314v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crossley MJ, Langford SJ, Parashar JK, Burn PL. J Chem Soc, Chem Commun. 1998:1921. [Google Scholar]
- 47.Hartwich GA, Fiedor L, Simonin I, Cmiel E, Schafer W, Noy D, Scherz A, Scheer H. J Am Chem Soc. 1998;120:3675. [Google Scholar]
- 48.Kozyrev AN, Perepyolkin PY, Mironov AF. Proc SPIE. 1993:186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.