Abstract
Experimental and clinical data suggest that aspirin and other non-steroidal inflammatory drugs may delay the progression of prostate cancer through inhibition of the cyclooxygenase (COX) pathway and its effects on cellular proliferation, apoptosis and angiogenesis. Epidemiological data support a reduced risk of prostate cancer incidence with aspirin use, yet no evidence exists regarding whether aspirin after diagnosis influences progression or survival. We conducted a prospective study of 3,986 participants of the Health Professionals Follow-up Study, with a prostate cancer diagnosis between January 1, 1990 and December 31, 2005. We used Cox proportional hazards regression to evaluate the association between aspirin use after diagnosis and the development of metastases or fatal prostate cancer through January 31, 2008, adjusting for risk factors associated with incidence and mortality in this cohort, pre-diagnostic aspirin use, Gleason score, TNM stage and primary treatment. In total, 265 men developed bony or other organ metastases or fatal prostate cancer during the 18 years of follow-up. We observed no association between updated aspirin use after diagnosis and lethal prostate cancer (tablets/week: <2, HR=1.12, 95% CI: 0.72, 1.72; 2-5, HR=1.05, 95% CI: 0.62, 1.80, ≥ 6, HR=1.08, 95% CI: 0.76, 1.54; p-trend=0.99). The results remained unchanged when we examined aspirin use at baseline only (p-trend=0.70) or frequency of use (days/week, p-trend=0.35); or limited the outcome to fatal prostate cancer (p-trend = 0.63). There was no association between aspirin use after a prostate cancer diagnosis and lethal disease in this cohort of prostate cancer survivors.
Introduction
Aspirin and other non-steroidal anti-inflammatory drugs (NSAID’s) inhibit the COX pathway and may affect cell proliferation, apoptosis, angiogenesis and tumor progression (1). Clinical data suggest that COX-2 inhibitors may delay the progression of disease in prostate cancer patients (2), (3), (4), (5), (6). Experimental data indicate that COX-2 dependent growth may mediate prostate cancer progression in an androgen independent manner, suggesting that hormone-refractory patients may benefit from COX-2 inhibitors (7). Clinical trials of NSAID’s - primarily COX-2 inhibitors - in prostate cancer patients have focused on biochemical recurrence as an endpoint (3), (4) or on molecular and gene expression profiles of treated prostate tumors (1), (6) but no studies have evaluated long-term outcomes such as metastases or cancer-specific mortality for aspirin or other NSAID’s after diagnosis. Aspirin may be useful for chemoprevention, given its cardiovascular benefits, safety and efficacy profiles (8) ((9)). We previously reported a reduced risk of pre-diagnostic aspirin use and high grade and lethal prostate cancer in this cohort ((10) and meta-analyses (11), (12), (9) also suggest that aspirin use is associated with a lower risk of incident prostate cancer, especially advanced disease. However, there are no epidemiological data on whether aspirin use after diagnosis influences prostate cancer progression and long-term survival.
Methods
Study Population
Study participants were members of the Health Professionals Follow-up Study (HPFS), a cohort of 51,529 US male dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians, who returned a mailed health questionnaire in 1986. Participants were 40-75 years of age at baseline and the questionnaire included a validated assessment of diet (13) and medical diagnoses, including cancer. Follow-up questionnaires are mailed biennially to update anthropometric, physical activity, smoking, medication, vitamin, diet (collected every four years) and other lifestyle factors. The response rate is 96%. The conduct of this cohort study and these analyses were approved by the Institutional Review Board of the Harvard School of Public Health.
We identified HPFS participants who reported a prostate cancer diagnosis on or after January 1, 1990, when we first collected detailed information on aspirin use. We did not consider men whose diagnosis date preceded the 1990 questionnaire, since quantity and frequency data were not available then. Medical records and pathology reports were reviewed to confirm the diagnosis and to determine clinical data, including Gleason score, prostate-specific antigen (PSA) levels at diagnosis, tumor-node-metastasis (TNM) stage (14) and primary treatment. Development of metastases was ascertained through mailed questionnaires to participants and their physicians. Deaths were identified through the National Death Index, postal system and next of kin, with virtually complete follow-up (15). A prostate cancer death was based on evidence of extensive metastatic disease and no other plausible cause of death and was determined by central adjudication of medical records and death certificates by study physicians.
Assessment of aspirin use and other covariates
Detailed information about aspirin use was first available in 1992 and updated through mailed questionnaires. Men were asked to report the number of days per week they used aspirin, and the number of adult-strength tablets (325 mg) consumed per day or per week (for dose levels, men were reminded that four 81mg tablets was the equivalent of one full-strength tablet). In any given cycle, men were considered non-users if they took aspirin less than two days per week. Information was updated every two years and individual medications or brand names were not ascertained (described in detail previously (10)). Information on aspirin use was completed by over 98% of participants during the study period (1990-2006), and missing data were retained as a separate stratum in regression models.
Statistical Analysis
We evaluated aspirin use using a 2-year lag analysis to avoid a potential bias due to changes in aspirin use close to the time of death. Men diagnosed on or after January 1, 1990 accrued follow-up time starting on the month of the questionnaire return date that followed their date of diagnosis, and ending on the date of metastases, the date of death or the end of follow-up (January 31, 2008), whichever came first. Eligible cases included men diagnosed with clinical stage T1, T2 or T3a. We considered two outcomes for these analyses – fatal and lethal prostate cancer. Lethal prostate cancer was defined as development of metastases to the bone or other organs, or death due to prostate cancer.
We used Cox proportional hazards regression to calculate the hazard ratio (HR) and 95% confidence intervals (CI) while adjusting for age (in years), time period (2-year intervals), established risk factors (race and family history) and other covariates shown to be associated with incidence or mortality in HPFS ((16)): height (<66, 66-67.9, 68-69.9, 70-71.9, ≥ 72 inches), body mass index (<21.0, 21-22.9, 23-24.9, 25-27.4, 27.5-29.9, ≥ 30+ kg/m2), smoking (never smoker or quit >10 years, current smoker or quit ≤10 years and <15 cigarettes/day, current smoker or quit ≤10 years and ≥15 cigarettes/day), intake of tomato sauce (<0.25, 0.25-1, 1-2, ≥ 2 servings/week), vitamin D intake (quintiles), total kilocalories (quintiles, kcal per day), fish (<2/month, 2/month-1/week, >1-<3/week, ≥ 3/week), red meat (quintiles, servings per week), vigorous physical activity (quintiles, hours), the use of statins (no, past, current user), Gleason score (4-6, 7, 8-10), TNM stage (T1, T2 vs. T3a) and initial treatment (radical prostatectomy (RP), external beam radiation and brachytherapy (RT), watchful waiting (WW), hormone therapy (HT) or other). We also adjusted for aspirin use prior to diagnosis in tablets per week (users, n=2111, non-users, n=1605) as it was previously associated with lethal disease in this cohort (10). As a secondary analysis, we also present data separately for non-aspirin NSAID use and the combination with aspirin as total NSAID’s. Except for race, family history, body mass index (BMI) at diagnosis, Gleason score, stage and treatment, covariates were updated every two or four years (dietary information was collected every four years). Tests for linear trend were conducted by assigning the median value in each category of aspirin use (p<0.05 used as the cutoff for statistical significance).
Results
A total of 3,986 men reported a prostate cancer diagnosis on or after January 1, 1990 and 265 of these men developed metastases or died of prostate cancer during the 18-year follow-up period. The average age at diagnosis was 68.6 years, and men were followed for an average of 8.4 years (Table 1). Aspirin use was reported by 39.8% of men on the first questionnaire after their prostate cancer diagnosis. These men were more likely than non-users to have low-grade (Gleason ≤ 6, 61.3%) or PSA-detected disease (T1 = 58.9%). Over four-fifths of men underwent either radical prostatectomy (n=1849) or radiation (n=1452, includes external beam radiation and brachytherapy) as their primary form of treatment.
Table I.
Clinical characteristics of men diagnosed with prostate cancer in the Health Professionals Follow-up Study reported on or after the 1992 biennial questionnaire* and status of aspirin use (user vs. non-user) based on first-post diagnosis questionnaire (≤ 2 years after diagnosis)
| Case characteristics | Aspirin user (n=1579) |
Non-user (n=1926) |
|---|---|---|
| % or Mean (SD) |
% or Mean (SD) |
|
| Age at diagnosis (yrs) | 69.0 (6.9) | 67.9 (7.5) |
| Body mass index (kg/m2, baseline) | 25.7 (3.5) | 25.6 (3.3) |
| Follow-up time (yrs) | 9.0 (3.8) | 9.4 (3.9) |
| PSA at dx (ng/mL) (median (IQR)) | 7.2 (5.1, 10.8) | 7.2 (5.0, 11.7) |
| missing (%) | 4.9 | 5.9 |
| Gleason score | ||
| 4-6 | 61.3 | 58.1 |
| 7 | 20.8 | 22.0 |
| 8-10 | 7.3 | 7.0 |
| missing (%) | 10.6 | 12.9 |
| Clinical stage | ||
| T1, NX/NO | 58.9 | 56.2 |
| T2, NX/NO | 38.2 | 40.0 |
| T3a or T3, NX/NO | 2.9 | 3.8 |
| Pre-diagnostic aspirin use** | ||
| Yes | 56.1 | 21.5 |
| No | 33.3 | 67.8 |
| Missing | 10.6 | 10.7 |
| Treatment | ||
| Prostatectomy | 44.4 | 50.3 |
| Radiation | 38.8 | 33.4 |
| Watchful waiting | 4.5 | 4.2 |
| Hormone | 7.4 | 7.4 |
| Other | 2.4 | 1.8 |
| Missing | 2.5 | 3.0 |
does not include n=481 persons with missing aspirin data on 1992 questionnaire (total survivors cohort, n=3986)
assessed at 2 cycles prior to diagnosis
We observed no association between updated aspirin use after diagnosis and the risk of lethal prostate cancer (p=0.99 for tablets/week for lethal disease, Table 2), after adjusting for prostate cancer risk factors in this cohort, pre-diagnostic aspirin use (tablets per week), Gleason score (4-6, 7, 8-10), stage of disease (T1, T2, T3a) and primary treatment (radical prostatectomy, radiation, hormone therapy, watchful waiting, other and missing). Similar null results were observed when we evaluated frequency of aspirin use (days per week). Also, when we evaluated the long-term effect of baseline use (first post-diagnostic report of aspirin tablets/week), the conclusions remained the same (p-for-trend = 0.70). For fatal prostate cancer, we also observed no association with aspirin use after diagnosis (updated or baseline only in tablets/week and days/week, Table 2). In analyses stratified by Gleason score, clinical stage (T1 vs. T2/T3) or pre-diagnostic aspirin use (yes/no), there were no significant differences in the associations between aspirin use after diagnosis and the risk of lethal prostate cancer (Figure 1). There was no association for men treated with a radical prostatectomy (n=87 events, p=0.59) or radiation treatment, which includes external beam radiation and brachytherapy (n=104 events, p=0.59); other treatment numbers were too small to evaluate separately (n=23, n=21 and n=9 for HT, WT and other; data not shown).
Table II.
Effects of post-diagnostic aspirin use on fatal and lethal (development of metastases to bone, other organs, or fatal) prostate cancer
| Fatal prostate cancer (n=177 events)
|
Lethal disease (n=265 events)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Aspirin Use | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Quantity, updated since diagnosis | ||||||||
| Non-user | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| < 2 tabs/week | 0.73 | 0.42, 1.26 | 0.93 | 0.52, 1.67 | 0.91 | 0.60, 1.36 | 1.12 | 0.72, 1.72 |
| 2 -5 tabs/week | 0.94 | 0.49, 1.78 | 1.08 | 0.55, 2.14 | 0.93 | 0.55, 1.56 | 1.05 | 0.62, 1.80 |
| ≥ 6 tabs/week | 0.99 | 0.67, 1.45 | 1.16 | 0.74, 1.82 | 0.94 | 0.68, 1.30 | 1.08 | 0.76, 1.54 |
| test-for-trend (p-value) | 0.63 | 0.99 | ||||||
| Quantity at baseline/diagnosis | ||||||||
| Non-user | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| < 2 tabs/week | 1.11 | 0.71, 1.73 | 1.39 | 0.85, 2.28 | 1.17 | 0.81, 1.68 | 1.37 | 0.92, 2.03 |
| 2 -5 tabs/week | 0.84 | 0.49, 1.42 | 1.08 | 0.61, 1.91 | 0.96 | 0.63, 1.45 | 1.20 | 0.78, 1.84 |
| ≥ 6 tabs/week | 0.92 | 0.62, 1.37 | 1.19 | 0.76, 1.87 | 0.91 | 0.65, 1.27 | 1.14 | 0.79, 1.64 |
| test-for-trend (p-value) | 0.62 | 0.70 | ||||||
| Frequency, updated since diagnosis | ||||||||
| < 2 days/week | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| 2 -3 days/week | 0.76 | 0.38, 1.52 | 0.92 | 0.44, 1.91 | 0.65 | 0.36, 1.19 | 0.72 | 0.39, 1.33 |
| 4 -5 days/week | 0.66 | 0.24, 1.87 | 0.67 | 0.23, 1.97 | 0.99 | 0.50, 1.99 | 1.07 | 0.52, 2.19 |
| 6 -7 days/week | 0.93 | 0.65, 1.32 | 1.14 | 0.76, 1.71 | 0.95 | 0.71, 1.26 | 1.13 | 0.82, 1.55 |
| test-for-trend (p-value) | 0.42 | 0.35 | ||||||
| Frequency at baseline/diagnosis | ||||||||
| < 2 days/week | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| 2 -3 days/week | 0.84 | 0.39, 1.81 | 0.96 | 0.43, 2.14 | 0.90 | 0.48, 1.67 | 1.02 | 0.54, 1.93 |
| 4 -5 days/week | 1.08 | 0.51, 2.30 | 1.03 | 0.45, 2.36 | 1.17 | 0.63, 2.15 | 1.19 | 0.63, 2.26 |
| 6 -7 days/week | 1.00 | 0.71, 1.39 | 1.33 | 0.90, 1.97 | 1.01 | 0.76, 1.33 | 1.26 | 0.92, 1.73 |
| test-for-trend (p-value) | 0.19 | 0.18 | ||||||
adjusted for age (months), period (2-year intervals), family history (yes/no), race (Asian, Caucasian or African-American), height (<66, 66-67.9, 68-69.9, 70-71.9, 72+ inches), BMI at dx (<21, 21-22.9, 23-24.9, 25-27.4, 27.5-29.9, 30+ kg/m2), tomato sauce (<0.25, 0.25-1, 1-2, 2+/wk), vigorous physical activity (quintiles, hours), smoking (never or curr or quit < 10 yrs & < 15 cigs/day), quit>10 yrs, vitamin D (quintiles,), fish (<2/mo, 2/mo-1/wk, >1-3/wk, 3+/wk), red meat (quintiles), cholesterol-lowering drugs (non-user, current user), total kcal (quintiles), gleason score (4-6, 7, 8-10), aspirin use prior to diagnosis (tabs/wk), TNM stage (T1/T2 and N0/M) vs. T3/T4 or N1 or M1) and initial treatment (WW, RP, RT, HT, other, missing)
first post-diagnostic measurement
Non-users are defined as those taking aspirin< 2 days/week in a given cycle
Figure 1.
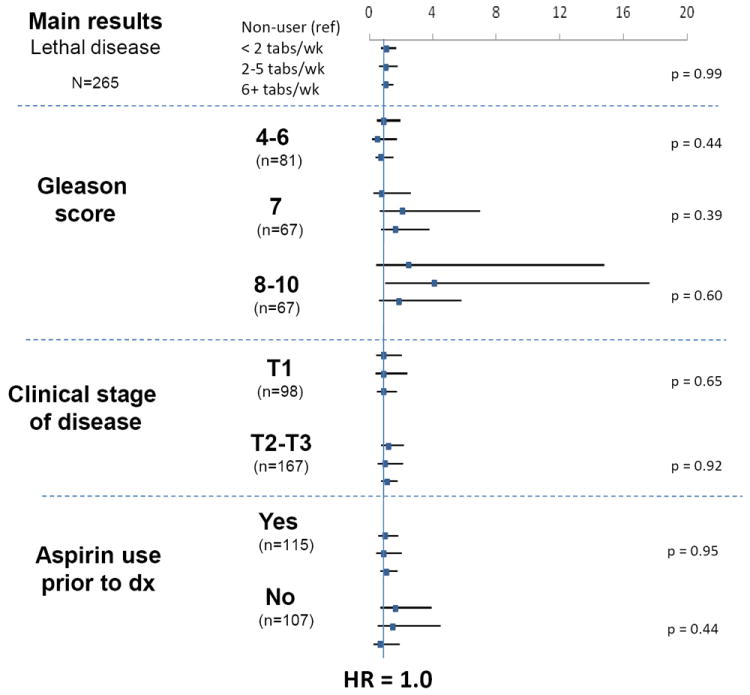
Post-diagnostic aspirin use and lethal prostate cancer, stratified by Gleason score, stage of disease and aspirin use prior to diagnosis in the Health Professionals Follow-up Study, 1992-2006
Limited data were available on non-aspirin NSAID’s, including ibuprofen (yes/no regular user) and COX-inhibitors (number of tablets per week), and when we evaluated the associations with lethal disease, separately as non-aspirin NSAID’s (< 2 t/wk, RR=1.12 (95% CI: 0.40, 3.14); 2-5 t/wk, RR=0.92 (0.60, 1.43); 6+ t/wk, RR=0.81 (0.39, 1.67); p test-for-trend=0.46) and in combination with aspirin as total NSAID’s (< 2 t/wk, RR=1.07 (0.67, 1.72); 2-5 t/wk, RR=1.16 (0.76, 1.77); 6+ t/wk, RR=1.02 (0.72, 1.45); p test-for-trend =0.84), the associations remained null.
Discussion
This is the first study reporting on aspirin consumption after a prostate cancer diagnosis and its association with the development of lethal prostate cancer. We observed no association between post-diagnostic aspirin use and metastatic or fatal prostate cancer. We, and others have reported inverse associations for pre-diagnostic aspirin use and the risk of incident prostate cancer (9) (10), (11), (12), (17), (18). Prior evidence suggests an approximate 10-33% reduction in the risk of prostate cancer, with greater reductions associated with more aggressive disease. In this analysis, we adjusted for pre-diagnostic patterns to focus on the potential influence of aspirin on prostate cancer progression after diagnosis.
Laboratory data suggest that inhibiting the COX pathway can suppress prostate cancer progression via the nuclear factor-kappa B pathway (19), in an androgen-independent manner (7), through suppression of the Wnt/beta-catenin signaling pathway (20) and more generally through up-regulating apoptosis, antioxidant processes and tumor suppressor functions (6). Experimental data also support an increased benefit of COX inhibitors with combined androgen blockade (7) although some data in humans suggest the contrary (21). Another potential mechanism for reducing fatal disease may be through aspirin’s anti-platelet effects to reduce blood-borne metastases. One may speculate that such metastases may have already occurred by the time of diagnosis (22), (23), eliminating aspirin’s potential effect through that mechanism. This is also consistent with another study in which 400 mg twice daily of celecoxib had no effect on biomarkers of prostate carcinogenesis (eg, Ki-67, p27, p21, factor VIII) despite having reached target levels in prostate tissue (as measured by prostaglandins, COX-1 & COX-2 mRNA) in a randomized trial (2).
Although our previous work in this cohort demonstrated a significantly reduced risk of lethal disease associated with long-term, aspirin tablets per week before diagnosis (10), the post-diagnostic data do not yield significant associations. We considered the possibility that men with pain due to undiagnosed metastatic disease might take more aspirin close to the date of metastases, or that men might stop taking aspirin because they were receiving chemotherapy. To address this potential confounding, we implemented a 2-year lag to avoid capturing aspirin use that was influenced by underlying metastatic disease or treatment. Residual confounding may persist with a 2-year lag given the extended time course of prostate cancer, even after the development of metastatic disease. Thus, we also examined aspirin use at baseline only (first post-diagnostic report after the date of diagnosis) as a predictor of long-term survival and still observed no association between aspirin use and lethal disease. Aspirin use after diagnosis may be associated with other lifestyle modifications (eg, diet, exercise, etc.) that are associated with prostate cancer mortality, so we adjusted for known risk factors for prostate cancer incidence and mortality in this cohort using updated biennial data until the end of follow-up; age-adjusted and multivariate models yielded similar conclusions, as well as models with clinical predictors only (data not shown).
Our data do not support an association between aspirin use after a prostate cancer diagnosis and the development of lethal prostate cancer. These results and accumulating evidence of a small to moderate benefit of aspirin use on prostate cancer risk, suggest that aspirin may play a stronger role in the early stages of carcinogenesis and in chemoprevention, rather than in established disease.
Acknowledgments
We thank Ms. Elizabeth Frost-Hawes, Ms. Mira Kaufman, Ms. Siobhan Saint Surin, Ms. Laura Sampson, Ms. Barbara Vericker, Ms. Lauren McLaughlin, and Ms. Tara Entwistle for their continuing help in the Health Professionals Follow-Up Study.
Statement of funding
Dr. Dhillon was supported by a Cancer Epidemiology Training Grant NCI T32 CA009001. This work was supported by the following National Institute of Health grants: R01 CA141298-02 (MJS), R01 CA55075 (ELG), and P01 CA055075-19.
Footnotes
Conflicts of interest
Nil
References
- 1.Sooriakumaran P, Coley HM, Fox SB, Macanas-Pirard P, Lovell DP, Henderson A, et al. A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer. Anticancer Res. 2009 May;29(5):1483–8. [PubMed] [Google Scholar]
- 2.Antonarakis ES, Heath EI, Walczak JR, Nelson WG, Fedor H, De Marzo AM, et al. Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. J Clin Oncol. 2009 Oct 20;27(30):4986–93. doi: 10.1200/JCO.2009.21.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006 Jun 20;24(18):2723–8. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 4.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2172–7. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 5.Ganswindt U, Budach W, Jendrossek V, Becker G, Bamberg M, Belka C. Combination of celecoxib with percutaneous radiotherapy in patients with localised prostate cancer - a phase I study. Radiat Oncol. 2006;1:9. doi: 10.1186/1748-717X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sooriakumaran P, Macanas-Pirard P, Bucca G, Henderson A, Langley SE, Laing RW, et al. A gene expression profiling approach assessing celecoxib in a randomized controlled trial in prostate cancer. Cancer Genomics Proteomics. 2009 Mar-Apr;6(2):93–9. [PubMed] [Google Scholar]
- 7.Cai Y, Lee YF, Li G, Liu S, Bao BY, Huang J, et al. A new prostate cancer therapeutic approach: combination of androgen ablation with COX-2 inhibitor. Int J Cancer. 2008 Jul 1;123(1):195–201. doi: 10.1002/ijc.23481. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009 May;10(5):501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012:1–11. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988-2006. Int J Cancer. 2011 May 15;128(10):2444–52. doi: 10.1002/ijc.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004 Jan 12;90(1):93–9. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003 Oct 31;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992 May 15;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 14.Schroder FH, Hermanek P, Denis L, Fair WR, Gospodarowicz MK, Pavone-Macaluso M. The TNM classification of prostate cancer. Prostate Suppl. 1992;4:129–38. doi: 10.1002/pros.2990210521. [DOI] [PubMed] [Google Scholar]
- 15.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the National Death Index. Am J Epidemiol. 1984 May;119(5):837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121(7):1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, et al. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005 Jul 6;97(13):975–80. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007 Apr 18;99(8):608–15. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 19.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008 Aug 15;68(16):6762–9. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Tinsley HN, Keeton A, Qu Z, Piazza GA, Li Y. Suppression of Wnt/beta-catenin signaling inhibits prostate cancer cell proliferation. Eur J Pharmacol. 2009 Jan 5;602(1):8–14. doi: 10.1016/j.ejphar.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Amico AV, Kantoff PW, Chen MH. Aspirin and hormone therapy for prostate cancer. N Engl J Med. 2007 Dec 27;357(26):2737–8. doi: 10.1056/NEJMc0706698. [DOI] [PubMed] [Google Scholar]
- 22.Murray NP, Calaf GM, Badinez L. Presence of prostate cells in bone marrow biopsies as a sign of micrometastasis in cancer patients. Oncol Rep. 2009 Mar;21(3):571–5. [PubMed] [Google Scholar]
- 23.Wood DP, Jr, Banks ER, Humphreys S, McRoberts JW, Rangnekar VM. Identification of bone marrow micrometastases in patients with prostate cancer. Cancer. 1994 Nov 1;74(9):2533–40. doi: 10.1002/1097-0142(19941101)74:9<2533::aid-cncr2820740922>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]


