Scheme 3.
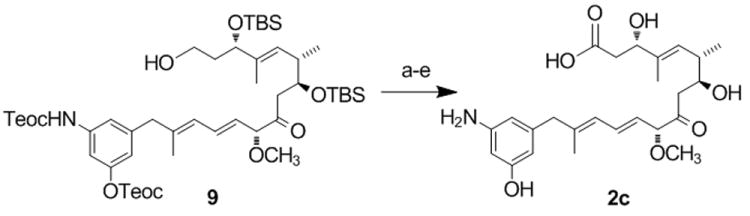
Synthesis of seco-proansamitocin 2c.[a]
[a] Reagents and conditions: (a) 9[13] DMSO, (COCl)2, -60 °C, 1 h then Et3N, -40°C (75%); (b) NaClO2, NaH2PO4 H2O, t-BuOH, 2-methyl-2-butene, 0 °C to rt, 30 min (86%); (c) HF-pyr, THF, rt, 6h, (48 %); (d) ZnCl2, MeNO2, ultrasound, rt, 1 h, (47%) (TBS= tert.-butyldimethylsilyl, Teoc= trimethylsilylethoxycarbonyl).
