Abstract
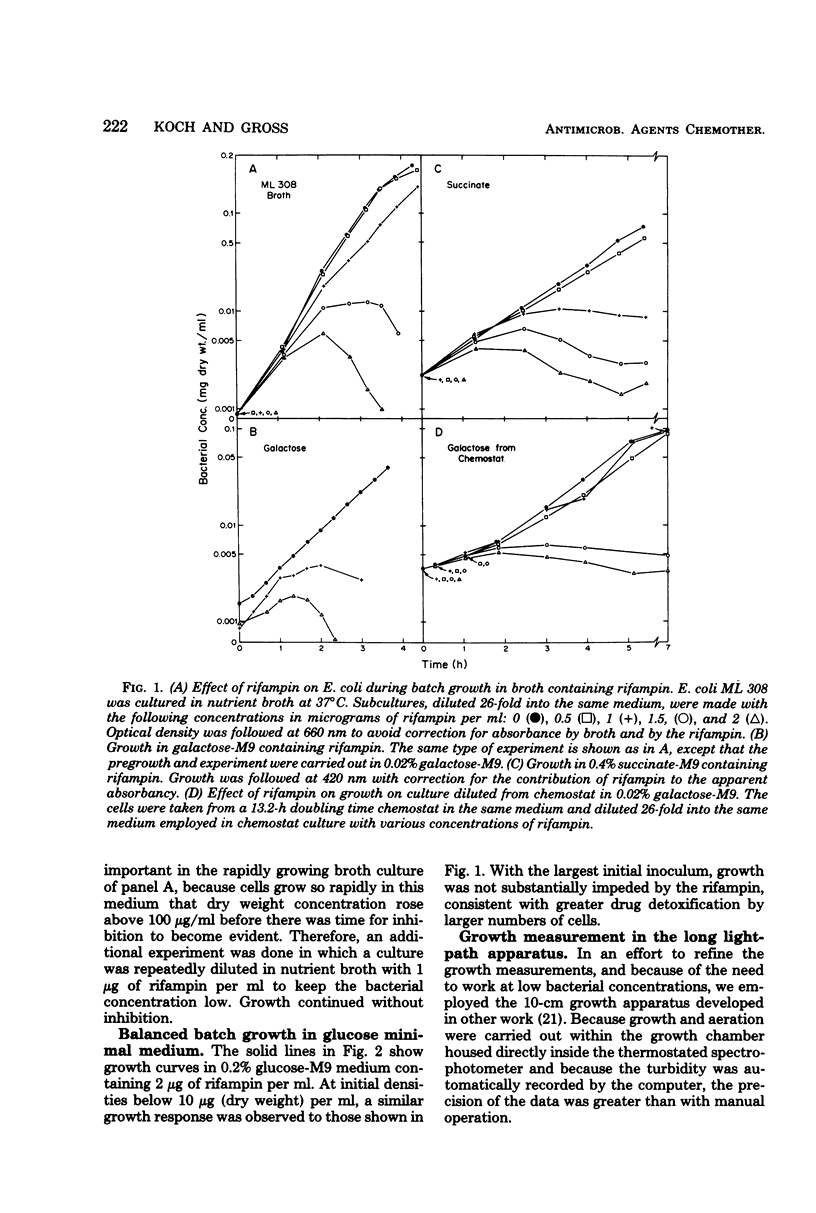
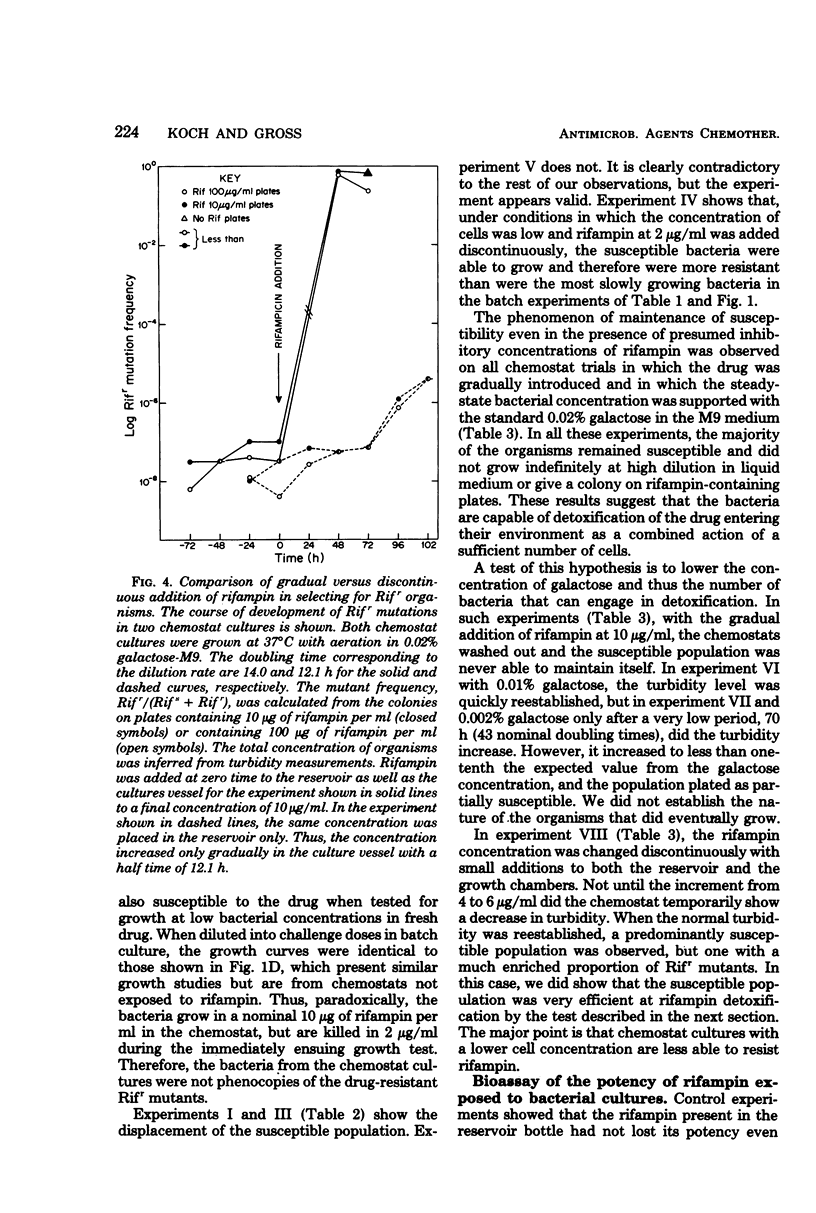
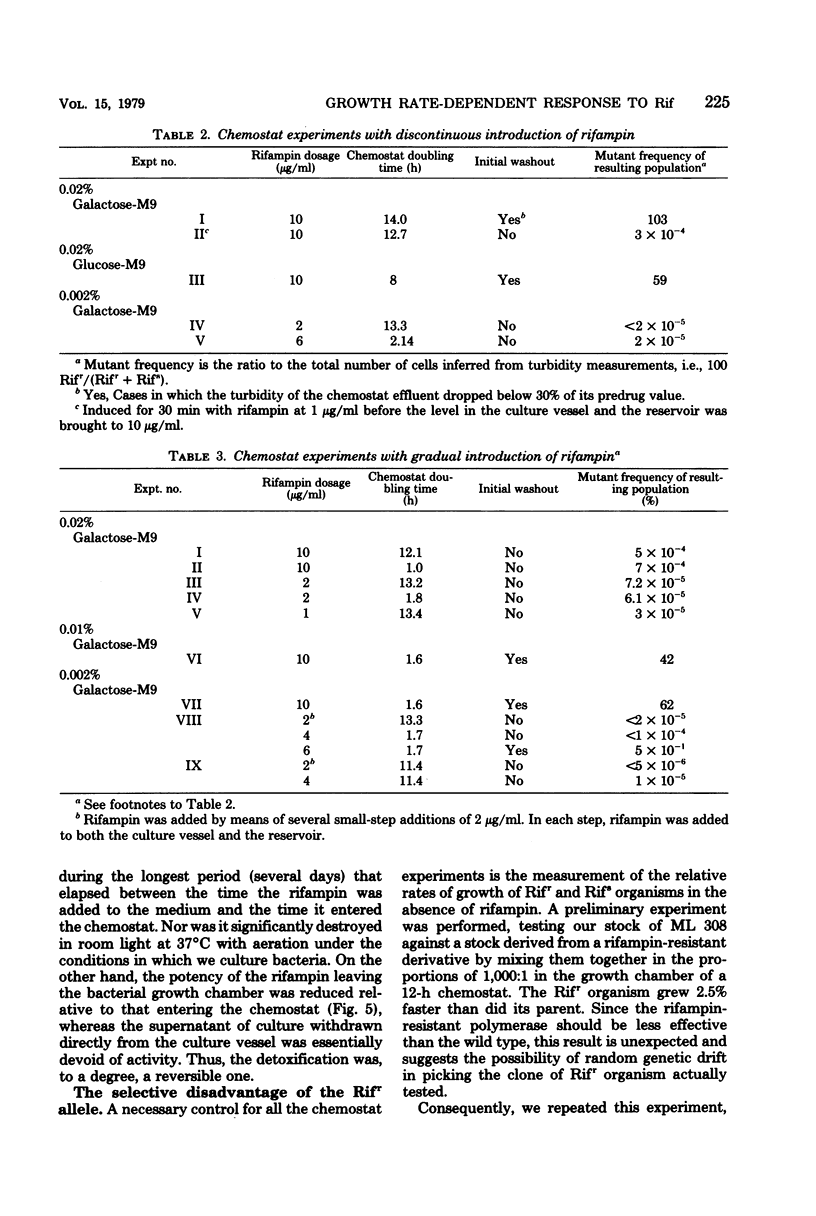
The susceptibility of Escherichia coli to rifampin was measured during unlimited growth in rich and poor media and during chemostat growth limited by the carbon source. During batch growth at low turbidities, the susceptibility of the bacteria increased as the growth rate decreased, consistent with the longer time available for drug penetration in the poorer media. During chemostat culture, the bacteria remained highly susceptible or became genetically resistant, dependent on the manner in which the bacteria were exposed to the antibiotic. If the concentration of rifampin was abruptly raised, susceptible cells were replaced by genetically resistant cells. However, if the concentration of antibiotic was raised slowly, the genetically susceptible cells continued to grow. This difference in response of chemostat cultures according to mode of drug administration was attributed to an inducible detoxification of the drug by the bacteria, because the susceptible genotype is maintained only when the concentration of rifampin is increased gradually and when a high population of cells is maintained. Direct evidence for the inactivation of the rifampin from the bioassay of culture supernatants is presented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S. J., Tittawella I. P., Hayward R. S., Scaife J. G. Amber mutations of Escherichia coli RNA polymerase. Nat New Biol. 1971 Aug 4;232(31):133–136. doi: 10.1038/newbio232133a0. [DOI] [PubMed] [Google Scholar]
- Binda G., Domenichini E., Gottardi A., Orlandi B., Ortelli E., Pacini B., Fowst G. Rifampicin, a general review. Arzneimittelforschung. 1971 Dec;21(12):1907–1977. [PubMed] [Google Scholar]
- Bochner B. R., Savageau M. A. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl Environ Microbiol. 1977 Feb;33(2):434–444. doi: 10.1128/aem.33.2.434-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Deppe C. S. In vivo assay of protein synthesizing capacity of Escherichia coli from slowly growing chemostat cultures. J Mol Biol. 1971 Feb 14;55(3):549–562. doi: 10.1016/0022-2836(71)90336-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Lag in adaptation to lactose as a probe to the timing of permease incorporation into the cell membrane. J Bacteriol. 1975 Oct;124(1):435–444. doi: 10.1128/jb.124.1.435-444.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Regulation of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Jul;115(1):107–116. doi: 10.1128/jb.115.1.107-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Reid P., Speyer J. Rifampicin inhibition of ribonucleic acid and protein synthesis in normal and ethylenediaminetetraacetic acid-treated Escherichia coli. J Bacteriol. 1970 Oct;104(1):376–389. doi: 10.1128/jb.104.1.376-389.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Wang C. H., Koch A. L. Constancy of growth on simple and complex media. J Bacteriol. 1978 Dec;136(3):969–975. doi: 10.1128/jb.136.3.969-975.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


