The colitis-associated glycome mediates CD4+ T cell expansion and contributes to the exacerbation of T cell–mediated intestinal inflammation.
Abstract
Immune responses are modified by a diverse and abundant repertoire of carbohydrate structures on the cell surface, which is known as the glycome. In this study, we propose that a unique glycome that can be identified through the binding of galectin-4 is created on local, but not systemic, memory CD4+ T cells under diverse intestinal inflammatory conditions, but not in the healthy state. The colitis-associated glycome (CAG) represents an immature core 1–expressing O-glycan. Development of CAG may be mediated by down-regulation of the expression of core-2 β1,6-N-acetylglucosaminyltransferase (C2GnT) 1, a key enzyme responsible for the production of core-2 O-glycan branch through addition of N-acetylglucosamine (GlcNAc) to a core-1 O-glycan structure. Mechanistically, the CAG seems to contribute to super raft formation associated with the immunological synapse on colonic memory CD4+ T cells and to the consequent stabilization of protein kinase C θ activation, resulting in the stimulation of memory CD4+ T cell expansion in the inflamed intestine. Functionally, CAG-mediated CD4+ T cell expansion contributes to the exacerbation of T cell–mediated experimental intestinal inflammations. Therefore, the CAG may be an attractive therapeutic target to specifically suppress the expansion of effector memory CD4+ T cells in intestinal inflammation such as that seen in inflammatory bowel disease.
The surface of all mammalian cells is covered by complex carbohydrate structures termed glycans (van Kooyk and Rabinovich, 2008). The glycan structure is determined by the enzymatic processes that produce glycosidic linkages of saccharides to other saccharides, and the expression profile of these glycan-modifying enzymes is altered by several factors, such as cell differentiation and activation, inflammatory insults, and the environment (Marth and Grewal, 2008; van Kooyk and Rabinovich, 2008; Baum and Crocker, 2009; Rabinovich and Toscano, 2009). Consequently, there is a diverse and abundant repertoire of glycan structures on the cell surface, which is known as the glycome (Marth and Grewal, 2008; van Kooyk and Rabinovich, 2008; Rabinovich and Toscano, 2009). The importance of the glycome in immune responses has been highlighted by its role in the control of cell homing, apoptosis, and microbial recognition (Marth and Grewal, 2008; van Kooyk and Rabinovich, 2008; Baum and Crocker, 2009; Rabinovich and Toscano, 2009). In addition, a recent human genetic study provides support for an alteration of the glycome in B cell signaling as a protective factor in some autoimmune diseases (Surolia et al., 2010). Therefore, understanding the functional role of each glycome motif in the immune response can potentially open up a new avenue for the treatment of immune-mediated diseases (Rabinovich and Toscano, 2009).
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory condition that develops in a genetically predisposed host. IBD is characterized by two major forms, Crohn’s disease (CD) and ulcerative colitis (UC), which are mediated by both common and distinct mechanisms (Xavier and Podolsky, 2007; Kaser et al., 2010; Mizoguchi and Mizoguchi, 2010). For example, Th1/Th17 responses have been implicated in the pathogenesis of CD, whereas UC has a significant contribution from Th2 cytokines (Xavier and Podolsky, 2007; Kaser et al., 2010; Mizoguchi and Mizoguchi, 2010). However, both diseases are characterized by a significant expansion of inflammatory memory CD4+ T cells in the inflamed intestine (Xavier and Podolsky, 2007; Kaser et al., 2010). Although much is known about the pathogenic effector mechanisms in these diseases, little information is currently available on how the carbohydrate structure of T cells contributes to these responses (Santucci et al., 2003; Hokama et al., 2004; Müller et al., 2006; Srikrishna et al., 2005). This is in contrast to the abundant amount of information available on the heavily O-glycosylated mucus that has been clearly demonstrated to play a protective role in IBD (An et al., 2007; Stone et al., 2009).
We herein demonstrate a colitis-associated glycome (CAG) on CD4+ T cells, which is characterized by immature core-1 O-glycan and induced through down-regulation of core-2 β1,6-N-acetylglucosaminyltransferase (C2GnT) 1 expression under intestinal inflammatory conditions. We also propose that the inducible glycome motif contributes to the exacerbation of colitis by enhancing the expansion of effector memory CD4+ T cells through stabilization of protein kinase C (PKC) θ activation.
RESULTS
Colitis-associated T cell glycome
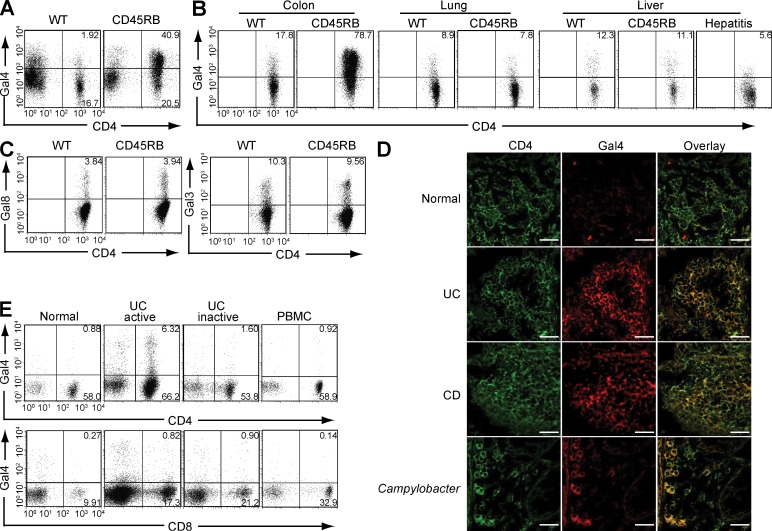
An endogenous glycan-binding protein family, the galectins, is composed of 15 members that recognize different carbohydrate epitopes and play different roles (e.g., anti- versus proinflammatory) in immune responses (Lowe, 2001; Baum and Crocker, 2009; Hsu et al., 2009; Rabinovich and Toscano, 2009). We previously demonstrated that an increased binding of galectin-4 is specifically seen on CD4+ T cells in the inflamed, but not normal, colon of Th2-mediated experimental colitis model (Hokama et al., 2004). We herein demonstrate that the binding of galectin-4 is also intensified on CD4+ T cells, but not CD4− cell populations, in the inflamed colon of Th1-mediated colitis model (CD45RB model) as compared with those in the normal colon of WT mice (Fig. 1 A). In contrast, intensified binding of galectin-4 was not observed on CD4+ T cells in other tissues (lung and liver) of this colitis model (Fig. 1 B). To see whether the intensified galectin-4 binding on CD4+ T cells is a specific feature of colitis or a common feature associated with any inflammatory conditions, we next examined a concanavalin A–induced hepatitis model. Interestingly, binding of galectin-4 on CD4+ T cells was decreased in the inflamed liver (Fig. 1 B, right).
Figure 1.
CAG. (A) Galectin-4 (Gal4) binding patterns on lamina propria cells (including CD4+ versus CD4− cells) in normal colon of WT mice and inflamed colon of CD45RB model are shown. Data shown are one representation of seven independent experiments. (B) Intensities of galectin-4 binding on CD4+ T cells from the colon, lung, and liver of WT mouse, CD45RB model, and concanavalin A–induced hepatitis model (right panel) are shown. The results are representative in 3–12 mice in each group. (C) Intensities of galectin-3 (Gal3) and galectin-8 (Gal8) binding on CD4+ T cells from the colon of WT mouse and from the inflamed colon of CD45RB model are shown. The results are representative in 3 mice in each group. (D) Colonic tissues from healthy individuals (n = 5) and from patients with UC (n = 6), CD (n = 4), or Campylobacter infection (n = 2) were co-stained with FITC–anti-CD4 (green, left) and Alexa Fluor 594–labeled recombinant human galectin-4 (red, middle). Overlap images are shown on the right. Bars, 25 µm. (E) Flow cytometric analysis shows the intensity of galectin-4 binding on CD4+ (top) or CD8+ (bottom) T cells from inflamed colon (active, n = 10), noninvolved colon (inactive, n = 5), and peripheral blood (PBMC, n = 2) of UC patients and from normal colon of control subjects (normal, n = 4).
Galectins are structurally classified into three groups (prototype, chimera type, and tandem-repeat type), and galectin-4 belongs to the tandem-repeat type (Lowe, 2001; Baum and Crocker, 2009; Hsu et al., 2009; Rabinovich and Toscano, 2009). To test whether the inflammation-inducible binding on colonic CD4+ T cells is specific for galectin-4, we used another tandem-repeat galectin (galectin-8) and a prototype galectin (galectin-3). Unlike galectin-4, there was no difference in the binding pattern of galectin-8 on CD4+ T cells from inflamed versus normal colon (Fig. 1 C, left). The different binding pattern between galectin-4 and -8 may be caused by the ability of galectin-8 to interact with more diverse arrays of glycan structures as compared with galectin-4 (Vokhmyanina et al., 2012). In addition, no difference in the binding pattern of galectin-3 was observed when CD4+ T cells from inflamed versus normal colon were examined (Fig. 1 C, right). Because galectin-3 interacts with complex N-glycans (Lowe, 2001), it is possible that O-glycan, rather than N-glycan, on colonic CD4+ T cells is more susceptible to inflammation-induced alteration.
As observed in mouse models, CD4+ T cells from the inflamed colon of both UC and CD patients also exhibited increased galectin-4 binding in comparison to noninflammatory controls (Fig. 1 D). Interestingly, this binding was also observed with CD4+ T cells from the inflamed colon of patients with Campylobacter infection (Fig. 1 D). Flow cytometric analysis confirmed a more intensified binding of galectin-4 on CD4+ T cells isolated from the inflamed area of UC patients as compared with those from noninflamed area (Fig. 1 E). Interestingly, in UC patients, galectin-4-binding was rarely observed on CD8+ T cells from the inflamed colon or on CD4+ T cells from the peripheral blood (Fig. 1 E), which is consistent with a previous study showing no galectin-4 binding on peripheral mononuclear cells (Paclik et al., 2008). Collectively, these findings suggest that a common glycome, which can be identified by intensified galectin-4 binding, is created on colonic CD4+ T cells under intestinal inflammatory conditions in mice and humans.
Decreased C2GnT expression in colitis
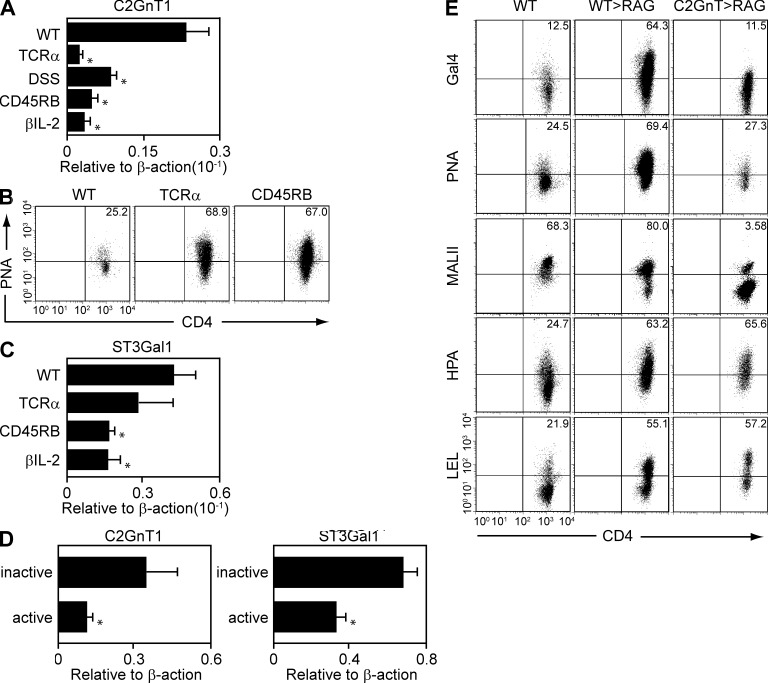
Glycome is produced by the coordinated action of glycan-modifying enzymes (Marth and Grewal, 2008; van Kooyk and Rabinovich, 2008; Baum and Crocker, 2009; Rabinovich and Toscano, 2009). We therefore performed real-time, PCR-based preliminary screening to examine the expression profile of 63 major glycan-modifying enzymes in purified CD4+ T cells from inflamed versus normal colon (unpublished data). As the result, we found that core-2 β1,6-N-acetylglucosaminyl transferase (C2GnT) 1 was commonly down-regulated in the CD4+ T cells obtained from the inflamed colon of various colitis models as compared with CD4+ T cells in the normal colon of WT mice (Fig. 2 A). The colitis models used include Th1-mediated colitis (CD45RB model) that was induced in RAG1−/− mice by adoptive transfer of CD4+ CD45RBhigh naive T cells from WT spleen, Th2-mediated colitis that spontaneously develops in TCRα−/− mice, memory T cell–induced colitis that was induced in TCRβ-deficient and IL-2–deficient double KO (βIL-2 DKO) mice by adoptive transfer of CD4+ CD45RBlow memory T cells from WT spleen, and acute intestinal injury that was induced by oral administration of 4% dextran sulfate sodium (DSS) for 4 d (Fig. 2 A).
Figure 2.
Development of CAG by down-regulation of C2GnT expressions. (A) Expression levels of C2GnT1 in purified CD4+ T cells from the normal colon of WT mice and from the inflamed colon of four kinds of colitis models were examined by real-time PCR. The colitis models include TCRα−/− mice, DSS model, CD45RB model, and βIL-2 DKO model. n = 6–7/each group. *, P < 0.001. (B) Binding intensities of PNA on colonic CD4+ T cells from WT mice, TCRα−/− mice, and CD45RB model are shown. The results are one representation of 3–5 independent experiments. (C) Expression levels of ST3Gal1 in purified CD4+ T cells from the normal colon of WT mice and from the inflamed colon of TCRα−/− mice, CD45RB model, and βIL-2 DKO model are shown. The results are representative in 6–8 mice in each group. *, P < 0.005. (D) Expression levels of C2GnT and ST3Gal1 in purified CD4+ T cells from noninflamed colon (inactive, n = 6) and from inflamed colon (active, n = 6) of UC patients are shown. **, P < 0.005; *, P < 0.05. (E) FACS analysis shows the binding intensities of galectin-4, PNA, MalII, HPA, and LEL on colonic CD4+ T cells from WT mice (as control, left) and from recipient Rag1−/− mice that were reconstituted with WT-derived (middle) versus C2GnT tg–derived (right) naive T cells. The data are one representation of three independent experiments.
C2GnT1 represents the key enzyme responsible for the production of core-2 O-glycan branch through addition of N-acetylglucosamine (GlcNAc) to a core-1 O-glycan structure (Tsuboi and Fukuda, 1997, 1998; Lowe, 2001). Because galectin-4 has previously been demonstrated to interact with selected glycomes, such as an immature (nonsialylated) core-1 O-glycan (Ideo et al., 2002; Blixt et al., 2004), this finding has prompted us to develop a hypothesis that colitis-associated glycome (CAG) represents the immature core-1 O-glycan. Indeed, an exogenous lectin peanut agglutinin (PNA), which interacts specifically with nonsialylated immature core-1 O-glycan (Toscano et al., 2007), was observed to bind to CD4+ T cells derived from the inflamed, but not normal, colon (Fig. 2 B). Competition of C2GnT1 and ST3Gal1 sialyltransferase for sialylation of core-1 O-glycan has previously been shown to control thymic CD8+ T cell homeostasis (Priatel et al., 2000). Therefore, we next examined the expression of ST3Gal-1 in colonic CD4+ T cells. Interestingly, no significant change or rather decrease in the ST3Gal1 expression was observed in colonic CD4+ T cells from inflamed colon as compared with those from normal colon (Fig. 2 C), suggesting that CAG may be created through a unique O-glycan biosynthesis pathway that favors generation of a nonsialylated immature core-1 O-glycan. As observed in mice, down-regulation of both C2GnT and ST3Gal1 expressions was seen in the purified CD4+ T cells from the involved areas (colon) in comparison to those obtained from the noninvolved areas of UC patients (Fig. 2 D).
To test whether the decreased C2GnT expression observed in mouse and human CD4+ T cells in the setting of inflammation is responsible for the development of CAG, we next restored C2GnT expression in CD4+ T cells by using T cell–specific C2GnT transgenic (T/C2GnT tg) mice that lack surface expression of core-1 O-glycan because of forced expression of this enzyme (Tsuboi and Fukuda, 1997, 1998). Indeed, PNA and galectin-4 were unable to bind to the C2GnT-expressing CD4+ T cells in the inflamed colon (Fig. 2 E, right). In addition, the restoration of C2GnT expression suppressed the binding of Maackia amurensis lectin II (MALII), which is capable of interacting with both sialylated and nonsialylated core 1 O-glycans (Geisler and Jarvis, 2011). Interestingly, binding of Helix pomatia lectin (HPA) and Lycopersicon esculentum lectin (LEL), which interact with other glycan structures, was intensified under inflammatory condition. However, the intensified binding on colonic CD4+ T cells was not suppressed by restoration of C2GnT expression (Fig. 2 E). Collectively, these findings suggest that down-regulation of C2GnT expression during inflammation is responsible for the generation of CAG recognized by galectin-4.
Pathogenic role of CAG in colitis of CD45RB model
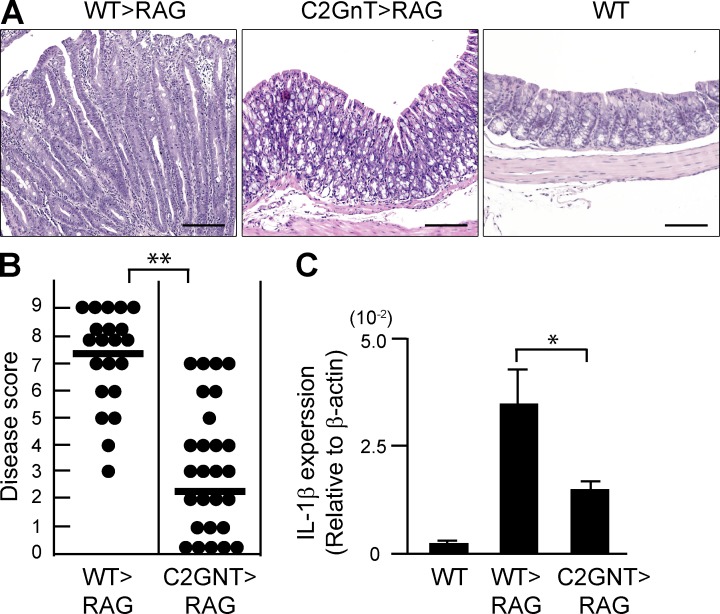
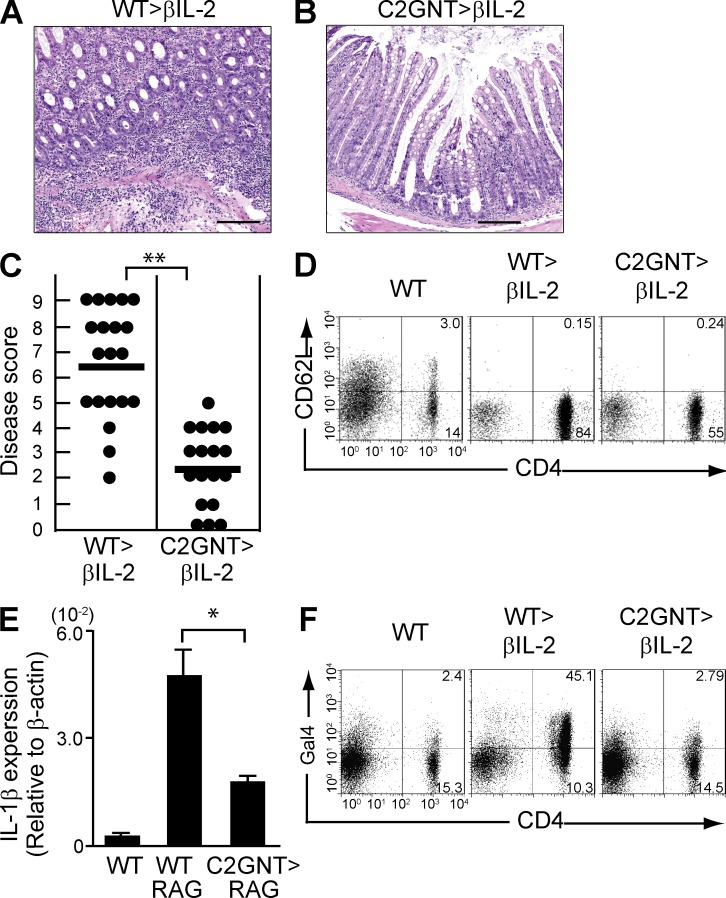
To examine whether the expression of CAG on CD4+ T cells plays any role in colitis, we examined a naive T cell–induced colitis model (CD45RB model; Izcue et al., 2009). In brief, CD4+CD45RBhigh naive T cells were purified from the spleen of WT versus T/C2GnT tg mice and transferred into Rag1−/− mice lacking T and B cells, and the recipient mice were sacrificed at 8 wk after cell transfer. As previously demonstrated (Izcue et al., 2009), the donor T cells from WT mice induced the development of severe colitis in the recipients (Fig. 3, A and B) and expressed the CAG in the inflamed colon (Fig. 2 E). In contrast, CD4+ T cells with restored expression of C2GnT were significantly less able to induce the development of colitis (Fig. 3, A and B), consistent with their inability to express the CAG (Fig. 2 E). As predicted, no colitis was found in control WT mice (Fig. 3 A). The disease severity was further supported by the colonic expression level of an inflammatory cytokine; significantly increased IL-1β expressions were observed in the colon of Rag1−/− mice reconstituted with WT-derived CD4+ T cells as compared with those reconstituted with T/C2GnT tg–derived CD4+ T cells (Fig. 3 C). These findings demonstrate that ability of CD4+ T cells to express CAG is a significant contributor to the exacerbation of this naive T cell–induced colitis.
Figure 3.
Pathogenic role of CAG in colitis of CD45RB model. CD4+CD45RBhigh naive T cells were purified from the spleen of WT (WT>RAG) versus T/C2GnT Tg (C2GnT>RAG) mice, and they were adoptively transferred i.v. into Rag1−/− mice. The recipients were sacrificed at 8 wk after transfer. (A) Representative colonic histologies (×10 objective) are shown. Colon of donor WT mouse is shown as normal control. Bars, 200 µm. (B) Disease scores evaluated by a combination of macroscopic and microscopic examinations are summarized. Each dot represents individual recipient. **, P < 0.0001. (C) Expression levels of IL-1β in the colonic tissues from WT mice and from Rag1−/− mice with transfer of WT-derived (WT>RAG) versus T/C2GnT tg–derived (C2GnT>RAG) CD4+ T cells are shown. n = 6/each group. *, P < 0.005.
Contribution of CAG for T cell proliferation
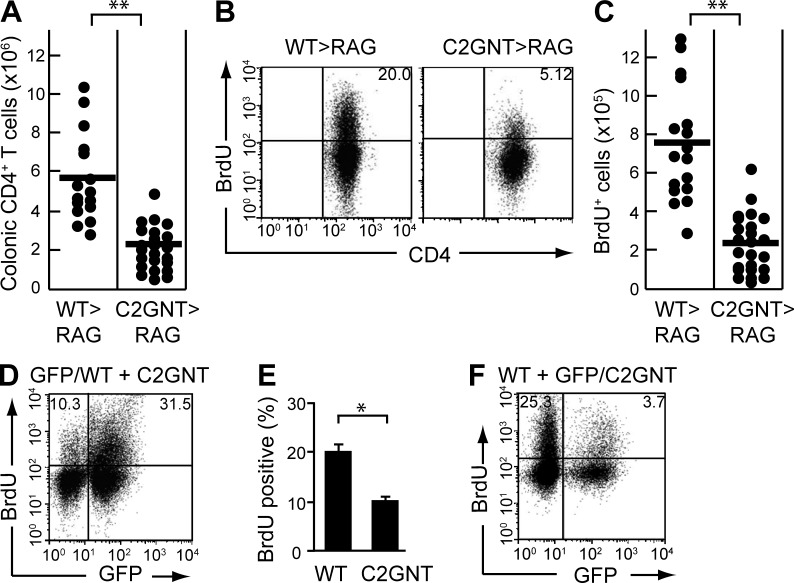
In the next series of studies, we examined the effect of CAG on colonic CD4+ T cell function during inflammation. We first noted that the improvement of colitis observed as a consequence of restored C2GnT expression was associated with a significant decrease in the absolute number of CD4+ T cells in the colon (Fig. 4 A). Indeed, in comparison to WT-derived CD4+ T cells, T/C2GnT tg–derived CD4+ T cells exhibited significantly less proliferation as judged by BrdU incorporation (Fig. 4, B and C).
Figure 4.
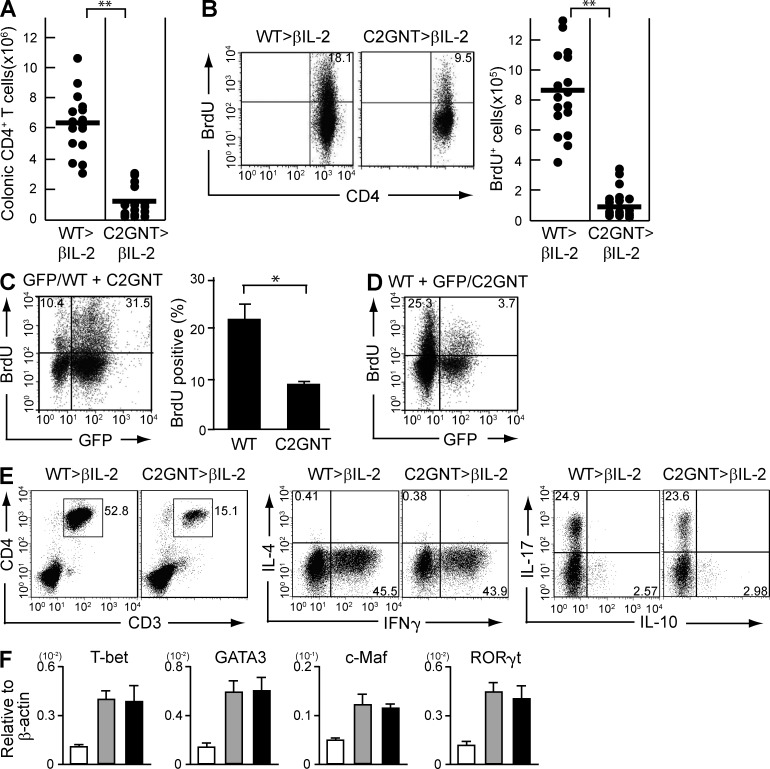
Effect of CAG on CD4+ T cell expansion. (A–C) CD4+CD45RBhigh naive T cells were purified from the spleen of WT (WT>RAG, n = 15) versus T/C2GnT Tg (C2GnT>RAG, n = 26) mice, and they were adoptively transferred i.v. into Rag1−/− mice. The recipients were sacrificed at 8 wk after transfer. Absolute numbers of colonic CD4+ T cells in the recipient colon are shown in A. Proliferative responses as judged by BrdU incorporation in the colonic CD4+ T cells are shown in B. The absolute number of colonic BrdU+ CD4+ T cells is summarized in C. (D and E) Purified CD4+CD45RBhigh T cells from the spleen of GFP Tg versus C2GnT Tg mice were co-transferred into Rag1−/− mice. The proliferative response of reconstituted GFP+ CD4+ (WT-derived) versus GFP−CD4+ (C2GnT tg–derived) cells in the same recipient colon are shown in (D; n = 5). The mean of the percentage of BrdU+ cells in the GFP+ versus GFP− CD4+ T cells is summarized in E. (F) Purified CD4+CD45RBhigh T cells from the spleen of WT versus GFP-expressing C2GnT double Tg mice were co-transferred into Rag1−/− mice. The proliferative response of reconstituted GFP− CD4+ (WT-derived) versus GFP+CD4+ (C2GnT tg–derived) cells in the same recipient colon are shown. Data shown are one representation of three independent experiments. *, P < 0.01; **, P < 0.0001.
It was possible that the decreased CD4+ T cell–proliferation observed was a secondary phenomenon associated with the improvement of colitis. Therefore, we next used a co-transfer system to clarify whether absence of CAG directly dampens the ability of CD4+ T cells to proliferate. To do so, CD4+ T cells were purified from a reporter mouse strain in which all cells are genetically engineered to express GFP (Shimomura et al., 2008) and from T/C2GnT tg mice. Both cell populations were then co-transferred at a 1:1 ratio into Rag1−/− mice. As predicted, the recipient mice developed severe colitis at 8 wk after co-transfer (unpublished data). Interestingly, in the same individual recipient colon, the GFP+ CD4+ (WT-derived) T cells were observed to proliferate more vigorously than the GFP− CD4+ (C2GnT tg–derived) T cells (Fig. 4, D and E).
To rule out the potential possibility that GFP expression, per se, influenced the outcome, T/C2GnT tg mice were next engineered to express GFP. Equal numbers of purified CD4+ T cells from WT mice and from GFP-expressing T/C2GnT tg mice were then co-transferred into Rag1−/− mice. As shown in Fig. 4 F, we observed that GFP− CD4+ (WT-derived) T cells exhibited significantly greater proliferation than seen in GFP+ CD4+ (C2GnT tg–derived) T cells. These findings propose that the expression of CAG directly contributes to the enhancement of colonic CD4+ T cell expansion under intestinal inflammatory condition.
Deleterious effect of CAG on memory T cell–induced colitis
A previous study has suggested that the initial priming of T cells necessary for maturation from naive to memory stages is impaired in T/C2GnT tg mice (Tsuboi and Fukuda, 1998). This finding raised a possibility that the decreased proliferation/expansion observed in T cells from T/C2GnT tg mice, and, consequently, protection from colitis, was a result of insufficient priming of the adoptively transferred naive T cells. To test this possibility, we next used another mouse model system in which colitis can be induced by adoptive transfer of memory CD4+ T cells (Nagahama et al., 2008). In brief, CD4+ CD45RBlow memory T cells were purified from the spleen of WT versus T/C2GnT tg mice, and they were adoptively transferred into TCRβ and βIL-2 DKO mice.
As previously demonstrated (Nagahama et al., 2008), WT memory CD4+ T cells induced severe colitis in the recipient mice at 8 wk after cell transfer (Fig. 5, A and C), and the reconstituted donor CD4+ T cells in the recipient colon all exhibited memory phenotype (Fig. 5 D). The development of severe colitis was further supported by the increased expression of colonic IL-1β (Fig. 5 E). Similar to the results obtained with adoptive transfer of naive T cells, CAG was observed on the memory-derived donor CD4+ T cells in the recipient colon (Fig. 5 F, middle panel). In contrast, T/C2GnT tg–derived memory CD4+ T cells induced significantly less severe colitis (Fig. 5 B and C), and they did not express CAG (Fig. 5 F, right panel). These findings suggest that CAG, which is expressed on memory CD4+ T cells, has the ability to exacerbate colitis.
Figure 5.
Pathogenic role of CAG in memory CD4+ T cell-mediated colitis. CD4+CD45RBlow memory T cells were purified from the spleen of WT (WT>βIL2, n = 20) versus T/C2GnT Tg (C2GnT>βIL2, n = 18) mice, and they were adoptively transferred i.v. into βIL-2 DKO mice. The recipients were sacrificed at 8 wk after T cell transfer. (A and B) Representative histology of recipient colons with transfer of WT (A) versus C2GnT Tg (B) CD4+ T cells are shown. Bars, 200 µm. (C) The disease scores are summarized. (D) Flow cytometric analysis shows the expressions of CD4 versus CD62L on splenic cells of WT mouse (left panel, as control) and colonic lamina propria cells from recipient βIL-2 DKO mice after transfer of memory CD4+ T cells from the spleen of WT (middle panel) or C2GnT tg (right panel) mice. Data are representative in 3 independent experiments. (E) Expression levels of IL-1β in colonic tissues from WT mice and from βIL-2 DKO mice with transfer of WT-derived (WT>βIL2, n = 6) versus T/C2GnT tg–derived (C2GnT>βIL2, n = 6) CD4+ T cells are shown. (F) Binding intensities of galectin-4 (Gal4) on colonic CD4+ T cells in WT mice (left, as control) and the recipients with transfer of WT-derived (middle) versus C2GnT Tg–derived (right) memory CD4+ T cells are shown. Data shown are one representation of five independent experiments. **, P < 0.0001; *, P < 0.005.
Effect of CAG on memory T cell proliferation
Consistent with the colitis severity, absolute numbers (Fig. 6 A) and proliferative responses (Fig. 6 B) of colonic CD4+ T cells were significantly lower in βIL-2 DKO recipient mice that received C2GnT tg–derived memory CD4+ T cells in comparison to those mice that received WT-derived memory CD4+ T cells. Co-transfer experiments of memory CD4+ T cells from GFP-reporter mice and from T/C2GnT tg mice into βIL-2 DKO mice confirmed that WT-derived memory T cells (GFP+) were more proliferative than C2GnT tg–derived memory T cells (GFP−) when examined in the same colons (Fig. 6 C). Another set of co-transfer experiments using memory CD4+ T cells from WT mice and from GFP-expressing T/C2GnT tg mice further confirmed the higher proliferative ability of WT-derived memory CD4+ T cells (GFP−) as compared with C2GnT tg–derived memory CD4+ T cells (GFP+) in the same individual colon of βIL-2 DKO mouse (Fig. 6 D).
Figure 6.
CAG-mediated stimulation of memory CD4+ T cell proliferation. (A and B) CD4+CD45RBlow memory T cells were purified from the spleen of WT (WT>βIL2) versus T/C2GnT Tg (C2GnT>βIL2) mice, and they were adoptively transferred i.v. into βIL-2 DKO mice. The recipients were sacrificed at 8 wk after T cell transfer. (A) Absolute numbers of colonic CD4+ T cells in the recipient are shown. Each dot represents an individual mouse. (B) Proliferative responses of colonic CD4+ T cells are shown. The absolute numbers of BrdU+CD4+ in the colon is summarized in right panel. (C) Purified CD4+CD45RBlow memory T cells from the spleen of GFP Tg versus C2GnT Tg mice were co-transferred into βIL-2 DKO mice. The proliferative response of reconstituted GFP+ CD4+ (WT-derived) versus GFP− CD4+ (C2GnT tg–derived) cells in the same recipient colon are shown. Right panel summarizes the average of percentages (n = 5) of BrdU+ cells in GFP+ versus GFP− CD4+ T cells. (D) Purified CD4+CD45RBlow memory T cells from the spleen of WT versus GFP-expressing C2GnT-expressing double Tg mice were co-transferred into βIL-2 DKO mice. The proliferative response of reconstituted GFP− CD4+ (WT-derived) versus GFP+CD4+ (C2GnT tg–derived) cells in the same recipient colon are shown. (E) Left panels show the proportion of CD3+CD4+ T cells in the colonic lamina propria cells of recipient βIL-2 DKO mice reconstituted with WT-derived (WT>βIL2) versus T/C2GnT tg–derived (C2GnT>βIL2) memory CD4+ T cells. The proportion of IL-4, IFN-γ, IL-17A, and IL-10–producing cells in the colonic CD4+ T cell population is then examined after ex vivo stimulation. Data shown are one representation of three independent experiments. (F) Expression levels of T-bet, GATA3, c-Maf, and RORγt in purified CD4+ T cells from the colon of WT mice (white bars, n = 6) and of βIL-2 DKO mice reconstituted with WT-derived (gray bars, n = 19) versus T/C2GnT tg–derived (black bars, n = 13) memory CD4+ T cells are shown. *, P < 0.01; **, P < 0.0001.
To test whether CAG promotes the expansion of selected T helper (Th) subset, colonic cells from βIL-2 DKO mice reconstituted with C2GnT tg–derived versus WT-derived memory CD4+ T cells were stimulated ex vivo with phorbor-12,13-dibutylate/ionomycin and subjected to intracellular staining of IFN-γ, IL-17A, IL-10, and IL-4. Interestingly, although absolute number of CD4+ T cells in the inflamed colon was significantly reduced by restoration of C2GnT expression (Fig. 6 E, left), there was no proportional difference in Th1 (IFN-γ), Th17 (IL-17A), Tr1 (IL-10), and Th2 (IL-4) subsets among the colonic CD4+ T cell population (Fig. 6 E). Indeed, restoration of C2GnT expression did not alter the expression level of T-bet, RORγt, GATA3, or c-Maf in the purified colonic CD4+ T cells (Fig. 6 F). Collectively, these findings demonstrate the ability of CAG to directly enhance the proliferative response of memory CD4+ T cells but not the differentiation of specific Th subsets.
Contribution of CAG to raft integrity
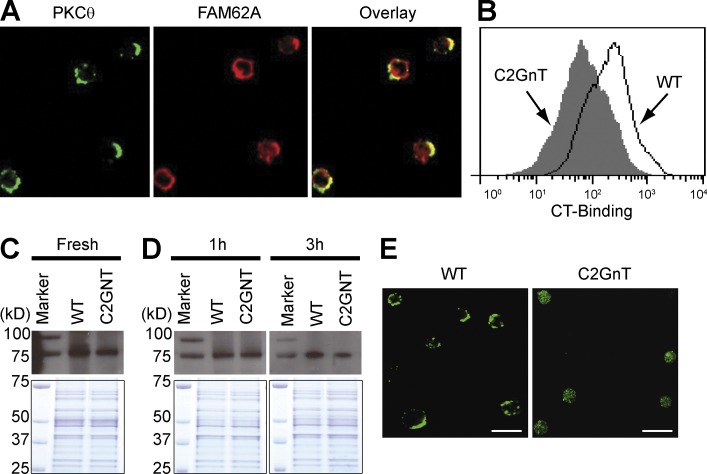
Galectin-4 has previously been demonstrated to stabilize lipid rafts on epithelial cells for generation of super rafts (Braccia et al., 2003). The immunological synapse (IS), which is built on the lipid raft characterized by detergent insolubility, serves as a key component providing signals for T cell stimulation and proliferation (Xavier et al., 1998; Arendt et al., 2002). We therefore biochemically determined whether galectin-4 binds to lipid rafts associated with the IS. In brief, purified CD4+ T cells were obtained from the inflamed colon, lysed as previously described (Xavier et al., 1998), and precipitated with galectin-4–coupled beads. The precipitants were then subjected to analysis with a liquid chromatography tandem mass spectrometry. The protein complex precipitated by galectin-4 included formin-like 1, vinculin, actin-polymerization Arpc3, and Rab11, all of which have been identified to localize within the IS (Gomez et al., 2007; Nolz et al., 2007). Interestingly, galectin-4 also precipitated Fam62a (five-C2 domain-containing protein); we confirmed Fam62a has accumulated within the IS by confocal microscopy (Fig. 7 A). These findings indicate that galectin-4 interacts with CAG in the detergent-insoluble region of the cell membrane (lipid raft) that is in association with the IS.
Figure 7.
Contribution of CAG for stabilization of PKCθ activity, (A) Purified CD4+ T cells from the inflamed colon were stained with anti-PKCθ (green, left) and ant-Fam62a (red, middle) antibodies. The merge image is shown on the right. Data shown are one representation of three 3 independent experiments. Bars, 15 µm. (B) Memory CD4+ T cells from GFP tg mice and T/C2GnT tg mice were co-transferred into βIL-2 DKO mice, and the intensity of CT-binding on GFP+ (line) versus GFP− (solid) CD4+ T cells from the same recipient colon was examined. Data shown are one representation of three independent experiments. (C–E) CD4+ T cells (Fresh in C) were purified from the colon of recipient βIL-2 DKO mice with transfer of WT-derived versus C2GnT Tg (C2GnT)–derived memory CD4+ T cells. Purified CD4+ T cells were stimulated with ant-CD3/CD28 coated beads, and after removal of beads they were cultured again in the presence of galectin-4 for 1 (1 h in D) and 3 h (3 h in D). Lipid rafts were precipitated using CT-coupled beads and subjected to detection of PKCθ (top). The bottom panels show the Coomassie blue staining of total protein subjected to CT-precipitation. The CD4+ T cells cultured for 3 h were stained with anti-PKCθ antibodies and subjected to confocal microscopic analysis (E). Bars, 15 µm. Data shown are one representation of three independent experiments.
We next examined whether the lack of CAG alters the formation of lipid raft/IS. To do so, CD4+ T cells from GFP mice and T/C2GnT tg mice were co-transferred into βIL-2 DKO mice, and the binding of cholera toxin (CT; a marker of lipid rafts) to the GFP+ (WT-derived) versus GFP− (T/C2GnT tg–derived) CD4+ T cells from the same inflamed colon was examined by flow cytometry. Interestingly, significantly more intense binding of CT was observed on WT-derived CD4+ T cells in comparison to that observed on the cell surface of T/C2GnT-derived CD4+ T cells (Fig. 7 B). These findings propose a contribution of CAG in the generation of super rafts on CD4+ T cells during the course of intestinal inflammation.
Sustained PKCθ activation through CAG
PKCθ, which represents a proximal molecule in IS-mediated signaling, exists diffusely within the cytoplasm of resting CD4+ T cells and is accumulated within the IS after TCR activation (Arendt et al., 2002). Therefore, we next examined whether absence of CAG altered the inflammation-induced activation of PKCθ. To do so, CD4+ T cells were purified from the inflamed colon of βIL-2 DKO mice with transfer of WT versus C2GnT tg–derived memory CD4+ T cells. After cell lysis, lipid rafts were precipitated with CT-coupled beads, and the precipitant subjected to immune-blot with an anti-PKCθ antibody. As shown in Fig. 7 C (top), more PKCθ could be detected in association with the precipitated detergent-insoluble lipid rafts obtained from the fresh WT-derived CD4+ T cells in comparison to that observed from the C2GnT-expressing CD4+ T cells. Coomassie blue staining of the precipitates showed that this was not caused by differences in the quantity of proteins (Fig. 7 C, bottom).
An in vitro approach was next conducted to confirm these in vivo data. To do so, purified CD4+ T cells from the inflamed colon were stimulated for 30 min with anti-CD3 and anti-CD28–coupled magnetic beads. After removal of the beads, the activated cells were cultured again in the presence of galectin-4 for 1 or 3 h, and then lysed. The accumulation of PKCθ within the lipid rafts was then assessed as described in Fig. 7 C. Interestingly, at 3 h after stimulation, activation of PKCθ (as indicated by the association within CT-precipitated lipid rafts) was lower in C2GnT tg–derived CD4+ T cells in comparison to WT-derived CD4+ T cells (Fig. 7 D, right). Indeed, significant capping of PKCθ was observed on the WT-derived CD4+ T cells as compared with C2GnT tg–derived CD4+ T cells (Fig. 7 E). In contrast, such differences in PKCθ activation were not detectable between CD4+ T cells from WT and C2GnT tg mice at 1 h after stimulation (Fig. 7 D, left). These findings suggest that CAG has the ability to sustain, but not initiate, the activation of PKCθ in colonic memory CD4+ T cells.
PKCθ-dependent proliferation of CAG-expressing T cells
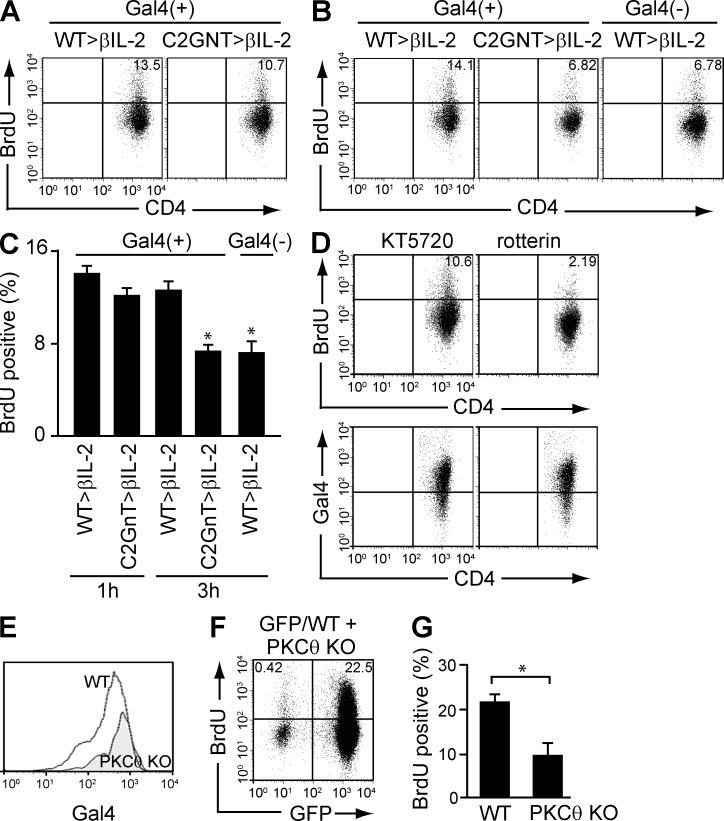
In a final set of experiments, we examined whether the sustained PKCθ activation in colonic CD4+ T cells by CAG development promotes the proliferative response. To do so, colonic memory CD4+ T cells were stimulated as shown in Fig. 7 D, and the activated cells were cultured again in the presence of galectin-4 for 1 or 3 h. At 1 h before analysis, cells were pulsed with BrdU. As shown in Fig. 8 (A and C), similar levels of proliferation were observed in WT- versus C2GnT tg–derived CD4+ T cells at 1 h after stimulation. However, at 3 h after stimulation, much less proliferation was seen in C2GnT tg–derived CD4+ T cells compared with WT-derived CD4+ T cells (Fig. 8, B and C). In addition, the proliferative response of WT-derived CD4+ T cells was reduced when galectin-4 was omitted from the culture (Fig. 8, B and C).
Figure 8.
Requirement of PKCθ for CAG-mediated proliferation. (A-C) Purified colonic CD4+ T cells from βIL-2 DKO mice with reconstitution of WT-derived (WT>βIL2) versus T/C2GnT tg–derived (C2GnT>βIL2) memory CD4+ T cells were stimulated with anti-CD3/CD28–coated beads, and after removal of beads they were cultured again in the presence or absence of galectin-4 for 1 (A) and 3 h (B). They were pulsed with BrdU 1 h before the analysis and subjected to flow cytometric analysis. The percentage of BrdU+ cells is summarized in C. *, P < 0.01 (n = 4–7). (D) Purified colonic CD4+ T cells from βIL-2 DKO mice with reconstitution of WT-derived (WT>βIL2) versus T/C2GnT tg–derived (C2GnT>βIL2) memory CD4+ T cells were pretreated with KT5720 (left) or rotterin (right), and they were then stimulated with anti-CD3/CD28–coated beads. After removal of beads, they were cultured in the presence of galectin-4 for 3 h, with 1-h pulse with BrdU, and subjected to flow cytometric analysis for evaluation of BrdU incorporation (top) and galectin-4 binding (bottom). Data shown are one representation of two independent experiments. (E and F) Purified CD4+CD45RBlow memory T cells from the spleen of GFP Tg versus PKCθ−/− mice were co-transferred into βIL-2 DKO mice. The intensity of galectin-4 binding on (E) and the proliferative response of (F) reconstituted GFP+ CD4+ (PKCθ-intact) versus GFP− CD4+ (PKCθ-deficient) cells in the same recipient colon are shown. The mean (n = 4) of percentages of BrdU+ cells in GFP+ (PKCθ-intact) versus GFP− (PKCθ-deficient) CD4+ T cells are summarized in G. *, P < 0.01.
We next examined whether the sustained proliferative response through development of CAG depends on PKCθ. To do so, colonic CD4+ T cells used in Fig. 8 B were pretreated with rotterin (an inhibitor of Ca2+-dependent PKC including PKCθ). As shown in Fig. 8 D, inhibition of PKC suppressed the galectin-4/CAG-induced proliferative response of colonic CD4+ T cells (Fig. 8 D, top right). In contrast, pretreatment with KT5720 (an inhibitor of PKA) had no effect on the proliferative response (Fig. 8 D, top left). In addition, these treatments did not alter the galectin-4 binding on the CD4+ T cells (Fig. 8 D, bottom). To further confirm the in vitro finding, in vivo co-transfer experiment using PKCθ−/− mice (Sun et al., 2000) was next conducted. To do so, CD4+CD45RBlow memory T cells were purified from the spleen of PKCθ−/− mice versus GFP-reporter mice, and they were co-transferred into βIL-2 DKO mice. In the same individual recipient colon, PKCθ-intact and PKCθ-deficient CD4+ T cells both developed CAG (Fig. 8 E). However, PKCθ-deficient CD4+ T cells (as indicated by GFP−), despite expressing CAG, exhibited significantly less proliferative response as compared with PKCθ-intact CD4+ T cells (as indicated by GFP+; Fig. 8 F). Collectively, these findings suggest that CAG-mediated expansion of memory CD4+ T cells depends on the activation of PKCθ.
DISCUSSION
In this manuscript, we propose that an inducible CAG, which can be identified by galectin-4 binding, is created on local memory CD4+ T cells under diverse intestinal inflammatory conditions that range from IBD to infection but not in healthy state. This CAG is characterized by the formation of an immature core-1–containing O-glycan, presumably through the loss of a C2GnT enzyme involved in the addition of N-acetylgalactosamine to create a core-2 O-glycan. It has been previously shown that CD4+ T cell subsets, including Th1, Th2, and Th17 express different glycomes depending on the cytokine profile (Toscano et al., 2007). Alternatively, as shown here, during intestinal inflammation from a variety of different causes and mechanistic pathways, a characteristic CAG is detectable that is functionally associated with promoting the inflammatory process. Therefore, intestinal inflammatory insults may further modify the Th-specific glycomes, creating a local inflammation-specific glycome. Interestingly, unlike intestinal inflammation, hepatic inflammation did not induce the development of CAG. Because host–microbial interaction is significantly enhanced in intestinal, but not hepatic, inflammation (Sartor, 2008), this raises a possibility that some microbial-derived factors may contribute to the development of CAG.
We show that the CAG can be recognized by galectin-4 that has previously been shown to interact with specific carbohydrate epitopes such as an immature (nonsialylated) core-1 O-glycan (Ideo et al., 2002; Blixt et al., 2004). In addition, we show that CAG development was abolished by restoration of C2GnT expression, an enzyme responsible for initiating the maturation (branching) of O-glycans from core-1 to core-2 (Tsuboi and Fukuda, 1997, 1998). Collectively, these findings propose that the CAG observed reflects an immature core-1 O-glycan caused by a down-regulation of expression of the responsible enzyme during inflammation from a variety of different causes. An important observation in our study is that the CAG observed on CD4+ T cells contributes significantly to the exacerbation, but not induction, of colitis. The data would thus indicate that impairment of O-glycan maturation in CD4+ T cells plays a deleterious role for progression of colitis and supports a previous notion that N-glycan maturation (branching) can safeguard against some autoimmune diseases (Green et al., 2007). Consistent with this, galectin-4, which interacts with immature glycan structures (Ideo et al., 2002; Blixt et al., 2004), has been shown to exacerbate an experimental chronic colitis (Hokama et al., 2004). In contrast, galectins-1 and -3, which interact with more mature glycan structures (Lowe, 2001; Baum and Crocker, 2009), contribute to the suppression of colitis (Santucci et al., 2003; Müller et al., 2006). Thus, during the course of inflammation, changes in the glycan structures that decorate pathogenic memory T cells results in alterations in cell surface interactions that further control intestinal inflammation. Alternatively, administration of galectin-4 delayed the recovery from DSS-induced colitis (Hokama et al., 2004), whereas the administration ameliorated the acute phase of this colitis (Paclik et al., 2008). DSS-induced acute colitis is mediated by innate immune response, and adaptive immune responses then participate in the recovery process from this colitis (Mizoguchi, 2012). Therefore, it is possible that the role of galectin-4 in colitis differs depending on innate versus adaptive immune responses involved. This possibility is supported by a recent report demonstrating the ability of galectin-4 to suppress innate immune response by killing blood group antigen-expressing bacteria (Stowell et al., 2010).
Galectin-4 has previously been demonstrated to promote the formation of super rafts on non–T cells (Braccia et al., 2003). Here, we propose that CAG, which interacts with galectin-4, contributes to super raft formation on colonic CD4+ T cells, leading to prolonged IS-mediated activation. In support of this, we show that formation of CAG led to sustained activation of PKCθ; a key proximal element in the IS-induced signaling machinery (Arendt et al., 2002). PKCθ is primarily responsible for the activation and proliferation of memory, but not naive, CD4+ T cells through consequent NF-κB1 activation (Sun et al., 2000). In addition, we have previously demonstrated that absence of PKCθ suppresses both Th1 and Th2 responses in the inflamed colon by reducing memory CD4+ T cell proliferation rather than by inhibiting the differentiation of naive T cells into Th1, Th17, or Th2 cells (Nagahama et al., 2008). Consistent with this, we herein show that the absence of CAG suppressed the proliferative responses of colonic memory CD4+ T cells, but it did not affect the differentiation of Th subsets in the inflamed colon. Therefore, our studies propose that formation of CAG serves to sustain PKCθ activation, resulting in enhanced expansion of memory CD4+ T cells in the inflamed colon. However, our current dataset may be unable to rule out a possibility that an alteration of retention/homing of CD4+ T cells is also involved in the CAG-mediated expansion in the inflamed colon.
PKCθ has been proposed as an attractive therapeutic target in IBD (Baier and Wagner, 2009). PKCθ-mediated signaling leading to consequent activation of NF-κB is used primarily by memory CD4+ T cells but not by naive CD4+ T cells, CD8+ T cells, or other cell types (Arendt et al., 2002; Kingeter and Schaefer, 2008). This is consistent with our observations that CAG is expressed by memory CD4+ T cells in the inflamed colon but not in other organs associated with models of colitis (Hokama et al., 2004). Interestingly, a recent study has suggested that PKCθ is not recruited to the IS of CD4+ Foxp3+ regulatory T cells, but rather inhibits the regulatory function of these cells (Zanin-Zhorov et al., 2010). Thus, collectively, blockade of CAG formation for inhibition of PKCθ activity may represent a specific therapeutic strategy to target colitogenic memory CD4+ T cells without affecting other T cell populations.
In summary, we propose that intestinal inflammation is associated with the formation of an inducible colitis-associated glycome (CAG) on local memory CD4+ T cells, which contributes to the exacerbation of intestinal inflammation, presumably by stimulating their expansion through prolonged PKCθ activation. This mechanistic pathway may provide a rationale to specifically target effector memory CD4+ T cells in the inflamed intestine.
MATERIALS AND METHODS
Mice.
Sperm of a transgenic mouse stain carrying human C2GnT gene under control of Lck promoter (Tsuboi and Fukuda, 1997) was obtained from M. Fukuda (Burnham Institute, San Diego, CA), and the mice were recovered through in vitro fertilization with the help of Charles River Laboratory. The recovered T cell–specific C2GnT (T/C2GnT) tg mice were further backcrossed to C57BL/6 mice five times. GFP tg, Tcrα−/−, Il-10−/−, Rag1−/−, and Tcrβ-βIL-2 DKO) mice were C57BL/6 background (Nagahama et al., 2008; Shimomura et al., 2008). GFP-expressing T/C2GnT tg mice were generated by crossing T/C2GnT tg mice with GFP tg mice. All mice were maintained under specific pathogen–free facility at Massachusetts General Hospital. All experiments were approved by the subcommittee on Research Animal Care of Massachusetts General Hospital.
Human samples.
Diagnosis for IBD was based on conventional clinical and endoscopic criteria. Surgically rejected or biopsy specimens were obtained with informed consent from patients who had active or inactive UC, active CD, Campylobacter infection, or polyps (as healthy control). All experiments were approved by local Ethics Committees of Shiga University of Medical Science and Keio University School of Medicine.
Induction and evaluation of colitis.
For CD45RB model, T cells were first enriched from the spleen using T cell enrichment column (R&D Systems). After staining with FITC–anti-CD4 and PE–anti-CD45RB mAbs, CD4+CD45RBhigh T cells were purified using flow cytometric sorting. The purity was >98%. CD4+CD45RBhigh T cells (5 × 105) were intravenously administrated into Rag1−/− mice, and the recipients were euthanized at 8 wk after cell transfer. For memory CD4+ T cell–induced colitis model, T cells enriched through T cell–enrichment column were stained with FITC–anti-CD4 and PE–anti-CD45RB mAbs, and CD4+CD45RBlow T cells were then purified using flow cytometric sorting. The purity was >99%. CD4+CD45RBlow T cells (5 × 105) were intravenously administrated into βIL-2 DKO mice, and the recipients were euthanized at 8 wk after cell transfer. The disease score of both models was assessed by a combination of gross and histological examinations as previously described (Nagahama et al., 2008).
Cell isolation and flow cytometric analysis.
In humans, lamina propria mononuclear cells were isolated as previously described (Okazawa et al., 2002; Kamada et al., 2008). In brief, dissected mucosa was incubated with 30 mM EDTA for 20 min, followed by incubation in medium containing 1.5 mg/ml collagenase type IV (Sigma-Aldrich) and 0.1 mg/ml DNaseI (Roche) for 60 min at 37°C. After passing glass wool column, cells were subjected to Ficoll density gradient, and CD4+ cells were magnetically separated using iMag CD4 MicroBeads (BD). Colonic LP cells from mice were isolated as previously described (Shimomura et al., 2008). They were blocked, stained, and analyzed using FACSCalibur (BD) and FlowJo software (Tree Star). For mice, FITC–anti-CD3ε (clone 145-2C11; BD), PE–anti-CD4 (clone RM4-4; eBioscience), FITC-PNA (Vector Laboratories), Biotinylated-MALII (Vector Laboratories)/Alexa Fluor 488–conjugated streptavidin (Invitrogen), Alexa Fluor 488–HPA (Invitrogen), and FITC-LEL (Vector Laboratories) were used. For humans, Alexa Fluor 488–human galectin-4, APC-anti–human CD4 (clone RPA-T4; BD), and APC-anti–human CD8 (clone SK1; BD) were used.
Recombinant galectins.
Human and mouse recombinant galectin-4 and galectin-3 were generated previously (Hokama et al., 2004). Full-length cDNA of mouse galectin-8 was synthesized by GenScript Inc. and subcloned into pGEX-6P vector (GE Healthcare). BL-21 stain carrying pGEX-6P was cultured in the presence of 0.1 mM IPTG. Fusion protein was purified with glutathione Sepharose 4B, and recombinant protein was separated from the GST moiety by using PreScission protease (GE Healthcare). After removal of endotoxin using endotoxin removal gel (Thermo Fisher Scientific), the protein was dialyzed to PBS and concentrated. 1 mg of galectin was labeled with Alexa Fluor 488 or Alexa Fluor 594 according to the manufacture’s instruction (Invitrogen).
Confocal microscopic analysis.
Human colon tissues were snap-frozen in O.C.T. compound, and 5 µm (in thickness) cryosections were prepared. After fixation with acetone for 5 min at 4°C and subsequent blocking with 2% BSA and 1% gelatin, sections were subjected to double staining with Alexa Fluor 594–conjugated recombinant galectin-4 and FITC-conjugated anti–human CD4 (BioLegend). The staining was analyzed using FV1000 confocal laser microscopy (Olympus) immediately after immunostaining.
For mouse cells, purified CD4+ T cells were incubated with rabbit anti-Fam62a antibody, which was generated by help from Affinity BioReagents. After washing, they were fixed by IC Fixation Buffer (eBioscience) and then incubated with goat anti-PKCθ antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 20 min. After washing with Permeabilization Buffer (eBioscience), they were stained with Alexa Fluor 488 anti-goat IgG and Alexa Fluor 568 anti-rabbit IgG (Invitrogen) at room temperature for 20 min. The stained cells were analyzed by Radiance 2000 (BioRad Laboratories).
In vivo BrdU incorporation assay.
1 mg of BrdU was administered intraperitoneally into mice at 48 and 24 h before experiment. After isolation of colonic LP cells, they were blocked with rat and hamster serum and anti-CD16/CD32 (BD), and stained with FITC–anti-CD3e antibody (BD) and PE–anti-CD4 antibody (eBioscience) on ice for 20 min. After surface staining, they were fixed with Cytofix/Cytoperm buffer (BD), washed, treated with DNase, and stained with APC–anti-BrdU mAbs (BD). The stained cells were analyzed using FACSCalibur immediately after staining.
Intracellular cytokine staining.
Colonic cells were stimulated with phorbor-12,13-dibutylate and Ionomycin for 1 h, GoldiStop (eBioScience) was added, and the incubation was continued for additional 4 h as previously described (Nishida et al., 2012). The stimulated cells were stained with surface markers, fixed with Cytofix/Cytoperm, and then stained with Alexa Fluor 647-IL-17, PE-IL-4, FITC-IL-10, and/or FITC-IFN-γ mAbs (eBioscience, BD, and BioLegend). The cells were subjected to flow cytometric analysis immediately after staining.
Real-time PCR analysis.
After staining with FITC–anti-CD4 (RM4.4) mAb, colonic cells were incubated with anti-FITC magnetic beads (Miltenyi Biotec) and CD4+ T cells were purified before RNA was extracted from them. In some experiments, RNA was extracted directly from colonic tissues. Total RNA were converted to cDNA using Superscript II (Invitrogen). The cDNA were initially amplified using Power SYBR Green PCR Master Mix (Applied Biosystems) with a primer set specific for β-actin, as previously described (Nagahama et al., 2008). The real-time PCR was performed by MX3000p QPCR machine (Strategene), and the cDNA concentration was further normalized to make the threshold cycle value of actin 20 cycles. The normalized cDNA samples were then subjected to real-time PCR for the measurement of expression levels of molecules described in the text.
Immunoprecipitation.
Purified CD4+ T cells from the colon were stimulated for 1 h with MACS iBead Particles coupled with anti-CD3ε and anti-CD28 mAbs (Miltenyi Biotec). After stimulation, the beads were removed through magnetic column, and cells were then cultured again in the presence of 1.0 µg/ml galectin-4 for 1 or 3 h. Freshly isolated and stimulated CD4+ T cells (3 × 107) were lysed in ice-cold MBS (25 mM MES and 150 mM NaCl), 0.5% Triton X-100, 1 mM NaVO4, 2 mM EDTA, 1 mM PMSF, and Complete Mini (Roche). The lysates were incubated with 4 µg CTB-conjugated µMACS MicroBeads on ice for 30 min, and then applied into the µ column (Miltenyi Biotec). After vigorous washing steps, proteins were eluted from the beads by heating in a loading buffer and subjected to immunoblot analysis for detection of PKCθ.
Statistical analysis.
Single comparisons were analyzed using the nonparametric Mann-Whitney U test.
Acknowledgments
We thank David Dombkowski and Ravi Mylvaganam for their expert helps for FACS sorting, Dr. Motohiro Nonaka for his useful advice, and Drs. Yasuyo Shimomura, Ken Sugimoto, and Katsunori Shirane for their technical help.
This study was supported primarily by National Institutes of Health (NIH) grant RO1DK064351, and partially by NIH grants RC1DK086242 and RO1AI081807, the Harry B. and Leona Helmsley Charitable Trust, Crohn’s and Colitis Foundation of America (A. Mizoguchi), and NIH grant RO1DK080070 (E. Mizoguchi).
All authors concur with the submission of this manuscript and have no conflicting financial interest.
Footnotes
Abbreviations used:
- βIL-2 DKO
- TCRβ-deficient and IL-2-deficient double knockout
- C2GnT
- core-2 β1,6-N-acetylglucosaminyltransferase
- CAG
- colitis-associated glycome
- CD
- Crohn’s disease
- GlcNAc
- N-acetylglucosamine
- HPA
- Helix pomatia lectin
- IBD
- inflammatory bowel disease
- IS
- immunological synapse
- LEL
- Lycopersicon esculentum lectin
- MALII
- Maackia Amurensis lectin
- PKC
- protein kinase C
- PNA
- peanut agglutinin
- UC
- ulcerative colitis
References
- An G., Wei B., Xia B., McDaniel J.M., Ju T., Cummings R.D., Braun J., Xia L. 2007. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 204:1417–1429 10.1084/jem.20061929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt C.W., Albrecht B., Soos T.J., Littman D.R. 2002. Protein kinase C-theta; signaling from the center of the T-cell synapse. Curr. Opin. Immunol. 14:323–330 10.1016/S0952-7915(02)00346-1 [DOI] [PubMed] [Google Scholar]
- Baier G., Wagner J. 2009. PKC inhibitors: potential in T cell-dependent immune diseases. Curr. Opin. Cell Biol. 21:262–267 10.1016/j.ceb.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Baum L.G., Crocker P.R. 2009. Glycoimmunology: ignore at your peril! Immunol. Rev. 230:5–8 10.1111/j.1600-065X.2009.00800.x [DOI] [PubMed] [Google Scholar]
- Blixt O., Head S., Mondala T., Scanlan C., Huflejt M.E., Alvarez R., Bryan M.C., Fazio F., Calarese D., Stevens J., et al. 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA. 101:17033–17038 10.1073/pnas.0407902101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braccia A., Villani M., Immerdal L., Niels-Christiansen L.L., Nystrøm B.T., Hansen G.H., Danielsen E.M. 2003. Microvillar membrane microdomains exist at physiological temperature. Role of galectin-4 as lipid raft stabilizer revealed by “superrafts”. J. Biol. Chem. 278:15679–15684 10.1074/jbc.M211228200 [DOI] [PubMed] [Google Scholar]
- Geisler C., Jarvis D.L. 2011. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 21:988–993 10.1093/glycob/cwr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez T.S., Kumar K., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D. 2007. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 26:177–190 10.1016/j.immuni.2007.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.S., Stone E.L., Tenno M., Lehtonen E., Farquhar M.G., Marth J.D. 2007. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity. 27:308–320 10.1016/j.immuni.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Hokama A., Mizoguchi E., Sugimoto K., Shimomura Y., Tanaka Y., Yoshida M., Rietdijk S.T., de Jong Y.P., Snapper S.B., Terhorst C., et al. 2004. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 20:681–693 10.1016/j.immuni.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Hsu D.K., Chen H.Y., Liu F.T. 2009. Galectin-3 regulates T-cell functions. Immunol. Rev. 230:114–127 10.1111/j.1600-065X.2009.00798.x [DOI] [PubMed] [Google Scholar]
- Ideo H., Seko A., Ohkura T., Matta K.L., Yamashita K. 2002. High-affinity binding of recombinant human galectin-4 to SO(3)(-)—>3Galbeta1—>3GalNAc pyranoside. Glycobiology. 12:199–208 10.1093/glycob/12.3.199 [DOI] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. 2009. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27:313–338 10.1146/annurev.immunol.021908.132657 [DOI] [PubMed] [Google Scholar]
- Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., et al. 2008. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 118:2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28:573–621 10.1146/annurev-immunol-030409-101225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingeter L.M., Schaefer B.C. 2008. Loss of protein kinase C theta, Bcl10, or Malt1 selectively impairs proliferation and NF-kappa B activation in the CD4+ T cell subset. J. Immunol. 181:6244–6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J.B. 2001. Glycosylation, immunity, and autoimmunity. Cell. 104:809–812 10.1016/S0092-8674(01)00277-X [DOI] [PubMed] [Google Scholar]
- Marth J.D., Grewal P.K. 2008. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8:874–887 10.1038/nri2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A. 2012. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 105:263–320 10.1016/B978-0-12-394596-9.00009-3 [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E. 2010. Animal models of IBD: linkage to human disease. Curr. Opin. Pharmacol. 10:578–587 10.1016/j.coph.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S., Schaffer T., Flogerzi B., Fleetwood A., Weimann R., Schoepfer A.M., Seibold F. 2006. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm. Bowel Dis. 12:588–597 10.1097/01.MIB.0000225341.37226.7c [DOI] [PubMed] [Google Scholar]
- Nagahama K., Ogawa A., Shirane K., Shimomura Y., Sugimoto K., Mizoguchi A. 2008. Protein kinase C θ plays a fundamental role in different types of chronic colitis. Gastroenterology. 134:459–469 10.1053/j.gastro.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Nishida A., Lau C.W., Zhang M., Andoh A., Shi H.N., Mizoguchi E., Mizoguchi A. 2012. The membrane-bound mucin Muc1 regulates T helper 17-cell responses and colitis in mice. Gastroenterology. 142:865–874: e2 10.1053/j.gastro.2011.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz J.C., Medeiros R.B., Mitchell J.S., Zhu P., Freedman B.D., Shimizu Y., Billadeau D.D. 2007. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol. Cell. Biol. 27:5986–6000 10.1128/MCB.00136-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa A., Kanai T., Watanabe M., Yamazaki M., Inoue N., Ikeda M., Kurimoto M., Ishii H., Hibi T. 2002. Th1-mediated intestinal inflammation in Crohn’s disease may be induced by activation of lamina propria lymphocytes through synergistic stimulation of interleukin-12 and interleukin-18 without T cell receptor engagement. Am. J. Gastroenterol. 97:3108–3117 10.1111/j.1572-0241.2002.07107.x [DOI] [PubMed] [Google Scholar]
- Paclik D., Danese S., Berndt U., Wiedenmann B., Dignass A., Sturm A. 2008. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS ONE. 3:e2629 10.1371/journal.pone.0002629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priatel J.J., Chui D., Hiraoka N., Simmons C.J., Richardson K.B., Page D.M., Fukuda M., Varki N.M., Marth J.D. 2000. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 12:273–283 10.1016/S1074-7613(00)80180-6 [DOI] [PubMed] [Google Scholar]
- Rabinovich G.A., Toscano M.A. 2009. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 5:338–352 10.1038/nri2536 [DOI] [PubMed] [Google Scholar]
- Santucci L., Fiorucci S., Rubinstein N., Mencarelli A., Palazzetti B., Federici B., Rabinovich G.A., Morelli A. 2003. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 124:1381–1394 10.1016/S0016-5085(03)00267-1 [DOI] [PubMed] [Google Scholar]
- Sartor R.B. 2008. Microbial influences in inflammatory bowel diseases. Gastroenterology. 134:577–594 10.1053/j.gastro.2007.11.059 [DOI] [PubMed] [Google Scholar]
- Shimomura Y., Ogawa A., Kawada M., Sugimoto K., Mizoguchi E., Shi H.-N., Pillai S., Bhan A.K., Mizoguchi A. 2008. A unique B-2 B cell subset in the intestine. J. Exp. Med. 205:1357–1368 10.1084/jem.20071572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikrishna G., Turovskaya O., Shaikh R., Newlin R., Foell D., Murch S., Kronenberg M., Freeze H.H. 2005. Carboxylated glycans mediate colitis through activation of NF-kappa B. J. Immunol. 175:5412–5422 [DOI] [PubMed] [Google Scholar]
- Stone E.L., Ismail M.N., Lee S.H., Luu Y., Ramirez K., Haslam S.M., Ho S.B., Dell A., Fukuda M., Marth J.D. 2009. Glycosyltransferase function in core 2-type protein O glycosylation. Mol. Cell. Biol. 29:3770–3782 10.1128/MCB.00204-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell S.R., Arthur C.M., Dias-Baruffi M., Rodrigues L.C., Gourdine J.P., Heimburg-Molinaro J., Ju T., Molinaro R.J., Rivera-Marrero C., Xia B., et al. 2010. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 16:295–301 10.1038/nm.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Arendt C.W., Ellmeier W., Schaeffer E.M., Sunshine M.J., Gandhi L., Annes J., Petrzilka D., Kupfer A., Schwartzberg P.L., Littman D.R. 2000. PKC-θ is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 404:402–407 10.1038/35006090 [DOI] [PubMed] [Google Scholar]
- Surolia I., Pirnie S.P., Chellappa V., Taylor K.N., Cariappa A., Moya J., Liu H., Bell D.W., Driscoll D.R., Diederichs S., et al. 2010. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 466:243–247 10.1038/nature09115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano M.A., Bianco G.A., Ilarregui J.M., Croci D.O., Correale J., Hernandez J.D., Zwirner N.W., Poirier F., Riley E.M., Baum L.G., Rabinovich G.A. 2007. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8:825–834 10.1038/ni1482 [DOI] [PubMed] [Google Scholar]
- Tsuboi S., Fukuda M. 1997. Branched O-linked oligosaccharides ectopically expressed in transgenic mice reduce primary T-cell immune responses. EMBO J. 16:6364–6373 10.1093/emboj/16.21.6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi S., Fukuda M. 1998. Overexpression of branched O-linked oligosaccharides on T cell surface glycoproteins impairs humoral immune responses in transgenic mice. J. Biol. Chem. 273:30680–30687 10.1074/jbc.273.46.30680 [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., Rabinovich G.A. 2008. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9:593–601 10.1038/ni.f.203 [DOI] [PubMed] [Google Scholar]
- Vokhmyanina O.A., Rapoport E.M., André S., Severov V.V., Ryzhov I., Pazynina G.V., Korchagina E., Gabius H.J., Bovin N.V. 2012. Comparative study of the glycan specificities of cell-bound human tandem-repeat-type galectin-4, -8 and -9. Glycobiology. 22:1207–1217 10.1093/glycob/cws079 [DOI] [PubMed] [Google Scholar]
- Xavier R.J., Podolsky D.K. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 448:427–434 10.1038/nature06005 [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity. 8:723–732 10.1016/S1074-7613(00)80577-4 [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A., Ding Y., Kumari S., Attur M., Hippen K.L., Brown M., Blazar B.R., Abramson S.B., Lafaille J.J., Dustin M.L. 2010. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 328:372–376 10.1126/science.1186068 [DOI] [PMC free article] [PubMed] [Google Scholar]