Abstract
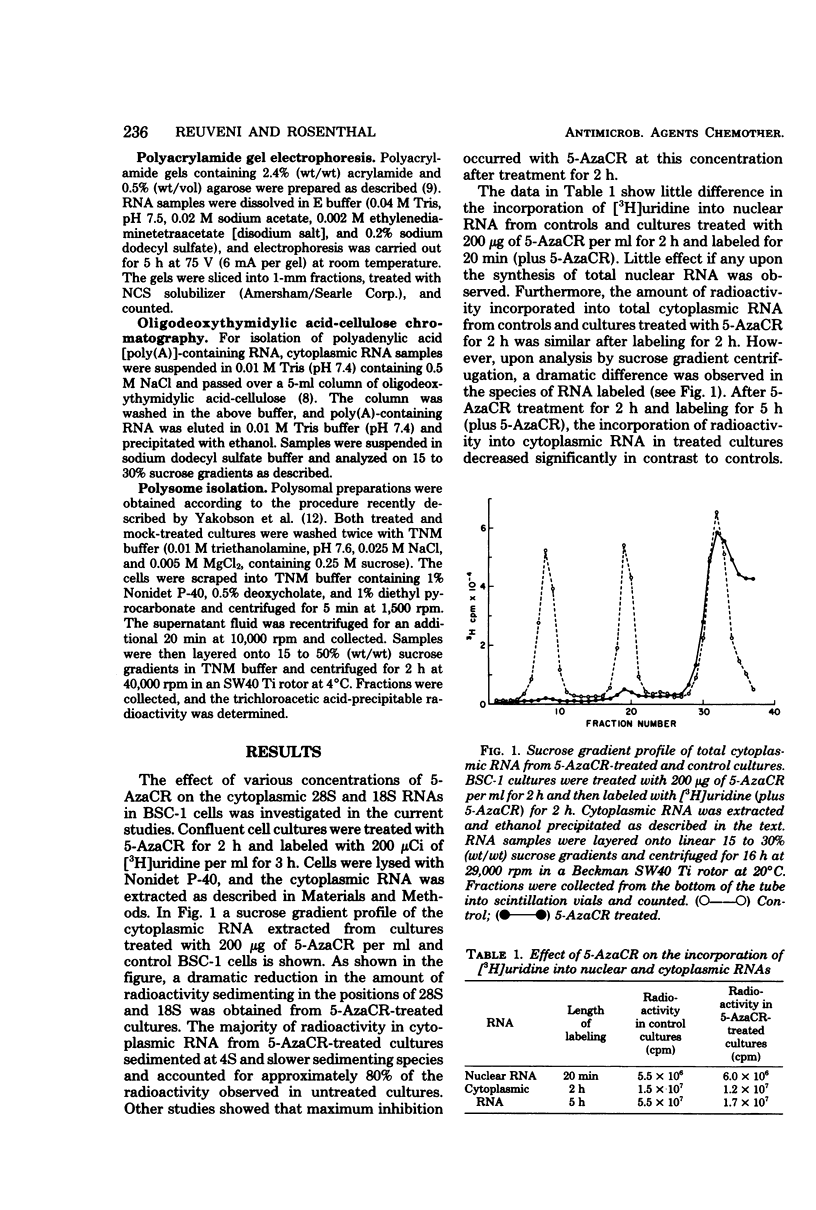
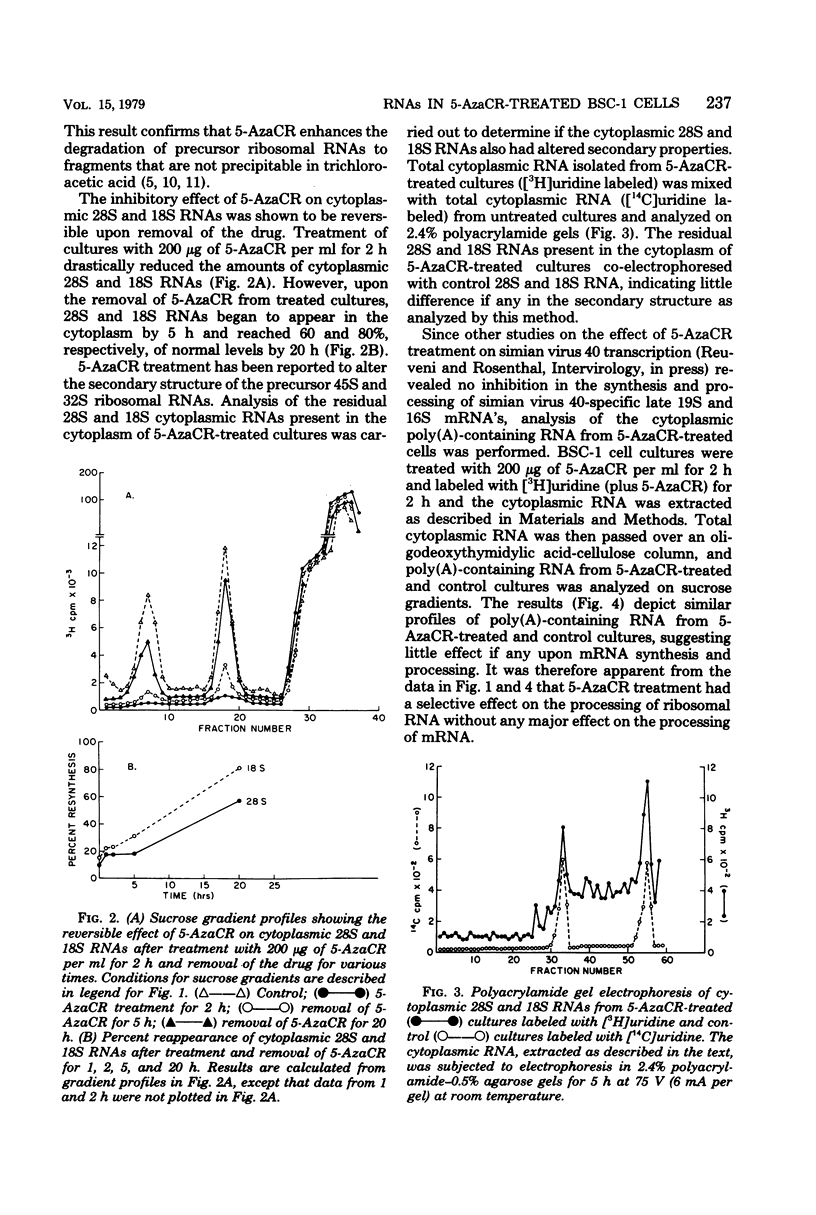
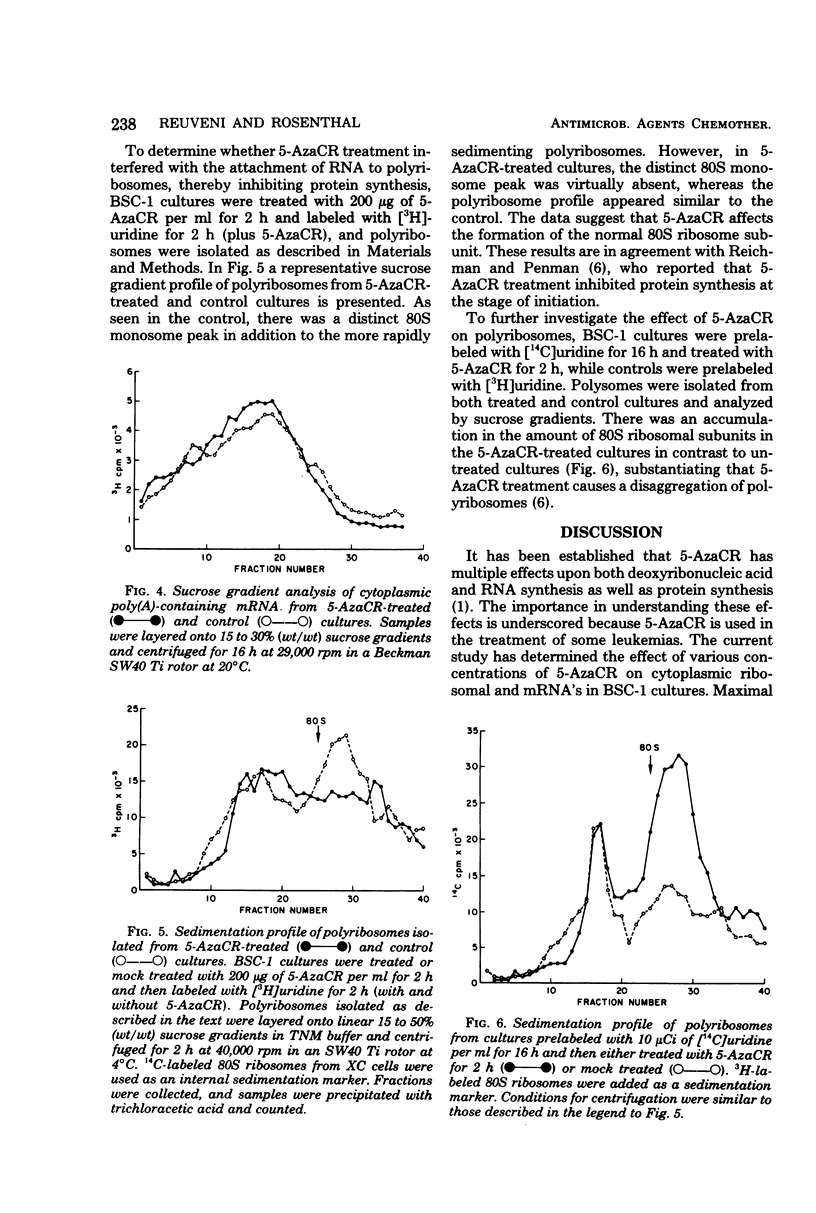
5-Azacytidine (5-AzaCR) inhibited the accumulation of 28S and 18S ribonucleic acids (RNAs) in the cytoplasm of treated cells. The inhibition of 28S and 18S RNAs in the cytoplasm of BSC-1 cells was dependent upon the concentration of 5-AzaCR employed and the time of exposure. At a concentration of 200 μg/ml for 2 h, 5-AzaCR inhibited the cytoplasmic 28S and 18S RNAs by 80 and 70%, respectively. The 28S and 18S RNAs that appeared in the cytoplasm of treated cultures had no altered secondary structure, as analyzed by polyacrylamide gel electrophoresis. The inhibitory effect on cytoplasmic 28S and 18S RNAs was found to be reversible, and removal of 5-AzaCR from treated cultures allowed the accumulation of cytoplasmic 28S and 18S RNAs to almost normal levels by 20 h. 5-AzaCR appeared to have no effect upon the synthesis and processing of polyadenylic acid-containing messenger RNA in treated cultures. However, the formation of the 80S ribosomal subunit appeared to be inhibited in drug-treated cells. Moreover, 5-AzaCR treatment caused a disaggregation of polyribosomes and an accumulation of 80S ribosomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cihák A. Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974;30(5):405–422. doi: 10.1159/000224981. [DOI] [PubMed] [Google Scholar]
- Levitan I. B., Morris H. P., Webb T. E. Tyrosine transaminase induction: differential effect of 5-azacytidine on liver and hepatoma 5123D. Biochim Biophys Acta. 1971 Jun 30;240(2):287–295. [PubMed] [Google Scholar]
- Li L. H., Olin E. J., Buskirk H. H., Reineke L. M. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970 Nov;30(11):2760–2769. [PubMed] [Google Scholar]
- Paces V., Doskocil J., Sorm F. Incorporation of 5-azacytidine into nucleic acids of Escherichia coli. Biochim Biophys Acta. 1968 Jul 23;161(2):352–360. [PubMed] [Google Scholar]
- Reichman M., Karlan D., Penman S. Destructive processing of the 45-S ribosomal precursor in the presence of 5-azacytidine. Biochim Biophys Acta. 1973 Feb 23;299(1):173–175. doi: 10.1016/0005-2787(73)90409-7. [DOI] [PubMed] [Google Scholar]
- Reichman M., Penman S. The mechanism of inhibition of protein synthesis by 5-azacytidine in HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):282–289. doi: 10.1016/0005-2787(73)90145-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. J., Brown M. The control of SV40 transcription during a lytic infection: late RNA synthesis in the presence of inhibitors of DNA replication. Nucleic Acids Res. 1977 Mar;4(3):551–565. doi: 10.1093/nar/4.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J. Isolation and characterization of poly(A)-containing polyoma "early" and "late" messenger RNAs. Nucleic Acids Res. 1976 Mar;3(3):661–676. doi: 10.1093/nar/3.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. II. Separation and characterization of virus-specific RNA species. Virology. 1972 Sep;49(3):766–783. doi: 10.1016/0042-6822(72)90533-8. [DOI] [PubMed] [Google Scholar]
- Weiss J. W., Pitot H. C. Alteration of ribosomal precursor RNA in Novikoff hepatoma cells by 5-azacytidine. Studies on methylation of 45S and 32S RNA. Arch Biochem Biophys. 1974 Dec;165(2):588–596. doi: 10.1016/0003-9861(74)90286-0. [DOI] [PubMed] [Google Scholar]
- Weiss J. W., Pitot H. C. Effects of 5-azacytidine on nucleolar RNA and the preribosomal particles in Novikoff hepatoma cells. Biochemistry. 1975 Jan 28;14(2):316–326. doi: 10.1021/bi00673a018. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Prives C., Hartman J. R., Winocour E., Revel M. Inhibition of viral protein synthesis in monkey cells treated with interferon late in simian virus 40 lytic cycle. Cell. 1977 Sep;12(1):73–81. doi: 10.1016/0092-8674(77)90186-6. [DOI] [PubMed] [Google Scholar]



