Abstract
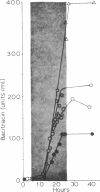
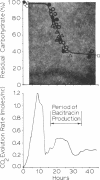
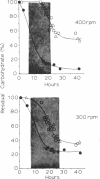
The physiological effects of controlling the dissolved oxygen tension at 0.01, 0.02, and 0.05 atm by the use of oxygen-enriched aeration were investigated during growth and bacitracin production by Bacillus licheniformis ATCC 10716. Up to a 2.35-fold increase in the final antibiotic yield and a 4-fold increase in the rate of bacitracin synthesis were observed in response to O2-enriched aeration. The increase in antibiotic production was accompanied by increased respiratory activity and an increase in the specific productivity of the culture from 1.3 to 3.6 g of antibiotic per g of cell mass produced. Oxygen enrichment of the aeration decreased medium carbohydrate uptake and the maximum specific growth rate of B. licheniformis from 0.6 h−1 to as low as 0.15 h−1, depending upon the level of enrichment and the conditions of oxygen transfer rate (impeller speed). The response of this culture to O2 enrichment suggests that this method of controlling the dissolved oxygen tension for antibiotic-producing cultures may simulate conditions that would occur if the carbon source were fed slowly, as is often employed to optimize antibiotic production. Analysis of the biologically active bacitracins produced by B. licheniformis ATCC 10716 suggested that the ratio of biologically active peptides was not changed by O2 enrichment, nor were any new biologically active compounds formed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S., Johnson B. A., Goldberg J., Meleney F. L. Bacitracin: Methods of Production, Concentration, and Partial Purification, with a Summary of the Chemical Properties of Crude Bacitracin. J Bacteriol. 1948 Feb;55(2):249–255. [PMC free article] [PubMed] [Google Scholar]
- BARTHOLOMEW J. W., MITTWER T. A simplified bacterial spore stain. Stain Technol. 1950 Jul;25(3):153–156. doi: 10.3109/10520295009110979. [DOI] [PubMed] [Google Scholar]
- Bylinkina E. S., Nikitina T. S., Biryukov V. V., Cherkasova O. N. Effect of dissolved carbon dioxide on life activity of antibiotic-producing microorganisms. Biotechnol Bioeng Symp. 1973;0(4-1):197–207. [PubMed] [Google Scholar]
- Cooney C. L., Wang H. Y., Wang D. I. Computer-aided material balancing for prediction of fermentation parameters. Biotechnol Bioeng. 1977 Jan;19(1):55–67. doi: 10.1002/bit.260190106. [DOI] [PubMed] [Google Scholar]
- Feren C. J., Squires R. W. The relationship between critical oxygen level and antibiotic synthesis of capreomycin and cephalosporin C. Biotechnol Bioeng. 1969 Jul;11(4):583–592. doi: 10.1002/bit.260110406. [DOI] [PubMed] [Google Scholar]
- Flickinger M. C., Perlman D. Application of Oxygen-Enriched Aeration in the Conversion of Glycerol to Dihydroxyacetone by Gluconobacter melanogenus IFO 3293. Appl Environ Microbiol. 1977 Mar;33(3):706–712. doi: 10.1128/aem.33.3.706-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: effect of glucose. J Gen Microbiol. 1974 Apr;81(2):383–390. doi: 10.1099/00221287-81-2-383. [DOI] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: role of catabolite repression and organic acids. J Gen Microbiol. 1974 Oct;84(2):321–326. doi: 10.1099/00221287-84-2-321. [DOI] [PubMed] [Google Scholar]
- Haavik H. I., Thomassen S. A bacitracin-negative mutant of Bacillus licheniformis which is able to sporulate. J Gen Microbiol. 1973 Jun;76(2):451–454. doi: 10.1099/00221287-76-2-451. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Silver R. S., Mateles R. I. Use of the glucose oxidase system to measure oxygen transfer rates. Biotechnol Bioeng. 1969 Jan;11(1):1–18. doi: 10.1002/bit.260110102. [DOI] [PubMed] [Google Scholar]
- Járai M., Györy E., Tombor J. Oxygen transfer in Streptomyces fermentation broths. Biotechnol Bioeng. 1969 Jul;11(4):605–622. doi: 10.1002/bit.260110408. [DOI] [PubMed] [Google Scholar]
- Kjaergaard L. Efficiency of microbial growth in biomass production. Biotechnol Bioeng. 1978 Oct;20(10):1691–1694. doi: 10.1002/bit.260201017. [DOI] [PubMed] [Google Scholar]
- Oura E. Effect of aeration intensity on the biochemical composition of baker's yeast. I. Factors affecting the type of metabolism. Biotechnol Bioeng. 1974 Sep;16(9):1197–1212. doi: 10.1002/bit.260160905. [DOI] [PubMed] [Google Scholar]
- Oura E. Effect of aeration intensity on the biochemical composition of baker's yeast. II. Activities of the oxidative enzymes. Biotechnol Bioeng. 1974 Sep;16(9):1213–1225. doi: 10.1002/bit.260160906. [DOI] [PubMed] [Google Scholar]
- Oura E. Energetics of yeast growth under different intensities of aeration. Biotechnol Bioeng Symp. 1973;0(4-1):117–127. [PubMed] [Google Scholar]
- ROLINSON G. N. Respiration of Penicillium chrysogenum in penicillin fermentations. J Gen Microbiol. 1952 May;6(3-4):336–343. doi: 10.1099/00221287-6-3-4-336. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Robertson J. H., Bach J. A. Quantitative high-pressure liquid chromatographic analysis of bacitracin, a polypeptide antibiotic. J Chromatogr. 1974 Nov 6;99(0):597–608. doi: 10.1016/s0021-9673(00)90888-4. [DOI] [PubMed] [Google Scholar]