Abstract
The distribution of species body size is critically important for determining resource use within a group or clade. It is widely known that non-avian dinosaurs were the largest creatures to roam the Earth. There is, however, little understanding of how maximum species body size was distributed among the dinosaurs. Do they share a similar distribution to modern day vertebrate groups in spite of their large size, or did they exhibit fundamentally different distributions due to unique evolutionary pressures and adaptations? Here, we address this question by comparing the distribution of maximum species body size for dinosaurs to an extensive set of extant and extinct vertebrate groups. We also examine the body size distribution of dinosaurs by various sub-groups, time periods and formations. We find that dinosaurs exhibit a strong skew towards larger species, in direct contrast to modern day vertebrates. This pattern is not solely an artefact of bias in the fossil record, as demonstrated by contrasting distributions in two major extinct groups and supports the hypothesis that dinosaurs exhibited a fundamentally different life history strategy to other terrestrial vertebrates. A disparity in the size distribution of the herbivorous Ornithischia and Sauropodomorpha and the largely carnivorous Theropoda suggests that this pattern may have been a product of a divergence in evolutionary strategies: herbivorous dinosaurs rapidly evolved large size to escape predation by carnivores and maximise digestive efficiency; carnivores had sufficient resources among juvenile dinosaurs and non-dinosaurian prey to achieve optimal success at smaller body size.
Introduction
The mass of an organism is fundamental to its biology, affecting physiology, ecology, metabolism and more [1], [2]. Knowledge of the mass of an adult individual, and by extension the species or genus to which it belongs, can therefore provide important information about the taxon in question. Much effort has thus been devoted to estimating the mass of the extinct non-avian dinosaurs (hereafter simply dinosaurs). As a group they are especially interesting as they feature numerous multi-ton taxa and include the largest terrestrial animals of all time [3]. Large size evolved early on in the Dinosauria, with multi-ton sauropodomorphs and basal sauropods appearing in the Late Triassic, and even the earliest dinosaurs show evidence for rapid growth [4]. However, while much research has been devoted to both mass estimates of dinosaurs (e.g. [5], [6]) and changes in body size (e.g. [7], [8], [9]), very limited attention has been paid to the distribution of dinosaur body size (but see [10], [11]), especially in the context of ecological implications [12], [13]. Dinosaurs may feature species that were considerably greater in maximum size to those of modern or other extinct animals, but this may only relate to the absolute size of a given taxon, rather than representing a fundamentally different distribution of body sizes within an entire group or clade.
The pattern of body size distribution is critically important for determining resource use: there is more usable space for small animals, so small-bodied species should be more prevalent in nature as they can better subdivide the habitat and co-exist in larger numbers [14], [15]. This phenomenon is highlighted by a skew towards small-sized species in many terrestrial groups [16], particularly mammals [17], [18], [19] and birds [20], [21], [22]. However, the positively-skewed distribution of these groups becomes less clear at the order level [18], [23] and at smaller spatial scales [19], [24]. This may be a product of small sample size [14], but it suggests that positively-skewed distributions are broad-ranging patterns, attributable to higher levels of taxonomic organisation at large biogeographical scales.
Maurer et al. [19] have also demonstrated that small body size is promoted by speciation, while extinctions are biased towards larger body size, leading to a higher probability of positively-skewed size distributions. These results were based on the models of McKinney [25], who suggested that, if most clades originate at small size, there is a lower limit on diversification toward small size, with size increases more likely. It has been shown that this lower constraint on species body size is a key factor driving the positively-skewed size distributions so often observed in nature [14]. The skew towards smaller species has also been linked to an optimum body size for a species based on the difference between assimilation and respiration [26], or energy that can be allocated to growth and reproduction. More recently, the concept of a size distribution around a common optimum for a taxon [26] has been rejected in favour of distributions of optimal sizes, different for each species and dependent on mortality and productivity [27], [28]. The latter phenomenon has been shown to produce a high prevalence of positively-skewed size distributions in simulated models, with occasional occurrence of negative skew [29], [30]. There is still, however, much uncertainty surrounding the mechanisms that lead to these exceptions to the rule.
As palaeontologists rely on modern analogues to inform our understanding of extinct ecologies, it is important to determine if dinosaurian size distributions were fundamentally similar or different to modern-day vertebrate groups. Here, we address this issue by comparing the body size distribution of dinosaurs to other known extant and extinct vertebrate groups. We also explore subdivisions of size distributions in dinosaurs by major clades, time periods and formations to tease apart the possible factors that facilitated the observed patterns.
Methods
Extensive datasets of maximum species body size were collated from the literature for eight major animal groups: extant birds, reptiles, amphibians, fish and terrestrial mammals and extinct dinosaurs and pterosaurs and Cenozoic terrestrial mammals. These categories represent the major vertebrate groups, forming a logical point of comparison.
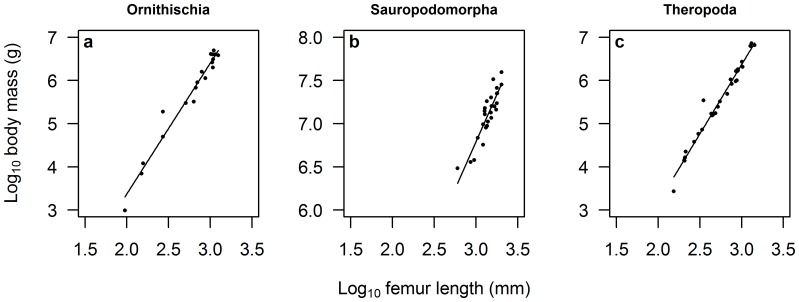
All dinosaur body masses were estimated from femur length-body mass relationships established during the study. Length-weight relationships were drawn separately for each of three clades (see Fig. 1): Ornithischia (19 data points, r 2 = 0.93), Sauropodomorpha (27 data points, r 2 = 0.73) and Theropoda (31 data points, r 2 = 0.97). The data collected to construct these relationships were the result of an extensive literature search spanning 41 separate publications and consist of all dependable published mass estimates for which a femur length could also be obtained (see Table 1). While some studies call into question the accuracy of volumetric models [31], these represent the best estimates of dinosaur body mass currently available. Femur lengths were acquired from the literature and museum specimens for a total of 329 out of approximately 1,350 dinosaur species (24% completeness). In cases where there were several individual femur length measurements available for a species, we chose to take the maximum femur length. While the use of limb bone circumference has been recommended for estimating mass [32], the combined data on femur lengths and body mass estimates were far more extensive. Additionally, a strong correlation (r 2 = 0.94) has been shown between femur length and diameter (a component of circumference) from a sample of 221 dinosaur individuals [33].
Figure 1. Log10(femur length)-log10(body mass) relationships for three major dinosaur clades: (a) Ornithischia (y = 3.0587x−2.7042; r 2 = 0.93), (b) Sauropodomorpha (y = 2.3459x−0.2935; r 2 = 0.73) and (c) Theropoda (y = 3.1854x−3.1840; r 2 = 0.97).
The data sources for these relationships are shown in Table 1.
Table 1. Dinosaur taxa constituting the femur length-body mass relationships shown in Fig. 1, along with specimen numbers (where available), mass (in kg), femur length (FL in mm), source of mass measurement and reference to the paper containing the FL and mass estimate.
| Clade | Genus | Species | Specimen | Mass (kg) | FL (mm) | Source | Reference |
| Ornithischia | Anatosaurus * | copei * | AMNH 5730 | 4000 | 1150 | Limb bone scaling | [70] |
| Ornithischia | Bactrosaurus | johnsoni | AMNH 6553 | 1588.9 | 790.5 | Polynomial | [6], [71] |
| Ornithischia | Corythosaurus | casuarius | AMNH 5240 | 3078.5 | 1080 | Polynomial | [6], [72] |
| Ornithischia | Edmontosaurus | annectens | USNM 2414 | 3990.8 | 1068.5 | Polynomial | [6], [72] |
| Ornithischia | Edmontosaurus | regalis | NMC 2289 | 3800 | 1245 | Limb bone scaling | [70] |
| Ornithischia | Gasparinisaura | cincosaltensis | MUCPc-208 | 0.98 | 94.7 | Polynomial | [6], [73] |
| Ornithischia | Huayangosaurus | taibaii | ZDM T7001 | 301.4 | 510 | Polynomial | [6], [74] |
| Ornithischia | Hypacrosaurus | altispinus | NMC 8501 | 2000 | 1074 | Limb bone scaling | [70] |
| Ornithischia | Hypsilophodon | foxii | NHM R196 | 7 | 150 | Polynomial | [6], [75], [76] |
| Ornithischia | Iguanodon | atherfieldensis | NHM R5764 | 678.4 | 670 | Polynomial | [6], [77] |
| Ornithischia | Kentrosaurus | * | HMN | 321.1 | 633 | 3D Slicing | [6], [78] |
| Ornithischia | Leptoceratops | gracilis | NMC 8889 | 190 | 270 | Limb bone scaling | [70] |
| Ornithischia | Muttaburrasaurus | langdoni | QM F6140 | 4100.4 | 1015 | Polynomial | [6], [79] |
| Ornithischia | Parkosaurus | warreni | ROM 804 | 50 | 270 | Limb bone scaling | [70] |
| Ornithischia | Psittacosaurus | mongoliensis | AMNH 6253 | 12.1 | 157 | Polynomial | [6], [80] |
| Ornithischia | Sauropelta | edwardsi | AMNH 3036 | 902.9 | 700 | Polynomial | [6], [81] |
| Ornithischia | Stegosaurus | armatus | USNM 4934 | 2610.6 | 1053 | Polynomial | [6], [75], [76] |
| Ornithischia | Triceratops | prorsus | USNM 4842 | 4964 | 1104 | Polynomial | [6], [82] |
| Ornithischia | Tuojiangosaurus | multispinus | CV00209 | 1134.3 | 875 | Polynomial | [6], [83] |
| Sauropodomorpha | Alamosaurus | sanjuanensis | 32663 | 1610 | Growth lines | [84] | |
| Sauropodomorpha | Amargasaurus | cazaui | 6852.9 | 1050 | Polynomial | [6], [85] | |
| Sauropodomorpha | Apatosaurus | * | 17273 | 1785 | 3D Slicing | [86] | |
| Sauropodomorpha | Apatosaurus | excelsus | 25952 | 1775 | Growth lines | [84] | |
| Sauropodomorpha | Apatosaurus | louisae | CM 3018 | 22407.2 | 1785 | Polynomial | [6], [87] |
| Sauropodomorpha | Barosaurus | * | 20039.5 | 1520 | Polynomial | [6], [87] | |
| Sauropodomorpha | Brachiosaurus | altithorax | FCM | 28264.6 | 2030 | Polynomial | [6], [88] |
| Sauropodomorpha | Brachiosaurus * | brancai * | 39500 | 2028 | Displacement | [89], [90] | |
| Sauropodomorpha | Camarasaurus | lewisi | BYU 9047 | 11652.2 | 1525 | Polynomial | [6], [87] |
| Sauropodomorpha | Camarasaurus | supremus | 9300 | 1341 | Displacement | [89], [90] | |
| Sauropodomorpha | Cetiosaurus | oxoniensis | 15900 | 1660 | Displacement | [89], [90] | |
| Sauropodomorpha | Dicraeosaurus | hansemanni | 5700 | 1220 | Displacement | [89], [90] | |
| Sauropodomorpha | Diplodocus | * | 13421 | 1506 | 3D Slicing | [5], [91] | |
| Sauropodomorpha | Diplodocus | carnegiei | 16000 | 1540 | Displacement | [89], [90] | |
| Sauropodomorpha | Euhelopus | zdanskyi | 3800 | 955 | Displacement | [90], [92] | |
| Sauropodomorpha | Haplocanthosaurus | * | 14528.6 | 1745 | Polynomial | [6], [87] | |
| Sauropodomorpha | Haplocanthosaurus | priscus | 12800 | 1275 | Displacement | [90], [92] | |
| Sauropodomorpha | Janenschia | robustus | 14029 | 1270 | Growth lines | [84] | |
| Sauropodomorpha | Jobaria | tiguidensis | 22448 | 1800 | 3D Slicing | [93], [94] | |
| Sauropodomorpha | Mamenchisaurus | hochuanensis | 18169.7 | 1350 | Polynomial | [6], [87], [95] | |
| Sauropodomorpha | Mamenchisaurus | hochuanensis | 15100 | 1275 | Displacement | [89], [90] | |
| Sauropodomorpha | Northampton* | sauropod* | 9000 | 1320 | Growth lines | [84] | |
| Sauropodomorpha | Omeisaurus | tianfuensis | 9800 | 1215 | Displacement | [89], [90] | |
| Sauropodomorpha | Opisthocoelicaudia | skarzynskii | ZPAL MgD-I/48 | 10522.2 | 1395 | Polynomial | [6], [96] |
| Sauropodomorpha | Patagosaurus | * | 9435.4 | 1360 | Polynomial | [6], [87] | |
| Sauropodomorpha | Riojasaurus | * | 3038.7 | 600 | Polynomial | [6], [97] | |
| Sauropodomorpha | Shunosaurus | lii | 3600 | 865 | Displacement | [89], [90] | |
| Theropoda | Afrovenator | abakensis | UCOBA1 | 826.6 | 760 | Polynomial | [6], [98] |
| Theropoda | Albertosaurus | * | 1685 | 905 | Displacement | [99] | |
| Theropoda | Allosaurus | fragilis | 1620 | 874 | Displacement | [99] | |
| Theropoda | Allosaurus | fragilis | USMN 4734 | 952 | 850 | Polynomial | [6], [100] |
| Theropoda | Anserimimus | * | 170 | 433 | Displacement | [99] | |
| Theropoda | Avimimus | portentosus | 14 | 205 | Displacement | [89], [101], [102] | |
| Theropoda | Carnotaurus | sastrei | 2070 | 1030 | Displacement | [89], [92], [103] | |
| Theropoda | Coelophysis | bauri | AMNH FR 7223 | 16 | 209 | Polynomial | [6], [102] |
| Theropoda | Daspletosaurus | * | 2700 | 1006 | Displacement | [99] | |
| Theropoda | Deinonychus | antirrhopus | MCZ 4371 | 73 | 336 | Displacement | [89], [102] |
| Theropoda | Deltadromeus | agilis | SGM-Din2 | 1048.9 | 740 | Polynomial | [6], [104] |
| Theropoda | Dilophosaurus | wetherilli | UCMP 37302 | 325 | 551 | Displacement | [99] |
| Theropoda | Dromiceiomimus | * | 160 | 454 | Displacement | [99] | |
| Theropoda | Elaphrosaurus | bambergi | HMN dd | 245 | 519 | Displacement | [99] |
| Theropoda | Eoraptor | lunensis | PVSJ 512 | 2.7 | 154 | Polynomial | [6], [105] |
| Theropoda | Gallimimus | bullatus | G.I.DPS 100/11 | 490 | 673 | Displacement | [99] |
| Theropoda | Gallimimus | bullatus | 38 | 270 | Displacement | [89] | |
| Theropoda | Giganotosaurus | carolinii | MUCPv-CH-1 | 6594.8 | 1430 | Polynomial | [6], [106] |
| Theropoda | Gorgosaurus | libratus | TMP ? | 1815 | 905 | Displacement | [89] |
| Theropoda | Herrerasaurus | ischigualastensis | 145 | 345 | Displacement | [89], [102] | |
| Theropoda | Ornitholestes | hermanni | AMNH 587 | 16.5 | 210 | Displacement | [99] |
| Theropoda | Ornithomimus | edmontonensis | TMP ? | 155 | 443 | Displacement | [99] |
| Theropoda | Oviraptor | philoceratops | 58 | 303 | Displacement | [99] | |
| Theropoda | Saurornitholestes | langstoni | TMP 88.121.39 | 22.5 | 214 | Displacement | [99] |
| Theropoda | Sinraptor | dongi | TMP 90.300.1 | 1700 | 884 | Displacement | [99] |
| Theropoda | Sinraptor | dongi | IVPP 10600 | 1009 | 876 | Polynomial | [6], [107] |
| Theropoda | Struthiomimus | altus | AMNH 5339 | 175 | 486 | Displacement | [99] |
| Theropoda | Syntarsus | rhodesiensis | QG/1 | 13.8 | 208 | Polynomial | [6], [102] |
| Theropoda | Tarbosaurus | * | 1650 | 854 | Displacement | [99] | |
| Theropoda | Tyrannosaurus | rex | CM 9780 (AMNH 5027) | 6300 | 1273 | Displacement | [99] |
| Theropoda | Tyrannosaurus | rex | 7224 | 1314.5 | 3D Slicing | [5], [108] |
Note that just 77 dinosaur species were identified in the literature with combined femur length and body mass estimates. Maximum femur lengths from a total of 329 species were used in the exploration of dinosaur body size distributions and these data are available on request from the authors.
Note that Anatosaurus copei is now identified as Edmontosaurus annectens and Brachiosaurus brancai is now known as Giraffatitan brancai. In this table, we report the species names as listed in the original referenced publication for ease of cross referencing. This includes a number of dinosaur genera that do not contain species names in the original paper.
All bird data were extracted from Dunning’s 2008 handbook of avian body masses [34]. We chose to take the maximum body mass listed for each species, irrespective of the sex of the bird. These measurements constitute 9,381 out of approximately 10,000 bird species (94% completeness).
Reptile data were collated from a number of sources. Snout-vent lengths (SVL) for 4,874 lizard species were taken from Shai Meiri’s dataset [35]. Here, maximum SVL is seen as a good measure of the size potential in a population and is tightly correlated with mean adult SVL and SVL at sexual maturity [35], [36]. Lizard body masses were obtained using the SVL-mass allometries listed in Table 2 of Meiri’s 2010 publication [37]. Body mass data for a further 1,330 reptile species were obtained from Guyer and Boback’s online published dataset [38]. This included 1,030 snake species, 260 turtle species, 22 crocodilian species and a further 18 lizard species. Snakes were measured as maximum total length (TL) and converted to body mass using the TL-mass allometry listed in Pough’s 1980 publication [39]. Turtles were measured as maximum carapace length (CL) and converted to body mass using the CL-mass allometry listed in Pough’s 1980 publication [39]. Crocodiles were measured as maximum TL and converted to body mass using the TL-mass allometry listed in Table 3 of Farlow et al.’s 2005 publication [40]. Body masses for the two existing species of tuatara were taken from two recent publications [41], [42]. This resulted in body mass estimates for a total of 6,206 out of approximately 8,700 reptile species (71% completeness).
Table 2. Exploration of body size distributions for major vertebrate groups, dinosaur clades, time periods and formations.
| Comments | Category | Skewness | Location of modes on x-axis of body size distribution | Lilliefors D | p value | ||||
| Major vertebrate groups | Dinosaurs | −0.758 | 6.3 | 0.105 | <0.001 | ||||
| (see Figure 2) | Birds | 0.837 | 1.3 | 4.6 | 5.2 | 0.091 | <0.001 | ||
| Reptiles | 1.077 | 0.9 | 6.1 | 0.109 | <0.001 | ||||
| Amphibians | 1.140 | 0.0 | 2.8 | 4.2 | 0.082 | <0.001 | |||
| Fish | 0.180 | −1.6 | 1.5 | 1.9 | 2.4 | 0.021 | <0.001 | ||
| Extant Mammals | 0.906 | 1.5 | 4.8 | 0.118 | <0.001 | ||||
| Pterosaurs | 0.226 | 3.0 | 4.5 | 0.117 | 0.084 | ||||
| Extinct Mammals | 0.333 | 2.0 | 4.7 | 0.089 | <0.001 | ||||
| Major dinosaur clades | Ornithischia | −0.909 | 6.1 | 0.157 | <0.001 | ||||
| (see Figure 4) | Sauropodomorpha | −1.501 | 7.1 | 0.161 | <0.001 | ||||
| Theropoda | −0.305 | 5.3 | 0.084 | 0.076 | |||||
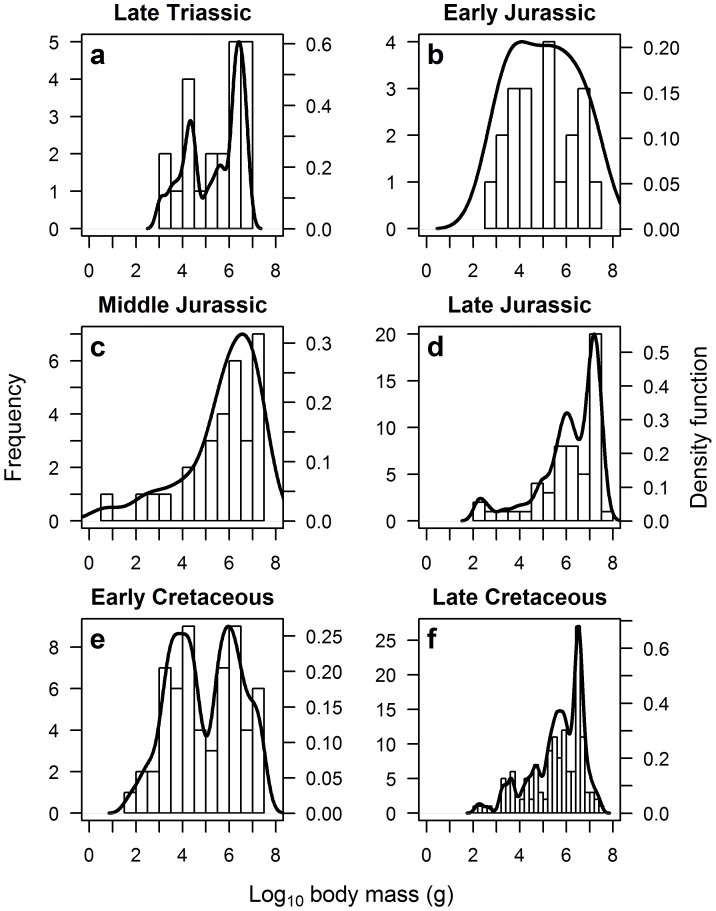
| Major time periods | Late Triassic | −0.432 | 4.3 | 5.6 | 6.4 | 0.195 | 0.029 | ||
| (see Figure 5) | Early Jurassic | 0.071 | 4.1 | 0.143 | 0.354 | ||||
| Middle Jurassic | −1.369 | 6.6 | 0.182 | 0.015 | |||||
| Late Jurassic | −1.294 | 2.3 | 6.0 | 7.2 | 0.142 | 0.008 | |||
| Early Cretaceous | −0.089 | 3.8 | 6.0 | 0.114 | 0.052 | ||||
| Late Cretaceous | −0.950 | 2.3 | 3.6 | 4.7 | 5.7 | 6.5 | 0.119 | <0.001 | |
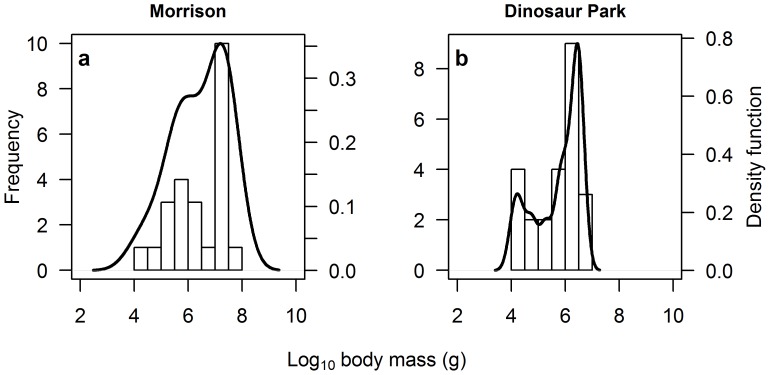
| Major Formations | Morrison | −0.558 | 7.2 | 0.223 | 0.003 | ||||
| (see Figure 6) | Dinosaur Park | −0.697 | 4.2 | 6.5 | 0.170 | 0.070 | |||
Values are given for skewness of the distribution, location of modes in the distribution, Lilliefors D statistic and the p value showing significant difference from a normal distribution.
Amphibian data were also obtained from Guyer and Boback’s online published dataset [38]. This included 1,424 anuran species, 244 Caudata and 101 Gymnophiona for a total of 1,769 out of approximately 6,500 species (37% completeness).
All fish data were collated from FishBase [43]. Fish body masses were calculated from maximum fish lengths (a mixture of total lengths, standard lengths and fork lengths) and their corresponding length-weight relationships. This resulted in body mass estimates for a total of 11,994 out of approximately 32,000 fish species (37% completeness).
Extant mammal body masses were taken from Smith et al.’s 2003 data paper [44], which provides body mass estimates for a total of 4,061 out of approximately 5,488 mammal species (74% completeness). Note that we considered only fully or predominantly terrestrial mammals. As such all chiropterans, cetaceans, sirenians and pinnipeds were excluded from this dataset.
Frequency distributions of maximum species body size were plotted from these data for each group, with size bins of 0.2 width on a log10 scale. A combination of kernel density estimation and smoothed bootstrap resampling (based on 1000 randomisations) was used to examine the modality of these body size distributions. This procedure (described in detail in [45], [46]) tests whether a distribution with k+1 modes fits significantly better than a distribution with k modes, thus determining the optimum modality of the data. Other arbitrary techniques (e.g. [47]) typically overestimate the number of modes and gaps in body size distributions [45], [48]. The location of each mode was recorded relative to the x-axis. A measure of skewness was also calculated for each distribution as g 1 = m 3/m 2 3/2, where m 3 is the sample third central moment and m 2 is the sample variance (after [49]). To determine if the distribution was significantly skewed, it was tested against normality using Lilliefors (Kolmogorov-Smirnov) test. The body size distribution of dinosaurs was also compared to all other groups using the Kolmogorov-Smirnov test.
To investigate the influence of taphonomic bias in the fossil record, body size distributions were also explored for extinct pterosaurs and Cenozoic mammals. Here, the existence of a similar pattern in other extinct groups would be convincing evidence for fossil bias. Pterosaurs are the sister-taxon to the dinosauromorphs and, like the dinosaurs, originated in the Late Triassic and went extinct at the end of the Cretaceous, occupying numerous common ecosystems. Pterosaur body mass estimates were taken from wingspan data in Ross Elgin’s appendix for the forthcoming Pterosauria book [50]. This gave a total of 50 species. While this is a small dataset, it encompasses approximately one third of known pterosaur species. Mass estimates were calculated from a wingspan-weight formula in [51]. Cenozoic mammals provide a well-sampled clade of fossilised terrestrial taxa and form an obvious point of comparison to extant mammals. Cenozoic mammal body masses were taken from John Alroy’s online paleobiology database [52] used in Clauset and Erwin’s 2008 publication [53]. This gave a total of 2,034 species. As for the extant mammals, we considered only fully or predominantly terrestrial mammals. Pterosaur and Cenozoic mammal data were analysed as described above for the other vertebrate groups. The body size distribution of extant and Cenozoic mammals were also compared using the Kolmogorov-Smirnov test.
To evaluate the consistency of observed patterns in the body size distribution of dinosaurs, the data were reanalysed after sub-dividing by clades, time periods and formations. Three major clades were employed in this analysis: Ornithischia, Sauropodomorpha and Theropoda, with 143, 86 and 100 data points, respectively. Six time periods were used: Late Triassic, Early, Middle and Late Jurassic, and Early and Late Cretaceous which used 23, 21, 31, 58, 61 and 135 data points, respectively. Finally, two major rock formations also had a sufficient number of species to be utilised: the Late Jurassic Morrison Formation in the western United States of America and the Late Cretaceous Dinosaur Park Formation in Alberta, Canada, each of which used 24 data points. Similar metrics to those described above were obtained for these sub-divisions (but plotted with size bins of 0.5 width on a log10 scale where the number of data points was less than 100). All analyses were performed with R 2.14.0 (R Development Core Team 2011).
Results
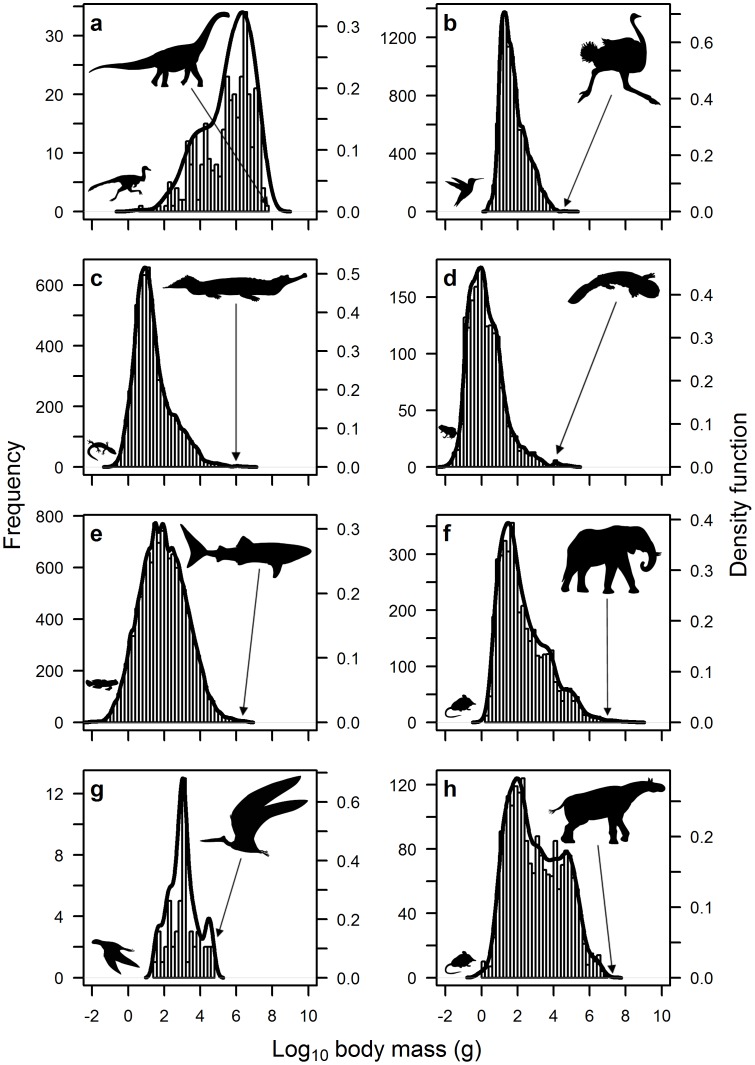
Dinosaurs exhibit a unimodal negatively-skewed frequency distribution of maximum species body size, which is significantly different from a normal distribution (Lilliefors test: D = 0.105, p<0.001; see Fig. 2a and Table 2). This is in contrast to all other major extant groups, i.e. birds, reptiles, amphibians, fish and terrestrial mammals, which exhibit positively-skewed frequency distributions that are significantly different from a normal distribution (Lilliefors test: p<0.001; see Fig. 2b–f and Table 2). Reptiles and extant mammals are characterised by a bimodal positively-skewed distribution (Fig. 2c, f and Table 2), with the second peak in reptiles occurring at very large body size due to the large mass of the Crocodilia relative to all other groups. Birds and amphibians are distinctly positively-skewed, but with a distribution exhibiting several modes (Fig. 2b,d and Table 2). The distribution of maximum fish species body size more closely resembles a bell-shaped curve, but is still positively-skewed and significantly different from a normal distribution, with several modes (Fig. 2e and Table 2). The body size distribution of dinosaurs is also significantly different from all other groups (Kolmogorov-Smirnov test: p<0.001).
Figure 2. Frequency distribution of species body size for eight different animal groups: (a) extinct dinosaurs; (b) extant birds; (c) extant reptiles; (d) extant amphibians; (e) extant fish; (f) extant mammals; (g) extinct pterosaurs; and (h) Cenozoic mammals.
Note that all distributions are positively-skewed except for dinosaurs, which are markedly negatively-skewed (see Table 2). A combination of kernel density estimation and smoothed bootstrap resampling was used to test for optimum modality of the body size distributions. Silhouettes of the largest and smallest animal in each group are also shown (provided by Matt van Rooijen).
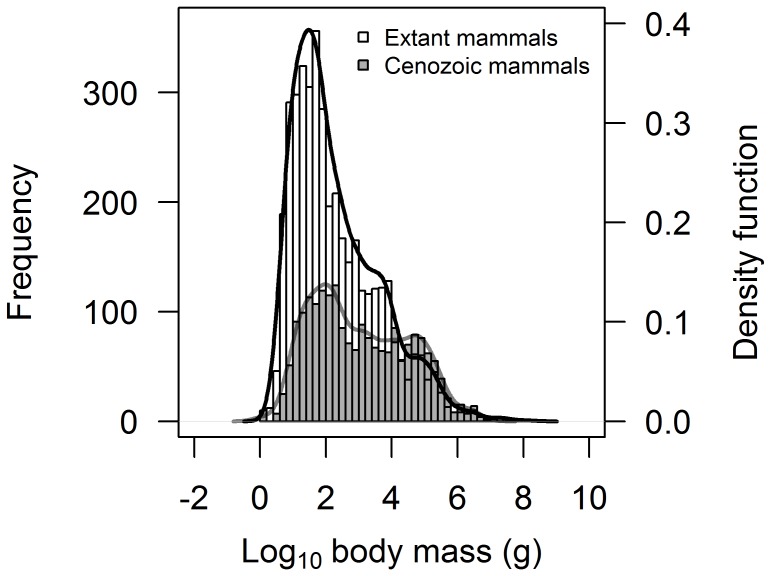
The exploration of other fossilised taxa, the extinct pterosaurs and Cenozoic mammals, revealed that both these groups have positively-skewed distributions of maximum species body size, in contrast to the dinosaurs (Fig. 2g–h and Table 2). The body size distribution for pterosaurs is not significantly different from a normal distribution (Lilliefors test: D = 0.117, p = 0.084; see Table 2). However, the existing dataset for pterosaur species body mass is very limited (n = 50), so these trends should be interpreted with caution. The Cenozoic mammals are characterised by markedly fewer small species compared to extant mammals as evidenced by the truncated peak around a body mass of log10(2) g (see Fig. 2h and Fig. 3). Additionally, the body size distribution of these two groups are significantly different from each other (Kolmogorov-Smirnov test: D = 0.218, p<0.001). This provides evidence of taphonomic bias against the discovery of smaller species in the fossil record and yet the distribution of Cenozoic mammals is still distinctly positively-skewed and significantly different from a normal distribution (Lilliefors test: D = 0.089, p<0.001; see Table 2).
Figure 3. Frequency distribution of maximum species body size for Cenozoic mammals (in grey) overlaid on the distribution for extant mammals (in white).
Curve fitting is based on a combination of kernel density estimation and smoothed bootstrap resampling. The figure clearly highlights the reduced frequency of small-bodied species in the Cenozoic mammal dataset, while the frequency of large-bodied species is comparable between both datasets.
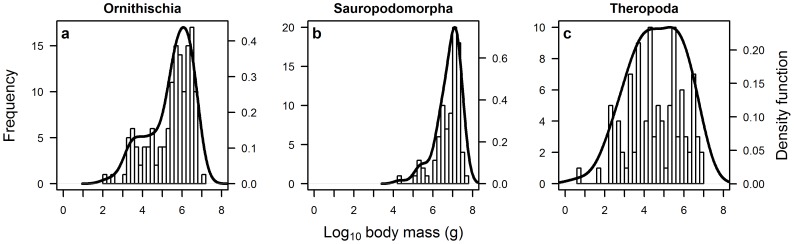
The negatively-skewed distribution of maximum dinosaur species body mass was only found to be consistent for two of the three major clades. Here, both the Ornithischia and Sauropodomorpha exhibit markedly negatively-skewed unimodal distributions, which are significantly different from a normal distribution (Lilliefors test: p<0.001; see Fig. 4a–b and Table 2). While the body size distribution of Theropoda is somewhat negatively-skewed, it does not differ significantly from a normal distribution (Lilliefors test: D = 0.084, p = 0.076; see Fig. 4c and Table 2).
Figure 4. Frequency distribution of species body size for three major dinosaur clades: (a) Ornithischia; (b) Sauropodomorpha; and (c) Theropoda.
The Sauropodomorpha and Ornithischia are significantly negatively-skewed, while the Theropoda exhibit a bell-shaped distribution (see Table 2). All three clades are best fitted by unimodal distributions.
The distribution of maximum dinosaur species body size was only found to be distinctly negatively-skewed towards the end of each major time period. Here, the Late Triassic, Late Jurassic and Late Cretaceous periods all display multi-modal negatively-skewed distributions, which are significantly different from a normal distribution (Lilliefors test: p<0.029; see Fig. 5a,d,f and Table 2). The additional modes may be partly explained by the reduced number of data points constituting these analyses. Dinosaur body size was also skewed towards larger species in the Middle Jurassic, with a unimodal distribution that is significantly different from a normal distribution (Lilliefors test: D = 0.182, p = 0.015; see Fig. 5c and Table 2). The Early Jurassic and Early Cretaceous periods showed many smaller as well as larger species of dinosaur, with unimodal and bimodal distributions, respectively, which are not significantly different from a normal distribution (Lilliefors test: p>0.052; see Fig. 5b,e and Table 2).
Figure 5. Frequency distribution of dinosaur species body size for six major time periods: (a) Late Triassic; (b) Early Jurassic; (c) Middle Jurassic; (d) Late Jurassic; (e) Early Cretaceous; and (f) Late Cretaceous.
The Early and Middle Jurassic were best fitted by unimodal distributions; the Early Cretaceous by a bimodal distribution. The Late Triassic, Jurassic and Cretaceous were all best fitted by negatively-skewed multi-modal distributions (see Table 2).
Finally, the two formations of dinosaur fossils with sufficient data for sampling, the Morrison and Dinosaur Park, again demonstrated negatively-skewed distributions of maximum species body size, with unimodal and bimodal distributions respectively (see Fig. 6 and Table 2). The body size distribution of the Morrison was found to be significantly different from a normal distribution (Lilliefors test: D = 0.223, p = 0.003), while the distribution for Dinosaur Park exhibited no significant difference from normality (Lilliefors test: D = 0.170, p = 0.070). Again, we caution about the small number of data points making up these analyses.
Figure 6. Frequency distribution of dinosaur species body size for two major formations: (a) the Morrison and (b) Dinosaur Park.
Both formations showed negatively-skewed distributions, with the Morrison formation approximately unimodal and the Dinosaur Park formation best fitted by a bimodal distribution (see Table 2). These patterns should be interpreted with caution, however, due to the small number of data points for each formation.
Discussion
Dinosaurs appear to be unique among vertebrates by demonstrating a strong skew in size distribution towards larger species. All other major extant vertebrate groups are dominated by a prevalence of smaller-bodied species (Fig. 2). Thus, it is not only absolute size, but also the size distribution that is skewed towards larger forms. While the fossil record suffers from a number of biases [10], [54], the distribution of dinosaurs here does not appear to be solely an artefact of the fossil record, as demonstrated by the similarity in positively-skewed data for extant and extinct mammals (see Fig. 3 and Table 2). Taphonomic processes are clearly at play, however, with a significant difference observed in the body size distribution of these two groups. Here, the peak in the distribution for large species remains largely unchanged in both data sets, while the peak for smaller species is suppressed and shifted to the right in Cenozoic mammals. Combined with existing knowledge of taphonomic biases in the dinosaur fossil record from a recent detailed study on the Dinosaur Park formation [10], this highlights the need to interpret the observed body size distribution for dinosaurs in Fig. 2a with caution.
However, it is also clear that taphonomic bias is unlikely to completely alter the interpretation of skewness of body size distributions. Brown et al. [10] identified a mass of 60 kg as marking the point below which taxa were vulnerable to being missed from the fossil record. In order to convert our overall dinosaur distribution dataset to match that of the extinct mammals, we would have to be missing around 90% of the non-avian dinosaurian diversity and all of it small (i.e. under 60 kg). To get our dinosaur distribution to match that of the extant mammals or birds, we would have to be missing 99.99% of diversity. Thus, we consider it implausible that taphonomic bias can be the sole force driving these results. Moreover, the pterosaurs (sister-taxon to the dinosauromorphs, living alongside them in the same environment and subject to similar conditions and taphonomic biases) display a more ‘typical’ vertebrate distribution in their body size (see Fig. 2g ), suggesting the dinosaurian signal is genuinely unique.
If the evolution of large body size was a product of long exposure to a stable environment, we would expect a steady progression from skew towards small species, to a bell-shaped distribution of species body size, before finally developing the skew towards large body size we observe in Fig. 2a . It is interesting that smaller species were more prevalent in the Early Jurassic and Early Cretaceous periods (see Fig. 5b, e ), in conjunction with large periods of species turnover in other groups [55]. By the end of both periods, dinosaurs exhibit a skew towards larger species once more (Fig. 5d, f ), providing some evidence for periods of stability leading to the evolution of larger size. However, the presence of marked left skew in the Late Triassic, just after the emergence of dinosaurs as a novel clade (Fig. 5a ), shows that this characteristic size distribution was acquired early in dinosaurian evolution and immediately became a fixture of dinosaur-dominated ecosystems, and indeed large sauropodomorphs are known from the Late Triassic [12], [56], [57]. There is also little evidence for a geographical bias in the prevalence of large body size in dinosaurs. Data from two species-rich formations, the Morrison and Dinosaur Park, both reveal a skew towards larger-bodied species (Fig. 6), although the data are too patchy to make a definitive judgement on this pattern. It should also be noted that other ecosystems may show different patterns (e.g. while untested here due to insufficient data, the Yixian Formation in China would appear to be dominated by smaller taxa).
Given the discrepancy between dinosaurs and all other vertebrates, the origin of this unusual body size distribution presumably lies in some major aspect of dinosaurian biology that distinguishes them from other taxa. It has been hypothesised that dinosaurs had a life history strategy unique to dominant terrestrial vertebrate clades [4]. Here, thanks to the small size at hatching and large size at adult, large dinosaurs grew through multiple orders of magnitude to reach adulthood. In consequence, they would have occupied multiple ecological niches during ontogeny and thus the apparent absence of small dinosaur species may in part be explained by the occupation of these niches by the juveniles of large species [4]. Furthermore, the size distribution of the theropods differs markedly from the ornithischians and sauropodomorphs (see Fig. 4). Most theropods were carnivorous (especially the larger forms e.g. tyrannosaurids, abelisaurids, allosauroids) and so their size could be considered contingent on the prey species available from the ranks of the herbivores. Notably, there were numerous small theropod species (Fig. 4c ) and although theropods as a whole might be expected to preferentially target juvenile dinosaurs for their prey [58], non-dinosaurian prey (e.g. lizards, mammals) would also have been available for smaller theropods. Thus, there is a disparity in the mechanisms driving size strategies in the various clades. The largely carnivorous theropods had sufficient animal resources to achieve optimal success (sensu [14]) at lower body size. In contrast, the herbivorous sauropodomorphs and ornithischians may have achieved optimal success through rapidly growing to a large body size that was outside the optimal foraging range of likely theropod predators [59], [60], [61], and provided a more beneficial feeding strategy (see below).
Comments on the giant size of dinosaurs have understandably tended to focus on the sauropodomorphs. For example, Sander et al. [12] noted a different body size distribution for sauropodomorphs versus a dataset of theropods and ornithischians combined. However, when separated out as shown in Fig. 4, the ornithischians have a more sauropodomorph-like distribution. In attempting to explain this apparent discrepancy, Sander et al. [12] focused on unique features of sauropodomorph paleobiology that might have facilitated or driven such large sizes and size distribution for the clade, but we suggest that the features driving large body size in the dinosaurs are not exclusive to the Sauropodomorpha. The ornithischians also featured numerous large taxa (37% of known species with available body mass estimates were greater than one ton), many of which exceeded the smaller sauropodomorphs in size. Burness et al. [62] showed that the body masses of the largest sauropods and theropods exceeded that predicted by the area of the land they occupied, yet no ornithischians were analysed as part of this work and at least some of these would similarly exceed the expected values. Thus, while Sander et al. [12] make a convincing case for the uniqueness of the sauropods with respect to their great size, a number of their supposedly unique features were also present in the ornithischians and may have similarly affected this clade. Both include species that grew through five orders of magnitude, from a few kilos to over ten tons. Sauropodomorphs and ornithischians also had similar reproductive strategies, with both capable of laying 20 or more eggs in a single nest [63] and achieving rapid growth to large body size [64]. Thus, although there were factors that may have helped promote extreme large size in sauropods not seen in ornithischians, such as their avian-like respiratory system and light skeletons [12], the potential strategies for optimal success were likely similar overall in the ornithischians.
One of the most notable factors affecting the herbivorous sauropodomorphs and ornithischians is digestive efficiency. Gut volume increases linearly [65] and basal metabolism is a fractional power [66] of body weight. These relationships produce a metabolic requirement to gut capacity ratio that decreases with body size, thus increasing the proportion of digested food particles in larger herbivores [67]. It is thought that this relationship may have played a major role in overcoming energetic issues through the optimisation of nutritive value from energy-rich, but slow fermenting pre-angiosperm plants [68]. This could be as true for multi-ton ornithischians as sauropodomorphs, where large size would also lead to increased gut volume and by extension greater digestive time. The largely carnivorous theropods would not have benefited from the same gut retention strategy and thus may not have exhibited the same evolutionary necessity for extreme large size. But this begs the question, why have other major groups not evolved similar divergent strategies?
It may be too energetically costly for endotherms to maintain a very large body mass and there is a danger of overheating [69]. As such, it is more beneficial for birds and mammals to possess a relatively small body size. If larger dinosaurs were ectothermic or gigantothermic as has been proposed [12], they would not be constrained in this way. Reptiles, amphibians and fish are also ectothermic or gigantothermic, however, and thus may be expected to show a similar response to dinosaurs. The vast majority of modern day reptiles and amphibians are carnivorous and will not benefit from increased digestive efficiency at large size, as argued for the Theropoda. While many fish are also carnivores, there is a sizeable proportion of planktivores and herbivores. It is interesting then that the body size distribution of fish is not as distinctly skewed towards small species as for the other major extant groups (see Fig. 2e and Table 2). This may reveal a possible trend towards increased body size in response to digestive efficiency. Future studies should examine the body size distribution of herbivorous relative to carnivorous fish species to explore this possibility in more detail.
Thus, while the exact evolutionary pressures and anatomical exaptations that led to large body size in dinosaurs is still a matter for debate [12], [13], the data presented here suggest a body size distribution that is unique among known vertebrate groups (see Fig. 2). While taphonomic processes may play a role in accentuating the negative skew of this distribution, there is also evidence for a divergence in optimal size strategies for the carnivorous theropod clade and the herbivorous sauropodomorph and ornithischian clades. This divergence is most likely related to the availability of small resources for the predatory theropods and the need to escape predation and maximise digestive efficiency in the herbivorous clades.
Acknowledgments
We are indebted to Phil Currie, Ross Elgin, Corwin Sullivan, Jonah Choiniere and the Open Dinosaur Project for generously sharing their data on dinosaurs and pterosaurs. Matt van Rooijen is thanked for producing the silhouettes used in Fig. 2. We also thank Peter Dodson and two anonymous reviewers for helpful comments on the manuscript.
Funding Statement
EJO was funded by the Irish Research Council for Science Engineering and Technology’s EMPOWER initiative when this work was carried out and is now a North American Electric Reliability Corporation funded Postdoctoral Research Fellow on grant NE/I009280/1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Woodward G, Ebenman B, Emmerson MC, Montoya JM, Olesen JM, et al. (2005) Body size in ecological networks. Trends in Ecology & Evolution 20: 402–409. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Nielsen K (1984) Scaling: why is animal size so important? Cambridge: Cambridge University Press. 241 p.
- 3. Alexander RM (1998) All-time giants: the largest animals and their problems. Palaeontology 41: 1231–1245. [Google Scholar]
- 4. Varricchio DJ (2011) A distinct dinosaur life history? Historical Biology 23: 91–107. [Google Scholar]
- 5. Henderson DM (1999) Estimating the masses and centers of mass of extinct animals by 3-D mathematical slicing. Paleobiology 25: 88–106. [Google Scholar]
- 6. Seebacher F (2001) A new method to calculate allometric length-mass relationships of dinosaurs. Journal of Vertebrate Paleontology 21: 51–60. [Google Scholar]
- 7. Hone DWE, Benton MJ (2005) The evolution of large size: how does Cope’s Rule work? Trends in Ecology & Evolution 20: 4–6. [DOI] [PubMed] [Google Scholar]
- 8. Turner AH, Pol D, Clarke JA, Erickson GM, Norell MA (2007) A basal Dromaeosaurid and size evolution preceding avian flight. Science 317: 1378–1381. [DOI] [PubMed] [Google Scholar]
- 9.Carrano MT (2005) Body-size evolution in the Dinosauria. In: Carrano MT, Blob RW, Gaudin TJ, Wible JR, editors. Amniote paleobiology: perspectives on the evolution of mammals, birds, and reptiles. Chicago: University of Chicago Press. 225–268.
- 10.Brown CM, Evans DC, Campione NE, O’Brien LJ, Eberth DA (In press) Evidence for taphonomic size bias in the Dinosaur Park Formation (Campanian, Alberta), a model Mesozoic terrestrial alluvial‐paralic system. Palaeogeography, Palaeoclimatology, Palaeoecology.
- 11. Peczkis J (1994) Implications of body-mass estimates for dinosaurs. Journal of Vertebrate Paleontology 14: 520–533. [Google Scholar]
- 12. Sander PM, Christian A, Clauss M, Fechner R, Gee CT, et al. (2011) Biology of the sauropod dinosaurs: the evolution of gigantism. Biological Reviews 86: 117–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Codron D, Carbone C, Müller DWH, Clauss M (2012) Ontogenetic niche shifts in dinosaurs influenced size, diversity and extinction in terrestrial vertebrates. Biology Letters 8: 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kozlowski J, Gawelczyk AT (2002) Why are species’ body size distributions usually skewed to the right? Functional Ecology 16: 419–432. [Google Scholar]
- 15. Morse DR, Lawton JH, Dodson MM, Williamson MH (1985) Fractal dimension of vegetation and the distribution of arthropod body lengths. Nature 314: 731–733. [Google Scholar]
- 16.Gaston KJ, Blackburn TM (2000) Pattern and Process in Macroecology. Oxford: Blackwell Science.
- 17. Caughley G (1987) The distribution of eutherian body weights. Oecologia 74: 319–320. [DOI] [PubMed] [Google Scholar]
- 18. Gardezi T, da Silva J (1999) Diversity in relation to body size in mammals: A comparative study. American Naturalist 153: 110–123. [DOI] [PubMed] [Google Scholar]
- 19. Maurer BA, Brown JH, Rusler RD (1992) The micro and macro in body size evolution. Evolution 46: 939–953. [DOI] [PubMed] [Google Scholar]
- 20. Blackburn TM, Gaston KJ (1994) The distribution of body sizes of the world’s bird species. Oikos 70: 127–130. [Google Scholar]
- 21. Gaston KJ, Blackburn TM (1995) The frequency-distribution of bird body weights - aquatic and terrestrial species. Ibis 137: 237–240. [Google Scholar]
- 22. Maurer BA, Brown JH (1988) Distribution of energy use and biomass among species of North-American terrestrial birds. Ecology 69: 1923–1932. [Google Scholar]
- 23. Maurer BA (1998) The evolution of body size in birds. I. Evidence for non-random diversification. Evolutionary Ecology 12: 925–934. [Google Scholar]
- 24. Bakker VJ, Kelt DA (2000) Scale-dependent patterns in body size distributions of neotropical mammals. Ecology 81: 3530–3547. [Google Scholar]
- 25.McKinney ML (1990) Trends in body-size evolution. In: McNamara KJ, editor. Evolutionary Trends. Tucson, Arizona.: University of Arizona Press. 75–118.
- 26. Brown JH, Marquet PA, Taper ML (1993) Evolution of body-size - consequences of an energetic definition of fitness. American Naturalist 142: 573–584. [DOI] [PubMed] [Google Scholar]
- 27. Jones KE, Purvis A (1997) An optimum body size for mammals? Comparative evidence from bats. Functional Ecology 11: 751–756. [Google Scholar]
- 28. Symonds MRE (1999) Insectivore life histories: further evidence against an optimum body size for mammals. Functional Ecology 13: 508–513. [Google Scholar]
- 29. Kindlmann P, Dixon AFG, Dostalkova I (1999) Does body size optimization result in skewed body size distribution on a logarithmic scale? American Naturalist 153: 445–447. [DOI] [PubMed] [Google Scholar]
- 30. Kozlowski J, Weiner J (1997) Interspecific allometries are by-products of body size optimization. American Naturalist 149: 352–380. [Google Scholar]
- 31.Bates KT, Falkingham PL, Breithaupt BH, Hodgetts D, Sellers WI, et al.. (2009) How big was ‘Big Al’? Quantifying the effect of soft tissue and osteological unknowns on mass predictions for Allosaurus (Dinosauria: Theropoda). Palaeontologia Electronica 12.
- 32.Campione NE, Evans DC (2012) A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. Bmc Biology 10. [DOI] [PMC free article] [PubMed]
- 33. Carrano MT (2001) Implications of limb bone scaling, curvature and eccentricity in mammals and non-avian dinosaurs. Journal of Zoology 254: 41–55. [Google Scholar]
- 34.Dunning JB (2008) CRC handbook of avian body masses: CRC Press.
- 35. Meiri S (2008) Evolution and ecology of lizard body sizes. Global Ecology and Biogeography 17: 724–734. [Google Scholar]
- 36. Greer AE (2001) Distribution of maximum snout-vent length among species of scincid lizards. Journal of Herpetology 35: 383–395. [Google Scholar]
- 37. Meiri S (2010) Length-weight allometries in lizards. Journal of Zoology 281: 218–226. [Google Scholar]
- 38.Guyer C, Boback SM (2011) Auburn University, COSAM collections: reptiles and amphibians project databases, http://www.auburn.edu/academic/science_math/cosam/collections/reptiles_amphibians/projects/vertbodysize.htm.
- 39. Pough FH (1980) Advantages of ectothermy for tetrapods. American Naturalist 115: 92–112. [Google Scholar]
- 40. Farlow JO, Hurlburt GR, Elsey RM, Britton ARC, Langston W (2005) Femoral dimensions and body size of Alligator mississippiensis: Estimating the size of extinct mesoeucrocodylians. Journal of Vertebrate Paleontology 25: 354–369. [Google Scholar]
- 41. Hoare JM, Pledger S, Keall SN, Nelson J, Mitchell NJ, et al. (2006) Conservation implications of a long-term decline in body condition of the Brothers Island tuatara (Sphenodon guntheri). Animal Conservation 9: 456–462. [Google Scholar]
- 42. Moore JA, Hoare JM, Daugherty CH, Nelson NJ (2007) Waiting reveals waning weight: Monitoring over 54 years shows a decline in body condition of a long-lived reptile (tuatara, Sphenodon punctatus). Biological Conservation 135: 181–188. [Google Scholar]
- 43.Froese R, Pauly D (2011) FishBase. World Wide Web electronic publication.
- 44. Smith FA, Lyons SK, Ernest SKM, Jones KE, Kaufman DM, et al. (2003) Body mass of late quaternary mammals. Ecology 84: 3403–3403. [Google Scholar]
- 45. Manly BFJ (1996) Are there clumps in body-size distributions? Ecology 77: 81–86. [Google Scholar]
- 46. Silverman BW (1981) Using kernel density estimates to investigate multimodality. Journal of the Royal Statistical Society Series B-Methodological 43: 97–99. [Google Scholar]
- 47. Holling CS (1992) Cross-scale morphology, geometry, and dynamics of ecosystems. Ecological Monographs 62: 447–502. [Google Scholar]
- 48. Siemann E, Brown JH (1999) Gaps in mammalian body size distributions reexamined. Ecology 80: 2788–2792. [Google Scholar]
- 49. Joanes DN, Gill CA (1998) Comparing measures of sample skewness and kurtosis. The Statistician 47: 183–189. [Google Scholar]
- 50.Elgin RA (In press) Appendix. In: Martill D, Unwin D, Loveridge R, editors. The Pterosauria: ISBN:9780521518956.
- 51. Witton MP (2008) A new approach to determining pterosaur body mass and its implications for pterosaur flight. Zitteliana Reihe B 28: 143–158. [Google Scholar]
- 52.Alroy J (2008) North American Fossil Mammal Systematics Database. Paleobiology Database Online Systematics Archive 3.
- 53. Clauset A, Erwin DH (2008) The evolution and distribution of species body size. Science 321: 399–401. [DOI] [PubMed] [Google Scholar]
- 54. Koch CF (1978) Bias in published fossil record. Paleobiology 4: 367–372. [Google Scholar]
- 55.Sookias RB, Butler RJ, Benson RBJ (In press) Rise of dinosaurs reveals major body-szie transitions are driven by passive processes of trait evolution. Proceedings of the Royal Society of London Series B-Biological Sciences. [DOI] [PMC free article] [PubMed]
- 56.Yates AM (2004) Anchisaurus polyzelus (Hitchcock): The smallest known sauropod dinosaur and the evolution of Gigantism among sauropodomorph dinosaurs. Postilla: 1–58.
- 57. Buffetaut E, Suteethorn V, Le Loeuff J, Cuny G, Tong HY, et al. (2002) The first giant dinosaurs: a large sauropod from the Late Triassic of Thailand. Comptes Rendus Palevol 1: 103–109. [Google Scholar]
- 58. Hone DWE, Rauhut OWM (2010) Feeding behaviour and bone utilization by theropod dinosaurs. Lethaia 43: 232–244. [Google Scholar]
- 59. Petchey OL, Beckerman AP, Riede JO, Warren PH (2008) Size, foraging, and food web structure. Proceedings of the National Academy of Sciences of the United States of America 105: 4191–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Charnov EL (1976) Optimal foraging, marginal value theorem. Theoretical Population Biology 9: 129–136. [DOI] [PubMed] [Google Scholar]
- 61. Cooper LN, Lee AH, Taper ML, Horner JR (2008) Relative growth rates of predator and prey dinosaurs reflect effects of predation. Proceedings of the Royal Society B-Biological Sciences 275: 2609–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burness GP, Diamond J, Flannery T (2001) Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proceedings of the National Academy of Sciences of the United States of America 98: 14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Horner JR (2000) Dinosaur reproduction and parenting. Annual Review of Earth and Planetary Sciences 28: 19–45. [Google Scholar]
- 64. Erickson GM, Rogers KC, Yerby SA (2001) Dinosaurian growth patterns and rapid avian growth rates. Nature 412: 429–433. [DOI] [PubMed] [Google Scholar]
- 65. Demment MW (1982) The scaling of ruminoreticulum size with body-weight in east-african ungulates. African Journal of Ecology 20: 43–47. [Google Scholar]
- 66. Kleiber M (1947) Body size and metabolic rate. Physiological Reviews 27: 511–541. [DOI] [PubMed] [Google Scholar]
- 67. Demment MW, Vansoest PJ (1985) A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. American Naturalist 125: 641–672. [Google Scholar]
- 68. Hummel J, Gee CT, Suedekum K-H, Sander PM, Nogge G, et al. (2008) In vitro digestibility of fern and gymnosperm foliage: implications for sauropod feeding ecology and diet selection. Proceedings of the Royal Society B-Biological Sciences 275: 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Speakman JR, Krol E (2011) Heat dissipation and hyperthermia risk as limiting factors in endotherm ecology. Integrative and Comparative Biology 51: E130–E130. [DOI] [PubMed] [Google Scholar]
- 70. Anderson JF, Hallmartin A, Russell DA (1985) Long-bone circumference and weight in mammals, birds and dinosaurs. Journal of Zoology 207: 53–61. [Google Scholar]
- 71. Godefroit P, Dong Z, Bultynck P, Li H, Feng L (1998) Sino-Belgian Cooperation Program “Cretaceous dinosaurs and mammals from Inner Mongolia” 1. New Bactrosaurus (Dinosauria: Hadrosauridae) material from Iren Dabasu (Inner Mongolia, P R. China). Bulletin de L’Institut Royal des Sciences Naturelles de Belgique Sciences de la Terre 68: 3–70. [Google Scholar]
- 72. Galton PM (1970) The posture of hadrosaurian dinosaurs. Journal of Paleontology 44: 464–473. [Google Scholar]
- 73. Coria RA, Salgado L (1996) A basal iguanodontian (Ornithischia: Ornithopoda) from the Late Cretaceous of south America. Journal of Vertebrate Paleontology 16: 445–457. [Google Scholar]
- 74.Zhou S (1984) Stegosaurs. The Middle Jurassic Dinosaurian Fauna from Dashanpu, Zidong, Sichuan, Vol. II. Chengdu, China: Sichuan Scientific and Technological Publishing House.
- 75. Galton PM (1974) The ornithischian dinosaur Hypsilophodon from the Wealden of the Isle of Wight. Bulletin of the British Museum of Natural History, Geology 25: 1–152. [Google Scholar]
- 76.Paul GS (1990) Chasmosaurus, Homocephale, Monoclonius, Pentaceratops, Bactrosaurus, Massospondylus, Omeisaurus, Stegosaurus. In: Dodson P, editor. Encyclopedia of Dinosaurs. New York: Beekman House. 58–215.
- 77. Norman DB (1980) On the ornithischian dinosaur Iguanodon bernissartensis from the Lower Cretaceous of Bernissart (Belgium). Institut Royal des Sciences Naturelles de Belgique Memoire 178: 1–103. [Google Scholar]
- 78.Galton PM (1997) Stegosaurs. In: Farlow JO, Brett-Surman MK, editors. The Complete Dinosaur. Indianapolis: Indiana University Press. 291–306.
- 79. Bartholomai A, Molnar RE (1981) Muttaburrasaurus langdoni new genus new species of Iguanodontid Ornithischia Ornithopoda dinosaur from the lower Cretaceous of Queensland, Australia. Memoirs of the Queensland Museum 20: 319–350. [Google Scholar]
- 80.Sereno PC (1990) New data on parrot-beaked dinosaurs (Psittacosaurus). In: Carpenter K, Currie PJ, editors. Dinosaur Systematics: Perspectives and Approaches. Cambridge: Cambridge University Press. 203–210.
- 81. Carpenter K (1984) Skeletal reconstruction and life restoration of Sauropelta (Ankylosauria: Nodosauridae) from the Cretaceous of North America. Canadian Journal of Earth Sciences 21: 1491–1498. [Google Scholar]
- 82. Ostrom JH, Wellnhofer P (1985) The Munich specimen of Triceratops with a revision of the genus. Zitteliana 14: 111–158. [Google Scholar]
- 83.Dong Z (1990) Stegosaurs of Asia. In: Carpenter K, Currie PJ, editors. Dinosaur Systematics: Perspectives and Approaches. Cambridge: Cambridge University Press. 255–268.
- 84. Lehman TM, Woodward HN (2008) Modeling growth rates for sauropod dinosaurs. Paleobiology 34: 264–281. [Google Scholar]
- 85. Salgado L, Bonaparte JF (1991) Amargasaurus cazaui new genus new species from the late Neocomian beds of La Amarga formation of the Neuquen Basin. Ameghiniana 28: 333–346. [Google Scholar]
- 86. Henderson DM (2004) Tipsy punters: sauropod dinosaur pneumaticity, buoyancy and aquatic habits. Proceedings of the Royal Society of London Series B-Biological Sciences 271: S180–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McIntosh JS, Brett-Surman MK, Farlow JO (1997) Sauropods. In: Farlow JO, Brett-Surman MK, editors. The Complete Dinosaur. Indianapolis: Indiana University Press. 264–290.
- 88. Riggs ES (1903) Brachiosaurus altithorax, the largest known dinosaur. American Journal of Science 15: 299–306. [Google Scholar]
- 89. Christiansen P (1999) Long bone scaling and limb posture in non-avian theropods: Evidence for differential allometry. Journal of Vertebrate Paleontology 19: 666–680. [Google Scholar]
- 90.Mazetta GV, Christiansen P, Farina RA (2004) Giants and bizarres: body size of some southern South American Cretaceous Dinosaurs. Historical Biology: 1–13.
- 91. Hatcher JB (1901) Diplodocus (Marsh): its osteology, taxonomy, and probable habits, with a restoration of the skeleton. Memoirs of the Carnegie Museum 1: 1–63. [Google Scholar]
- 92.Paul GS (1997) Dinosaur models: the good, the bad, and using them to estimate the mass of dinosaurs. In: Wolberg DL, Stump E, Rosenberg GD, editors. DinoFest International Proceedings. Philadelphia: The Academy of Natural Sciences. 129–154.
- 93. Henderson DM (2006) Burly gaits: Centers of mass, stability, and the trackways of sauropod dinosaurs. Journal of Vertebrate Paleontology 26: 907–921. [Google Scholar]
- 94. Sereno PC, Beck AL, Dutheil DB, Larsson HCE, Lyon GH, et al. (1999) Cretaceous sauropods from the Sahara and the uneven rate of skeletal evolution among dinosaurs. Science 286: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 95.Mallison H (2010) CAD assessment of the posture and range of motion of Kentrosaurus aethiopicus HENNIG 1915. Swiss Journal of Geoscience 103.
- 96. Borsuk-Bialynicka M (1977) A new camarasaurid sauropod Opisthocoelicaudia skarzynskii gen. N., sp. N. from the upper Cretaceous of Mongolia. Palaeontologie Polonica 37: 5–64. [Google Scholar]
- 97.Van Heerden J (1997) Prosauropods. In: Farlow JO, Brett-Surman MK, editors. The Complete Dinosaur. Indianapolis: Indiana University Press. 330–346.
- 98. Sereno PC, Wilson JA, Larsson HCE, Dutheil DB, Sues HD (1994) Early Cretaceous dinosaurs from the Sahara. Science 266: 267–271. [DOI] [PubMed] [Google Scholar]
- 99. Christiansen P, Farina RA (2004) Mass Prediction in Theropod Dinosaurs. Historical Biology 16: 85–92. [Google Scholar]
- 100. Gilmore CW (1920) Osteology of the Carnivorous Dinosauria in the United States National Museum, with Special Reference to the Genera Antrodemus (Allosaurus) and Ceratosaurus. Bulletin of the United States National Museum 110: 1–159. [Google Scholar]
- 101. Kurzanov SM (1987) Avimimidae and the problem of the origin of birds. Joint Soviet-Mongolian Paleontological Expedition Transactions 31: 1–95. [Google Scholar]
- 102.Paul GS (1988) Predatory dinosaurs of the world: a complete and illustrated guide. New York: Simon and Schuster. 464 p.
- 103. Bonaparte JF, Novas FE, Coria RA (1990) Carnotaurus sastrei Bonaparte, the horned, lightly built carnosaur from the Middle Cretaceous of Patagonia. Contributions in Science of the Natural History Museum of Los Angeles County 416: 1–41. [Google Scholar]
- 104. Sereno PC, Dutheil DB, Iarochene M, Larsson HCE, Lyon GH, et al. (1996) Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation. Science 272: 986–991. [DOI] [PubMed] [Google Scholar]
- 105. Sereno PC, Forster CA, Rogers RR, Monetta AM (1993) Primitive dinosaur skeleton from Argentina and the early evolution of Dinosauria Nature. 361: 64–66. [Google Scholar]
- 106. Coria RA, Salgado L (1995) A new giant carnivorous dinosaur from the Cretaceous of Patagonia. Nature 377: 224–226. [Google Scholar]
- 107. Currie PJ, Zhao XJ (1993) A new Carnosaur (Dinosauria, Theropoda) from the Jurassic of Xinjiang, Peoples Republic of China Canadian Journal of Earth Sciences. 30: 2037–2081. [Google Scholar]
- 108.Brochu CA (2003) Osteology of Tyrannosaurus rex: Insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. Journal of Vertebrate Paleontology 22.