Abstract
Background
Gastroenteropancreatic neuroendocrine neoplasm (GEP-NEN) is the most common type of neuroendocrine tumors accounting for 65–75% of neuroendocrine neoplasms (NENs). Given the fact that there are few studies on GEP-NENs among Chinese patients, we performed a retrospective study in South China.
Methods
Totally 178 patients with GEP-NENs treated at the First Affiliated Hospital of Sun Yat-sen University between January 1995 and May 2012 were analyzed retrospectively.
Results
Pancreas was found the most common site of involvement (34.8%). 149 patients (83.7%) presented as non-functional tumors with non-specific symptoms such as abdominal pain (33.7%); carcinoid syndrome was not found in this study. Several methods are useful for localization of GEP-NENs, yielding varied detection rates from 77.8% to 98.7%. Positive rates of chromogranin A (CgA) and synaptophysin (Syn) immunhistochemically were 69.1% and 90.2%, respectively. 87 patients (51.5%) had G1 tumors, 31(18.3%) G2 tumors and 51 (30.2%) G3 tumors. Neuroendocrine tumor (NET), neuroendocrine carcinoma (NEC) and mixed adenoendocrine carcinoma (MANEC) were 69.8%, 27.2% and 3.0%, respectively. 28.1% of patients presented with distant disease. Surgery was performed in 152 (85.4%) patients, and overall 5-year survival rate was 54.5%. Functionality, G1 grading and NET classification were associated with favorable prognosis in univariate analysis. Distant metastasis contributed to unfavorable prognosis of these tumors.
Conclusions
Nonfunctional tumors with non-specific symptoms account for the majority of GEP-NENs. Diagnosis depends on pathological classification. Multidisciplinary treatments could help improve the outcome.
Keywords: Gastroenteropancreatic neuroendocrine neoplasms, Clinical pathological characteristics, Survival
Background
Neuroendocrine neoplasms, which originate from neuroendocrine cells distributed throughout the body, comprise a heterogeneous family with a wide and complex clinical behaviors [1]. The incidence of NENs ranges from 2.5 to 5 cases per 100,000 in the United States, and the gastrointestinal tract is the most commonly affected site [2,3]. According to an analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database (SEER, http://seer.cancer.gov/data/index.html), which is the largest epidemiologic series nowadays, the incidence of NENs has been rising substantially in the past 30 years.
NENs have been the subject of debate regarding optimal nomenclature, grading, staging and classification of these tumors for many years. A uniform World Health Organization (WHO) classification greatly facilitates the comparison of clinical, pathological and prognostic features and results of treatment in GEP-NENs, and so do the China Consensus Guidelines for the standards of histopathologic diagnosis as well [4,5].
The incidence of NENs, the treatments and survival of Caucasians have been well studied in western countries such as United States, Norway, Spain, German and the United Kingdom [2,3,6-9]. But for Asian population [10-13], especially for Chinese population, available information on these cancers is rather limited [14]. Therefore, it requires detailed data for comprehensive knowledge of NENs in China. Based on the 17-year data of our hospital, a comprehensive retrospective study was performed to examine the relationship between clinical pathological characteristic and survival of GEP-NENs. To our knowledge, it is the first study providing information on these tumors using the latest histopathologic diagnosis consensus from an Asian country.
Methods
178 patients with histologically confirmed sporadic GEP-NENs from The First Affiliated Hospital, Sun Yat-sen University (1995–2012) were enrolled in this study to collect clinical information including age, gender, locations, clinical syndromes, endoscopic and radiographic features, histopathological characteristics, metastasis patterns, treatment modalities and outcomes.
The histology of each patient was reviewed according to the WHO classification [4] and China Consensus Guidelines [5]: First, immunohistochemical staining of CgA and Syn, which are all neuroendocrine markers, were performed to recognize the histological patterns of these tumors. Specific peptide hormones (eg. insulin, glucagon and somatostatin) staining methods were not regularly used only when a functional neuroendocrine neoplasm was considered. Second, the Ki-67 index (≤2%, 3–20%, and >20% per 500–2000 tumor cells in the most active regions or hot spots, respectively) or mitotic rate (1, 2–20, and >20 mitoses per 10 high-power field in the most active regions or hot spots, respectively), which was re-stained or recounted, was used to estimate the tumor proliferative activities. Tumors with a Ki-67 index of <2% were classified as G1 tumors, index of 3–20% were classified as G2, greater than 20% as G3. Likewise, tumors with mitotic rates of <2/10 HPF were classified as G1, those of 2 to 20/10 HPF were classified as G2, greater than 20/10 HPF as G3. Once the grading of Ki-67 index disaccorded with the mitotic rate, the higher one was preferred. Thus, GEP-NENs were classified as NET (G1 and G2), NEC (G3) and MANEC (G3).
Overall survival was defined as the time from diagnosis to death or last follow-up in living patients. Survival rate was estimated according to the Kaplan–Meier product limit method, and differences between subgroups were assessed by the log-rank test with P < 0.05 as statistically significant. SPSS 16.0 was used for statistical analysis.
The study was approved by the ethics committee of The First Affiliated Hospital Sun Yat-sen University (with a reference number: [2012]317) and complied with the Declaration of Helsinki.
Results
Clinical features
Among the 178 Chinese patients with GEP-NENs, 108 (60.7%) were men and 70 (39.3%) were women; male-to-female ratio was 1.54. The mean age was 50.96 ± 15.01 years. The most common sites were the pancreas (62/178, 34.8%), followed by rectum (36/178, 20.2%), stomach (25/178, 14.0%), duodenum (13/178, 7.3%), metastatic NENs of unknown primary (12/178, 6.7%) and esophagus (7/178, 3.9%). Other sites included appendix, jejunum/ileum, Vater’s ampulla at 12.9% (23/178). Non-functional tumors comprised the majority of GEP-NENs (149/178, 83.7%), whereas functional tumors accounted for the other 16.3%. A variety of gastrointestinal manifestations were caused by the effect of local compression on nearby tissues in nonfunctional tumors. The most common initial presentation was abdominal pain (60/178, 33.7%), which was not specific for the diagnosis of tumor. Other non-specific symptoms were gastrointestinal bleeding (29/178, 16.3%), jaundice (16/178, 9.0%), progressive dysphagia (9/178, 5.1%), diarrhea (8/178, 4.5%), abdominal distension (6/178, 3.4%) and so on. Incidental diagnosis occurred in 10.1% of cases which were usually asymptomatic. Insulinoma comprised 93.1% of functional tumors, which mainly occurred in pancreas, occasionally followed by the substantially rarer glucagonoma and vasoactive intestinal peptidoma (only 1 case respectively in our study). Typical symptoms included hypoglycemia, epileptic seizure and secondary diabetes mellitus, which heralded functional NENs, but carcinoid syndrome did not present in our study. The demographics and presenting symptoms of GEP-NENs are listed in Table 1.
Table 1.
Characteristics of study population (N = 178 patients)
|
Site |
All patients |
Men,
n(%) |
Women,
n(%) |
Clinical symptoms |
Main signs |
|
|---|---|---|---|---|---|---|
| N | % | |||||
| Pancreas |
62 |
34.8 |
32(51.6) |
30(48.4) |
Abdominal pain, Jaundice, Hypoglycaemia |
Jaundice |
| Rectum |
36 |
20.2 |
29(80.6) |
7(19.4) |
Gastrointestinal bleeding, Abdominal pain, Diarrhea |
Rectum mass |
| Somach |
25 |
14.0 |
16(64.0) |
9(36.0) |
Abdominal pain, Gastrointestinal bleeding, Dysphagia |
Abdominal tenderness |
| Duodenum |
13 |
7.3 |
10(76.9) |
3(23.1) |
Abdominal pain, Jaundice, Gastrointestinal bleeding |
Jaundice |
| Metastasis of
unknown primary |
12 |
6.7 |
6(50.0) |
6(50.0) |
Abdominal pain, Asymptomatic, Fatigue |
Hepatomegaly |
| Esophagus |
7 |
3.9 |
5(71.4) |
2(28.6) |
Progressive dysphagia |
No signs |
| Appendix |
6 |
3.4 |
2(33.3) |
4(66.7) |
Abdominal pain, Abdominal distension |
Rebound pain in the
Mcburney’s point |
| Jejunum/ileum |
4 |
2.2 |
3(75.0) |
1(25.0) |
Gastrointestinal bleeding, Small bowel obstruction |
Anemia |
| Gallbladder |
4 |
2.2 |
1(25.0) |
3(75.0) |
Jaundice, Asymptomatic |
Jaundice |
| Vater’s ampulla |
3 |
1.7 |
3(100) |
0(0) |
Jaundice, Abdominal pain, |
Jaundice |
| Peritoneum |
3 |
1.7 |
0(0) |
3(100) |
Abdominal pain, Asymptomatic |
Abdominal mass |
| Cecum |
1 |
0.6 |
1(100) |
0(0) |
Abdominal pain |
Abdominal mass |
| Choledoch |
1 |
0.6 |
1(100) |
0(0) |
Jaundice |
Jaundice |
| Greater omentum | 1 | 0.6 | 0(0) | 1(100) | Asymptomatic | No signs |
Imaging studies
The most frequently used examination procedures included endoscopy, ultrasound, endoscopic ultrasonography (EUS), computed tomography (CT) scan, magnatic resonance imaging (MRI), and positron emission computed tomography imaging (PET-CT, using with 16 F-FDG). Endoscopy provided the highest detection rate of 98.7% (74/75). EUS was performed on 37 patients, of which a lesion was found in 34 patients, promised a detection rate of 91.9%. MRI and PET-CT, was performed in only about 10% of patients, respectively. Tumors usually appeared as polypoid prominences, ulcer type or cauliflower-like neoplasm under endoscopy; whereas on CT scan, they appeared as local space-occupying lesions which were significantly enhanced by iodinated contrast. Ultrasound and EUS usually demonstrated the tumors as rounded, homogeneous, hypoechoic, well-defined and well-vascularized masses (Table 2).
Table 2.
Characteristics of imaging studies
|
Imaging studies |
Site |
Manifestation |
Case tested |
Positive tests |
|
|---|---|---|---|---|---|
| n | % | ||||
| Endoscopy |
gastrointestinal tract |
|
75 |
74 |
98.7 |
| Gastroscope |
esophagus, stomach, duodenum |
ulcer type, bulge type, invasive type |
34 |
34 |
100 |
| Duodenoscope |
duodenum |
bulge type |
3 |
3 |
100 |
| Small intestinal endoscope |
jejunum/ileum |
small intestinal hemorrhage |
2 |
1 |
50.0 |
| Colonoscope |
rectum, appendix |
polypoid prominences, submucosal uplift,
cauliflower-like neoplasm |
36 |
36 |
100 |
| Ultrasound |
pancreas, liver, gallbladder, cho- ledoch |
hypoechoic masses, well delimited and
vascularized |
63 |
49 |
77.8 |
| EUS |
pancreas, duodenum, stomach |
hypoechoic masses |
37 |
34 |
91.9 |
| CT scan |
pancreas, liver, stomach |
local space-occupying lesions |
123 |
98 |
91.9 |
| MRI |
pancreas, duodenum, biliary |
local space-occupying lesions |
20 |
19 |
95.0 |
| PET-CT | pancreas, rectum | local space-occupying lesions | 20 | 19 | 95.0 |
EUS, endoscopic ultrasonography; CT, computed tomography; MRI, magnatic resonance imaging; PET-CT, positron emission computed tomography imaging.
Pathologic characteristics
Overall, the mean diameter of tumors was 3.95 cm (0.4–25 cm): 38.6% were smaller than 2 cm in diameter, 29.7% ranging from 2 to 4 cm, and 31.7% larger than 4 cm. Immunohistochemistry staining determined a 69.1% positive rate of CgA and a 90.2% positive rate of Syn. Ki-67 index and mitotic rate were assessed in 127 and 118 specimens to estimate the proliferative activities. Over half (51.5%) of the tumors were G1, 18.3% were at G2 and 30.2% at G3. The most common tumor type was NET (69.8%), followed by NEC (27.2%) and MANEC (3.0%). Approximately half of the assessed tumors (53/100, 53.0%) originated from gastrointestinal tract and biliary system with muscularis or serosa infiltration at diagnosis. Local infiltration and lymphatic metastasis occurred in 23.0% and 27.0% of patients respectively. Distant metastasis was a frequent event at diagnosis with an occurrence of 23.0% (41/178), which increased to 28.1% (55/178) during follow up. The liver was one of most frequently involved organs: liver metastasis occurred in 44 (80.0%) of 55 patients in the disease courses. Among the 44 patients, 29 presented with synchronous liver metastasis, whereas other 15 presented with metachronous liver metastasis during follow-up. Other locations that tumors involved were the peritoneum (12.7%, 7/55), cavitas pelvis (9.1%, 5/55), bone (7.3%, 4/55) and ovary (5.5%, 3/55). The most common site of primary tumor associated with widespread disease at diagnosis was cecum (100.0%), followed by jejunum/ileum (75.0%), gallbladder (50.0%), duodenum (38.5%), Vater’s ampulla (33.3%) and stomach (28.0%) (Table 3).
Table 3.
Pathologic characteristics
|
Characteristics |
Case
tested |
Positive tests |
|
|---|---|---|---|
| n | % | ||
| Immunohistochemistry |
|
|
|
| CgA |
149 |
103 |
69.1 |
| Syn |
143 |
129 |
90.2 |
| Tumor grading |
|
|
|
| G1 |
169 |
87 |
51.5 |
| G2 |
169 |
31 |
18.3 |
| G3 |
169 |
51 |
30.2 |
| Tumor type |
|
|
|
| NET |
169 |
118 |
69.8 |
| NEC |
169 |
46 |
27.2 |
| MANEC |
169 |
5 |
3.0 |
| Infiltration/Metastasis |
|
|
|
| Muscularis/Serosa infiltration |
100 |
53 |
53.0 |
| Adjacent tissue/Capsule infiltration |
178 |
41 |
23.0 |
| Lymphatic metastasis |
178 |
48 |
27.0 |
| Distant metastasis |
|
|
|
| At initial diagnosis |
178 |
41 |
23.0 |
| During follow-up | 178 | 55 | 28.1 |
CgA, Chromogranin A; Syn, Synaptophysin;NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MANEC, mixed adenoendocrine carcinoma.
Therapeutic interventions
85.4% patients underwent a surgery with curative intent (75.9%) or for palliative purpose (9.6%). Different types of endoscopic radical surgery were performed, including endoscopic mucosa resection (EMR), endoscopic submucosal dissection (ESD) and endoscopic electroexcision. Local-regional therapies such as transcatheter hepatic arterial chemoembolization (TACE), radiofrequency or other ablative techniques were carried out only in 11 cases (6.2% of the population). Chemotherapy and biological therapy were performed in 31 patients, among which 15 received chemo regimen, 8 received biological therapy and 8 received both. The most common first-line chemo combinations included platinum-etoposide (6 patients, 3.4%), oxaliplatin- capecitabine (2 patients, 1.3%), oxaliplatin-TS-1 (2 patients, 1.3%) and so on. Octreotide, a somatostatin analogue, was frequently administered at a dose of 20–40 mg/month as a biological therapy, combined with chemotherapy in 1 patient (0.6%) after surgery and in 7 patients (3.9%) with unresectable tumors. 14 (7.9%) cases with progressive malignant disease were treated only with supportive care.
Survival and prognostic factors
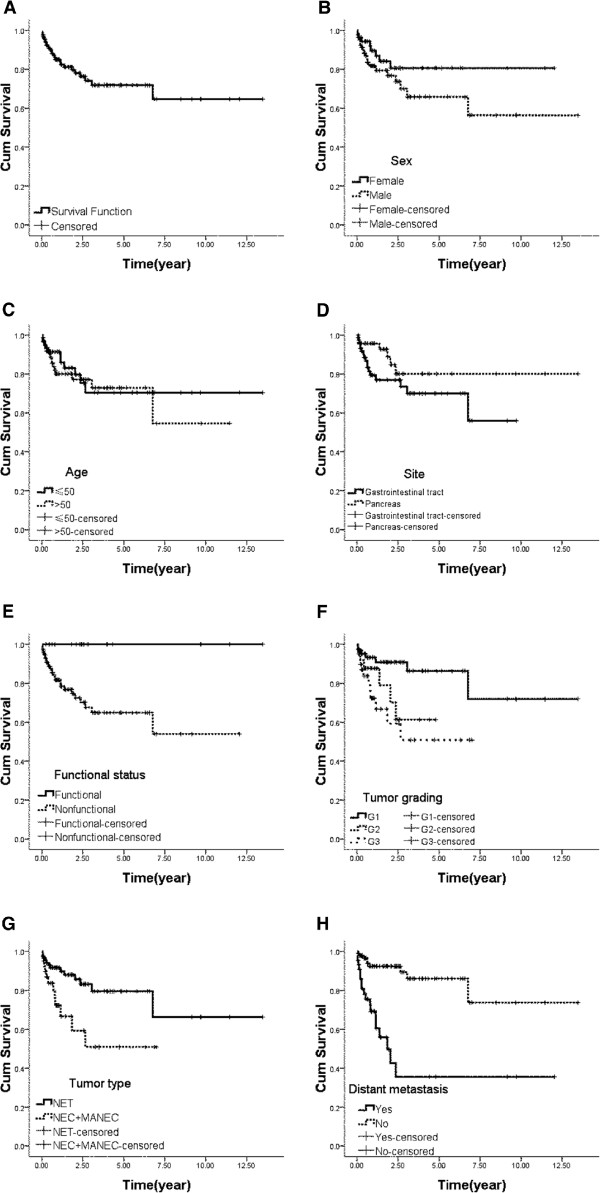
136 out of 178 patients received long-term follow up with a median duration of 8.6 years (range 0.03–13.48 years). Median survival was not obtained during the observation period. The 1-, 3- and 5-year survival rates was 74.4%, 66.7% and 54.5% respectively, and 25 patients had died at the last follow-up (14.0%). The major causes of death were tumor-related complications (84.0%), and treatment-related adverse events (12.0%); other disease contributed the other 4.0%. An analysis was performed on patients’ age, gender, primary tumor site, histopathological grading, classification and condition of metastasis to identify prognostic factors for survival. Univariate analysis confirmed that functional tumors, patients were at G1 phase and classified as NET were superior to other types of NENs in survival. Distant metastasis also contributed to the prognosis of these neuroendocrine tumors. However, age, sex, primary tumor site had little impact on overall survival. The mean survival time and statistic data were provided in Table 4. Survival curves were displayed in Figure 1.
Table 4.
Overall survival
|
Factors |
Overall survival |
||||
|---|---|---|---|---|---|
| Number | Mean (years) | 95% CI | χ2 | P | |
| All patients |
136 |
9.5 |
8.1-11.0 |
|
|
| Sex |
|
|
|
2.053 |
0.152 |
| Female |
54 |
9.9 |
8.5-11.3 |
|
|
| Male |
82 |
8.7 |
6.7-10.6 |
|
|
| Age |
|
|
|
0.259 |
0.611 |
| ≤50 |
60 |
9.9 |
8.0-11.8 |
|
|
| >50 |
76 |
7.8 |
5.9-9.6 |
|
|
| Site |
|
|
|
2.385 |
0.123 |
| Gastrointestinal
tract |
77 |
6.7 |
5.4-8.0 |
|
|
| Pancreas |
46 |
11.1 |
9.3-12.9 |
|
|
| Functional status |
|
|
|
6.691 |
0.006 |
| Functional |
22 |
NR |
NC |
|
|
| Nonfunctional |
114 |
7.7 |
6.1-9.2 |
|
|
| Tumor grading |
|
|
|
9.087 |
0.011 |
| G1 |
63 |
10.8 |
8.7-12.9 |
|
|
| G2 |
25 |
3.5 |
2.6-4.3 |
|
|
| G3 |
41 |
4.1 |
2.8-5.4 |
|
|
| Tumor type |
|
|
|
6.634 |
0.010 |
| NET |
88 |
10.1 |
8.1-12.1 |
|
|
| NEC + MANEC |
41 |
4.1 |
2.8-5.4 |
|
|
| Distant metastasis |
|
|
|
23.773 |
0.000 |
| Yes |
44 |
5.0 |
2.7-7.3 |
|
|
| No | 92 | 11.0 | 9.2-12.8 | ||
CI, confidence interval; NR, not reached; NC, not computable; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MANEC, mixed adenoendocrine carcinoma.
Figure 1.
Overall survival (A) Overall survival in all patients. (B) Overall survival by sex. (C) Overall survival by age at diagnosis. (D) Overall survival by site of tumors. (E) Overall survival by functional status. (F) Overall survival by histological grading. (G) Overall survival by tumor type. (H) Overall survival by condition of distant metastasis.
Discussion
The WHO classification system of gastroenteropancreatic neuroendocrine tumors was adopted in previous studies [3,7,8,10-12,14]. Some of these studies only focused on particular types of GEP-NENs such as well-differentiated endocrine tumors, poorly differentiated endocrine carcinomas or a single site of tumors (pancreas, colon or rectum). Our study investigated the pathologic features of GEP-NENs by using the latest histopathologic diagnosis consensus for the first time. It also analyzed any possible tumor site of digestive system including pancreas, biliary and peritoneal cavity. This study should contribute to establishing a database of the epidemiology, clinical pathological features, treatment and prognosis of GEP-NENs in China.
It is confirmed in our study that GEP-NENs comprise a heterogeneous group in relation to their primary locations. Previous researches indicated that the small intestine and appendix were the most predominant NENs locations [2,15-17]. But according to our study, pancreas is the principal site of GEP-NENs. The rectum is the most frequent sites of gastrointestinal tract, followed by the stomach and duodenum, whereas the jejunum/ileum accounts for no more than 2% tumor cases. A similar distribution of NENs was also found from a Korean study [10], which observed that rectum was the most common primary site of tumor in 470 available cases, followed by the pancreas, stomach and duodenum. Results from another three registries including SEER, National Cancer Registry for Gastroenteropancreatic Neuroendocrine Tumors (RGETNE, http://www.retegep.net) and Norwegian Registry of Cancer (NRC) significantly differed from that in our series: Rectum and jejunum/ileum were the most common sites for NENs in the SEER Program tumor registry, pancreas NENs were only the third most common NENs; The pancreas and jejunum/ileum were the most frequent positions in RGETNE; whereas the small intestine was the most frequent sites of origin, followed by the colon and rectum in NRC. These inconsistencies may be due to the racial disparities, as well as the selection bias among population based data and hospital series. So a larger patient population is required to carry on further investigation.
NENs can be classified into functional and nonfunctional tumors according to the presence or absence of symptoms associated with hormones overproduction [18]. The current study demonstrated that the majority of nonfunctional NENs usually presented with non-specific symptoms, which may give rise to misdiagnosis of the tumors as irritable bowel syndrome or digestive adenocarcinomas. Our study also showed that insulinomas were the most frequently encountered functional tumors in the pancreas, accounting for 93.1% of pancreatic NENs. No case, however, presented with carcinoid syndrome in this study. Interestingly, the incidence of carcinoid syndrome (10–32%) in the Western population [8,17,19-21] is significantly different from our report, with the fact that ileal tumors account for the vast majority.
Assessments of the locations and extents of GEP-NENs were crucial for management. The present study analyzed imaging methods, which is commonly used in current clinical practice, in this patient population. Conventional imaging procedures include endoscopy, ultrasound, EUS, CT scan, MRI and PET-CT, with detection rates ranging from 77.8 to 98.7%. CT scan was one of the most widely used imaging modalities (123/178) whereas endoscopy promised the highest yields of tumor detection (98.7%). The introduction of EUS provides unique advantages in evaluating the pancreatic biliary system, especially in tumors <1.0 cm in diameter and micrometastasis. The typical EUS patterns of NENs includes rounded, homogeneous, hypoechoic, well defined and vascularized masses, with the detection rate of 91.9% in our study, rather comparable to the results achieved in other series [22-24]. Small tumors and liver metastasis (i.e., tumors <0.5 cm in diameter) may be missed, resulting in underestimate of the exact disease extent. No single technique is 100% sensitive and accurate. Therefore, multiple imaging modalities should be combined to detect small, biochemically diagnosed tumors.
Despite the advances in both morphology and biology, the classification of NENs is still under debate. The lack of a uniform classification system for NENs hampers evaluation of therapy and comparison between clinical trials [25]. European Neuroendocrine Tumor Society (ENETS) and the North American Neuroendocrine Tumor Society (NANETS) have published diagnosis standard and pathology reports of NENs in 2009 and 2010 [18,26], respectively. Furthermore, the WHO revised the nomenclature and classification of GEP-NENs in 2010, version 4 [4]. In 2011, China established her own classification system for NENs [5]. Chinese Pathologic Consensus Group suggested the term “Neuroendocrine neoplasm (NEN)” instead of “Neuroendocrine Tumor (NET)” and formulated the classification criteria by the use of Ki-67 index/mitotic rate and histology. The pathologic features of NENs in our hospital were reviewed according to this diagnosis consensus in the current analysis, which to our knowledge, is the first study using the newest consensus. Overall, G1 tumors accounted for 51.5% of 169 available cases, followed by G3 (30.2%) and G2 (18.3%). The occurence of NET, NEC and MANEC were 69.8%, 27.2% and 3.0%, respectively. The availability of this uniform system for NETs greatly facilitates classification of the tumors, evaluation of treatment, and comparison of clinical trials.
In our series, distant disease at initial diagnosis occurred at the rate of 23.0%, which increased to 28.1% during follow up. Liver was the most frequent site tumor involved and the distribution of distant metastasis was wider than that either in SEER or in NRC (18–22%). In RGETNE, however, a significant proportion of patients (44%) with widespread disease were reported compared with our series. The frequency of primary tumor sites associated with distant disease varied in different series: in our cohort, the most common sites was cecum (100.0%), followed by jejunum/ileum (75.0%), gallbladder (50.0%), duodenum (38.5%), Vater’s ampulla (33.3%) and stomach (28.0%); in the SEER Registry, the most common site was pancreas (64%), followed by cecum/colon (44%/32%) and jejunum/ileum (30%); and in the RGETNE Registry, it was jejunum/ileum (65%), followed by colon (48%) and rectum (40%). Therefore, jejunum/ileum tumors appear to have a greater propensity for distant metastasis. However, the diversity should be taken into account.
Among the many therapeutic options for NENs, surgery is the treatment of choice. A variety of operations are available to reduce load of tumor and improve survival. The extent of surgical resection depends on the tumor size and origin and approximately 75.9% of patients have undergone a radical surgery. Radiofrequency ablation or TACE is usually adopted to treat liver involvement, accounting for 6.2% of the cases.
Besides surgery, other therapeutic options such as chemotherapy, biological therapy and targeted therapy can be used for NENs. According to the new WHO 2010 classification, well-differentiated NENs are classified as G1 and G2 neuroendocrine tumors (NETs) and poor-differentiated NENs are referred to as G3 neuroendocrine carcinomas (NECs). It has been reported that existing cytotoxic chemotherapy agents have been of limited value for the treatment of well-differentiated gastrointestinal NENs (with response rates 10% ~ 15%) [27-29], but has been the standard of care for well-differentiated metastatic pancreatic endocrine tumors (with response rates 40% ~ 70%) [30-32]. However, chemotherapy is generally considered active in poor-differentiated NENs (with response rates 50% ~ 70%) [33-35]. According to the published documents, several chemotherapeutic regimens are available, most of them are either platinum based or flurouracil based [29,34,36,37]. For the GEP-NEC, platinum-based combination regimens with etoposide or paclitaxel [33,34,36] are recommended. In our cohort, chemotherapy was performed in 23 patients. The most frequently used chemo regimen was etoposide–platinum combination. During follow-up, 3 of them died of tumor progression. It has been noticed that biological therapy and targeted therapy promise some effect on NENs in recent years [38-43]. Somatostatin analogues are effective therapeutic option for functional neuroendocrine tumors because they reduce hormone-related symptoms [44-46]. They have also been shown to stabilize tumor growth over long periods, even to inhibit tumor growth in patients with well-differentiated metastatic neuroendocrine midgut tumors [40,47,48]. Although the treatment effect of somatostatin analogues on foregut and hindgut tumors remain to be confirmed, 16 patients including 2 patients with functional neuroendocrine tumors and 14 patients with well-differentiated metastatic GEP-NENs received long-term administration of octreotide LAR at a dose of 20–40 mg monthly in our study.
The prognosis of GEP-NENs is more favorable than that of the adenocarcinomas of the digestive system. The overall 5-year survival rate in our series was 54.5%, rather comparable to that of SEER or NRC registry [2,3,6] (50–59%), but it was lower than that in some European countries [7,9] (75–79%). The inconsistencies of survival rates may be due to the racial and geographical disparities. We also proved that prognosis differed statistically according to functional status, pathological grading and classification. As the great majority of functional tumors were insulinomas which are benign in most cases in our study, that may lead to the conclusion that functionality may be a favorable prognostic marker. The result obtained above may be caused by small sample in this series. We also confirmed that metastasis represented a worse outcome with a mean survival of 5.0 years (P = 0.000). Multivariate analysis was not done due to the small size of our series. Therefore, further evaluation in a larger patient population is required to estimate the independent prognostic factors of GEP-NENs.
A broad range of this heterogeneous tumors was reviewed in the current study, which to our knowledge, is the first report using the latest pathological diagnosis consensus of these tumors. We also confirmed that GEP-NENs may originate from any part of the digestive system, and the majority of them are nonfunctional tumors with non-specific symptoms. Endoscopy and radiographic examination play an important role in tumor detection. However, final diagnosis should be based on pathological detection. The prognosis of these tumors was more favorable compared with gastrointestinal carcinomas. Nonetheless, the outcome was extremely poor for patients with high grading tumor and distant metastasis. Further understanding of the molecular mechanisms should facilitate management of the disease. Early diagnosis is crucial for radical resection before development of local invasion or distant disease, and interdisciplinary cooperation is the direction of future.
Conclusions
Nonfunctional tumors with non-specific symptoms account for the majority of GEP-NENs. Diagnosis depends on pathological classification. Multidisciplinary treatments could help improve the outcome.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WYH and LY contributed equally to this work; WYH and LY: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing; LY and XL: Pathological data collection and analysis; WJH: Collection of clinical data; Chen J and Chen MH: Conception and design, Financial and administrative support, manuscript editing. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Yu-hong Wang, Email: wangyuhong1106@163.com.
Yuan Lin, Email: linyuan618@126.com.
Ling Xue, Email: xuel@mail.sysu.edu.cn.
Jin-hui Wang, Email: jinhuiwang100@msn.com.
Min-hu Chen, Email: chenminhu@vip.163.com.
Jie Chen, Email: chenjie7209@yahoo.com.
Acknowledgments
This study was supported by the grants from National Natural Science Foundation of China (No. 30871145 and No. 81072048), the Junior Teacher Cultivation Project of Sun Yat-sen University (No. 09ykpy22), grants for major projects and emerging interdisciplinary studies of Sun Yat-sen University (No.10ykjc23) supported by the Fundamental Research Funds for the Central Universities.
References
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- Chinese Pathologic Consensus Group for Gastrointestinal and Pancreatic Neuroendocrine Neoplasm. China Consensus Guidelines for the standards of histopathologic diagnosis in Gastroenteropancreatic Neuroendocrine neoplasm. Chin J Pathol. 2011;40(4):257–262. [PubMed] [Google Scholar]
- Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, Modlin IM. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113(10):2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Diaz-Perez JA, Martinez Del Prado MP, Alonso Orduna V, Sevilla-Garcia I, Villabona-Artero C, Beguiristain-Gomez A, Llanos-Munoz M. et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE) Ann Oncol. 2010;21(9):1794–1803. doi: 10.1093/annonc/mdq022. [DOI] [PubMed] [Google Scholar]
- Ploeckinger U, Kloeppel G, Wiedenmann B, Lohmann R. The German NET-registry: an audit on the diagnosis and therapy of neuroendocrine tumors. Neuroendocrinology. 2009;90(4):349–363. doi: 10.1159/000242109. [DOI] [PubMed] [Google Scholar]
- Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology. 2007;132(3):899–904. doi: 10.1053/j.gastro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lim T, Lee J, Kim JJ, Lee JK, Lee KT, Kim YH, Kim KW, Kim S, Sohn TS, Choi DW. et al. Gastroenteropancreatic neuroendocrine tumors: incidence and treatment outcome in a single institution in Korea. Asia Pac J Clin Oncol. 2011;7(3):293–299. doi: 10.1111/j.1743-7563.2011.01423.x. [DOI] [PubMed] [Google Scholar]
- Li AF, Hsu CY, Li A, Tai LC, Liang WY, Li WY, Tsay SH, Chen JY. A 35-year retrospective study of carcinoid tumors in Taiwan: differences in distribution with a high probability of associated second primary malignancies. Cancer. 2008;112(2):274–283. doi: 10.1002/cncr.23159. [DOI] [PubMed] [Google Scholar]
- Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56(6):863–868. doi: 10.1136/gut.2006.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Tanaka M, Sasano H, Osamura YR, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. et al. Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors. J Gastroenterol. 2007;42(6):497–500. doi: 10.1007/s00535-007-2056-6. [DOI] [PubMed] [Google Scholar]
- Wang DS, Zhang DS, Qiu MZ, Wang ZQ, Luo HY, Wang FH, Li YH, Xu RH. Prognostic factors and survival in patients with neuroendocrine tumors of the pancreas. Tumour Biol. 2011;32(4):697–705. doi: 10.1007/s13277-011-0170-9. [DOI] [PubMed] [Google Scholar]
- Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240(1):117–122. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gompel JJ, Sippel RS, Warner TF, Chen H. Gastrointestinal carcinoid tumors: factors that predict outcome. World J Surg. 2004;28(4):387–392. doi: 10.1007/s00268-003-7019-3. [DOI] [PubMed] [Google Scholar]
- Onaitis MW, Kirshbom PM, Hayward TZ, Quayle FJ, Feldman JM, Seigler HF, Tyler DS. Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg. 2000;232(4):549–556. doi: 10.1097/00000658-200010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gonen M, Jensen RT, Kidd M, Kulke MH, Lloyd RV. et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34(3):300–313. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- Pape UF, Bohmig M, Berndt U, Tiling N, Wiedenmann B, Plockinger U. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann N Y Acad Sci. 2004;1014:222–233. doi: 10.1196/annals.1294.025. [DOI] [PubMed] [Google Scholar]
- Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229(6):815–821. doi: 10.1097/00000658-199906000-00008. discussion 822–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik AI, Thompson N, Eckhauser F, Moattari AR. Clinical features of carcinoid syndrome and the use of somatostatin analogue in its management. Acta Oncol. 1989;28(3):389–402. doi: 10.3109/02841868909111212. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95(9):2271–2277. doi: 10.1111/j.1572-0241.2000.02480.x. [DOI] [PubMed] [Google Scholar]
- Zimmer T, Scherubl H, Faiss S, Stolzel U, Riecken EO, Wiedenmann B. Endoscopic ultrasonography of neuroendocrine tumours. Digestion. 2000;62(Suppl 1):45–50. doi: 10.1159/000051855. [DOI] [PubMed] [Google Scholar]
- Varas Lorenzo MJ, Miquel Collell JM, Maluenda Colomer MD, Boix Valverde J, Armengol Miro JR. Preoperative detection of gastrointestinal neuroendocrine tumors using endoscopic ultrasonography. Rev Esp Enferm Dig. 2006;98(11):828–883. doi: 10.4321/s1130-01082006001100004. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst. 2008;100(18):1282–1289. doi: 10.1093/jnci/djn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, Scarpa A, Scoazec JY, Wiedenmann B, Papotti M. et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90(2):162–166. doi: 10.1159/000182196. [DOI] [PubMed] [Google Scholar]
- Bukowski RM, Tangen CM, Peterson RF, Taylor SA, Rinehart JJ, Eyre HJ, Rivkin SE, Fleming TR, Macdonald JS. Phase II trial of dimethyltriazenoimidazole carboxamide in patients with metastatic carcinoid. A Southwest Oncology Group study. Cancer. 1994;73(5):1505–1508. doi: 10.1002/1097-0142(19940301)73:5<1505::AID-CNCR2820730530>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Pitot HC, Burch PA, Kvols LK, Mahoney MR, Rubin J. A Phase II study of high-dose paclitaxel in patients with advanced neuroendocrine tumors. Cancer. 2001;91(8):1543–1548. doi: 10.1002/1097-0142(20010415)91:8<1543::AID-CNCR1163>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23(22):4897–4904. doi: 10.1200/JCO.2005.03.616. [DOI] [PubMed] [Google Scholar]
- Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303(21):1189–1194. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]
- Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326(8):519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–232. doi: 10.1002/1097-0142(19910715)68:2<227::AID-CNCR2820680202>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Spigel DR, Litchy S, Greco FA. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol. 2006;24(22):3548–3554. doi: 10.1200/JCO.2005.05.0575. [DOI] [PubMed] [Google Scholar]
- Fjallskog ML, Granberg DP, Welin SL, Eriksson C, Oberg KE, Janson ET, Eriksson BK. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. 2001;92(5):1101–1107. doi: 10.1002/1097-0142(20010901)92:5<1101::AID-CNCR1426>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufie P, Aparicio T, Lasser P, Elias D, Duvillard P, Schlumberger M. et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81(8):1351–1355. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22(23):4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A. et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26(26):4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X. et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26(20):3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Blaker M. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D. et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28(1):69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15(6):966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- Karashima T, Cai RZ, Schally AV. Effects of highly potent octapeptide analogs of somatostatin on growth hormone, insulin and glucagon release. Life Sci. 1987;41(8):1011–1019. doi: 10.1016/0024-3205(87)90690-4. [DOI] [PubMed] [Google Scholar]
- Oberg K. Future aspects of somatostatin-receptor-mediated therapy. Neuroendocrinology. 2004;80(Suppl 1):57–61. doi: 10.1159/000080743. [DOI] [PubMed] [Google Scholar]
- Faiss S, Rath U, Mansmann U, Caird D, Clemens N, Riecken EO, Wiedenmann B. Ultra-high-dose lanreotide treatment in patients with metastatic neuroendocrine gastroenteropancreatic tumors. Digestion. 1999;60(5):469–476. doi: 10.1159/000007693. [DOI] [PubMed] [Google Scholar]
- Welin SV, Janson ET, Sundin A, Stridsberg M, Lavenius E, Granberg D, Skogseid B, Oberg KE, Eriksson BK. High-dose treatment with a long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol/European Federation of Endocrine Societies. 2004;151(1):107–112. doi: 10.1530/eje.0.1510107. [DOI] [PubMed] [Google Scholar]