Abstract
The pituitary gland regulates numerous physiological functions including growth, reproduction, temperature and metabolic homeostasis, lactation, and response to stress. Pituitary organogenesis is dependent on signaling factors that are produced in and around the developing pituitary. The studies described in this report reveal that the forkhead transcription factor, Foxd1, is not expressed in the developing mouse pituitary gland, but rather in the mesenchyme surrounding the pituitary gland, which is an essential source of signaling factors that regulate pituitary organogenesis. Loss of Foxd1 causes a morphological defect in which the anterior lobe of the pituitary gland protrudes through the cartilage plate that is developing ventral to the pituitary at embryonic days (e)14.5, e16.5, and e18.5. The number of proliferating pituitary cells is increased at e14.5 and e16.5. Loss of Foxd1 also results in significantly decreased levels of Lhb expression at e18.5. This decrease in Lhb expression does not appear to be due to a change in the number of gonadotrope cells in the pituitary gland. Previous studies have shown that loss of the LIM homeodomain factor, Lhx3, which is activated by the FGF signaling pathway, results in loss of LH production. Although there is a difference in Lhb expression in Foxd1 null mice, the expression pattern of LHX3 is not altered in Foxd1 null mice. These studies suggest that Foxd1 is indirectly required for normal Lhb expression and cartilage formation.
Introduction
The pituitary gland is a highly specialized organ that is essential for normal endocrine function. This essential gland secretes hormones that regulate growth, metabolism, reproduction, lactation, and response to stress [1]. Pituitary organogenesis begins in mice on embryonic day (e)8.5. By e10.5 the oral ectoderm invaginates and will form Rathke’s pouch by e12.5. The early stages of pituitary development are characterized by rapid proliferation. This is evident at e14.5 by the significant expansion of the anterior lobe of the pituitary. The last day of mouse embryonic development is e18.5 (the day before birth) [2].
The anterior lobe of the pituitary gland contains five specialized hormone-secreting cell types. Somatotropes produce growth hormone (GH) that targets the liver and bone. Lactotropes secrete prolactin (PRL) that acts on the mammary glands. Gonadotropes produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH) that regulate function of the gonads. Thyrotropes secrete thyroid-stimulating hormone (TSH) that targets the thyroid. FSH, LH, and TSH are dimeric hormones consisting of a common α-subunit (CGA) and a unique β-subunit (FSHB, LHB, TSHB). Finally, corticotropes produce adrenocorticotropic hormone (ACTH) that acts on the adrenal gland. The posterior lobe of the pituitary gland is stimulated by direct innervation from the hypothalamus and secretes oxytocin and anti-diuretic hormone. The intermediate lobe produces melanocyte-stimulating hormone. Input from the hypothalamus stimulates the pituitary to secrete hormones that act on a number of target organs throughout the body to regulate a diverse range of physiological functions [2].
Differentiation of the different cell types is dependent on dorsal-ventral morphogenetic gradients that result in overlapping dorsal-ventral patterns of transcription factor expression. Signaling molecules such as bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), and sonic hedgehog (SHH) are involved in initiating pituitary development [3], [4].
Several forkhead factors have roles in pituitary development and function. Foxl2 (Pfrk) is the first forkhead to be described in the pituitary gland [5]. FOXL2 protein is detected in the prospective anterior lobe of the developing pituitary gland starting at e11.5 and continuing into adulthood in gonadotrope and thyrotrope cells of the anterior pituitary [6]. FOXL2 plays a role in regulating several gonadotropin genes including those coding for gonadotropin-releasing hormone receptor, the glycoprotein hormone α-subunit (Cga), and Fshb [6], [7], [8], [9], [10]. In fact, expression of Fshb is severely impaired in the absence of Foxl2, suggesting that Foxl2 is required for normal Fshb expression [10]. The forkhead factor, FOXP3, has a well-established role in the development and function of helper T cells [11], [12]. While Foxp3 is not expressed in or even near the pituitary gland, it is important for pituitary function [13]. Scurfy mice have a mutation in Foxp3 and have drastically reduced levels of Lhb and Fshb expression resulting in infertility [13], [14], [15]. FOXA1 represses growth hormone expression in mouse and human pituitary [16]. In cell culture studies with a gonadotrope-derived cell line, FOXO1 represses expression of Lhb [17]. Foxf1 is expressed in the mesenchyme surrounding the developing pituitary gland and in the adult posterior and anterior pituitary [18]. Finally, Foxe1 is expressed in Rathke’s pouch from e10.5–e11.5, however pituitary hormones are normal in Foxe1 null pups [19].
Foxd1 was originally known as brain factor-2 (Bf2) and is important for proper kidney formation [20], [21]. Heterozygous null mice have no obvious phenotype and are fertile [20]. Foxd1 homozygous null mice have small kidneys, decreased ureteric branching and die within 24 hours after birth due to renal failure. This is due, in part, to ectopic bone morphogenetic (BMP) signaling, which causes mis-patterning of the kidney [20]. Foxd1 is also expressed in the retina and is required for normal development of the retina and optic chiasm [22]. The following studies demonstrate that while Foxd1 is not expressed in the developing pituitary gland, it is expressed in the mesenchyme surrounding the pituitary gland, which produces signaling factors that are essential for normal pituitary development.
Materials and Methods
Mice
Foxd1 heterozygous null mice were generated by Hatini et al. [20] and provided to us by Cathy Mendelsohn of Columbia University. To create the null Foxd1 allele, a LacZ cassette was inserted in place of the Foxd1 coding sequence. A phosphoglycerokinase (PGK)-neor cassette was inserted in the same orientation [20]. As Foxd1 is a single exon gene, this procedure eliminated nearly all of the Foxd1 coding sequence. Mice were maintained in a 12-hour dark-light cycle. Embryos were obtained from an intercross of Foxd1LacZ/+ mice. Foxd1 mutant mice are on a mixed Swiss Webster and C57BL/6J background. The morning the copulatory plug is detected is determined to be e0.5. Littermates were used for all experiments in which normal and mutant embryos were compared. Live mice were genotyped using PCR with primers that amplify a region of the neor gene (5′- ACCTTGCTCCTGCCGAGAAAGTAT and 5′- ATGTTTCGCTTGGTGGTCGAATGG). Embryos were genotyped by PCR with the following primers: 5′ - TCC CTT TAG CCC GGT TAG TCC AGG, 5′ - GCT CTG ACG TGC ACA CCA TGT GAC AG, 5′ - ATT CAG GCT GCG CAA CTG TTG GGA with DMSO and hot start.
The Southern Illinois University Animal Care and Use Committee approved all procedures using mice (Protocol Number: 10-020). All experiments were conducted in accord with the principles and procedures outlined in the NIH Guidelines for the Care and Use of Experimental Animals.
β-galactosidase Staining
Foxd1LacZ/+ embryos (e12.5-e18.5) were frozen and cryo-sectioned (5 µm). Frozen sections were post-fixed in 4% formaldehyde for 5 min, rinsed in PBS and stained overnight in β-galacotosidase staining solution in 1X PBS (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/mL X-gal). Foxd1LacZ/+ embryos (e10.5) and adult pituitaries were stained whole mount for β-galactosidase as follows. Embryos and adult pituitaries were fixed in 4% formaldehyde for 1 hour, rinsed in PBS and stained overnight in β-galactosidase staining solution. After a series of graded ethanol washes, samples were embedded in paraffin and sectioned (5 µm).
Histology and Immunohistochemistry
Embryos were dissected and fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) (pH 7.2) for 45 min to 24 h (depending on stage of development). All samples were washed in PBS, dehydrated in a graded series of ethanol, and embedded in paraffin. Sections (5 µm) were deparaffinized in xylene, rehydrated through a series of graded ethanol washes, and stained in hematoxylin (Fisher Scientific) and eosin (Sigma) or used for immunohistochemistry.
To detect cell proliferation in embryonic pituitaries, pregnant mice were given an intraperitoneal injection of bromodeoxyuridine (BrdU) at 100 µg/g body weight 2 h before the embryos were harvested [23].
To visualize BrdU and LHX3, tissue sections were deparaffinized in xylene, rehydrated in ethanol, and 1.5% peroxide in water was used to remove endogenous peroxidases. After epitopes were unmasked by boiling in 10 mM citric acid for 10 min, tissue sections were blocked using the Mouse on Mouse (M.O.M.) kit (Vector Laboratories) according to the manufacturer's directions. Tissue sections were incubated overnight at 4°C with antibodies for BrdU (Invitrogen, clone ZBU30, 1∶100) or LHX3 (Developmental Studies Hybridoma bank, University of Iowa, 1∶1000). Tissue sections were incubated with anti-mouse secondary (MOM kit, Vector Laboratories) 30 min at room temperature. Next, sections were incubated sequentially with strept-avidin-horseradish peroxidase and fluorescein from the Tyramide Signal Amplification kit (PerkinElmer). Following a 5 min incubation with water, sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (167 nM, Molecular Probes). One mid-sagittal section from each individual was photographed at a magnification of 200X. The total number of BrdU-positive cells in the intermediate and anterior lobe of each section was counted manually in three individuals per group.
To visualize pituitary hormones, tissue sections were deparaffinized and rehydrated as described above. Tissue sections were incubated with antibodies against GH (1∶10,000; National Hormone and Peptide Program (NHPP)), POMC (1∶500; NHPP), TSHB (1∶2000, NHPP), LHB (1∶500, NHPP), CGA (1∶300), or FSHB (1∶250) for 1 hour at room temperature and then the appropriate secondary antibodies: anti-rabbit-TRITC (1∶100, Jackson ImmunoResearch), anti-guinea pig-FITC (1∶100, Jackson ImmunoResearch), or biotinylated anti-rabbit followed by horseradish peroxidase and FITC as described above. One mid-coronal section was photographed at a magnification of 200X for each individual. One half of the section was pictured in each photograph. Because coronal pituitary sections are symmetrical, this gives a good representation of cell types of the anterior lobe. The total number of LHB-positive cells in each photograph was counted manually. Three individuals per group were analyzed and values were set relative to wild type controls.
Programmed cell death in the pituitaries was also detected by the TUNEL method using the in situ cell detection kit POD (Roche) according to manufacturer’s instructions.
Cartilage was stained by Gomori’s aldehyde fuchsin stain as follows. Tissue sections were deparaffinized and rehydrated before incubating 10–60 min in 0.5% iodine. Tissue sections were then decolorized with 0.5% sodium bisulfite, washed in water and transferred to 70% alcohol. Tissue sections were stained in aldehyde fuchsin solution (0.5% basic fuchsin, 70% ethanol, 1% paraldehyde, pH 1.0) for 1–2 hr and rinsed in 70% ethanol.
Digital images of pituitary sections were captured with a Leica DM 5000B fluorescent microscope and Retiga 2000R digital camera. FITC and DAPI pictures were merged using Adobe Photoshop CS3.
RT-PCR
Pituitaries were dissected from e18.5 embryos. Total RNA was isolated with the RNAqueous-Micro kit (Ambion, Inc.) according to manufacturer’s directions. RNA concentrations were determined by spectrophotometry. RNA was treated with DNase I and DNase inactivating reagent from the TURBO DNase-free kit (Ambion, Inc.) as per manufacturer’s instructions. ImPromII reagents and random primers (Promega) were used to synthesize cDNA.
Real time RT-PCR was performed on a CFX96 Real Time System (BioRad). Amplification was accomplished using Taqman Gene Expression Assays (Applied Biosystems) as per manufacturer’s instructions. Five ng of cDNA was used in a 15 µL reaction volume. Samples and controls were run in triplicate. No-template controls and no-reverse transcriptase controls were used to assure the absence of contamination and efficacy of the DNase treatment, respectively. Five to six individuals were included in each group. Data were analyzed by the ΔΔCT method [24], [25]. The values for ΔΔCT were calculated by subtracting the average ΔCT of wild type controls from the ΔCT for each sample. CT values over 30 were considered unreliable and were not included in our analyses.
Statistical Analysis
All results are expressed as mean ± SEM. Data were analyzed by Student’s t-test using Microsoft Excel. P-values less than 0.05 were considered significant (*). P-values less than 0.01 were considered very significant (**).
Results
Normal Foxd1 Expression
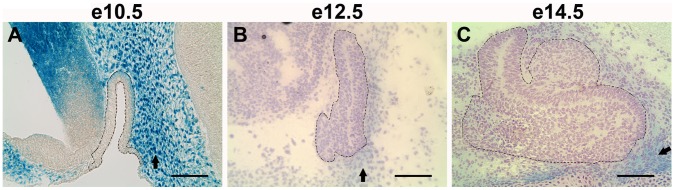
Foxd1 was identified in an expression library developed from pituitary at e14.5, suggesting that it may be important for pituitary development [26]. Foxd1 expression was confirmed by real time RT-PCR (data not shown). To visualize Foxd1 expression, mice in which the Foxd1 allele has been replaced with the coding sequence for LacZ were analyzed [20]. Sections from mouse embryos that were heterozygous for the engineered Foxd1 allele (Foxd1+/LacZ) were stained for β-galactosidase. This study reveals that Foxd1 is not expressed in the pituitary gland during development, but rather in the mesenchyme surrounding the pituitary (Fig. 1A–C). Many signaling factors that are important for pituitary organogenesis are expressed in the mesenchyme surrounding the pituitary, thus FOXD1 could contribute to transcriptional regulation of these factors [27], [28].
Figure 1. Foxd1 is expressed in the mesenchyme surrounding the developing pituitary gland.
X-gal staining of mid-sagittal sections from Foxd1+/LacZ embryos shows Foxd1 expression (blue) in the mesenchyme (arrows) surrounding the developing pituitary gland at e10.5 (A), e12.5 (B), and e14.5 (C). Foxd1 expression is not apparent in the pituitary gland itself (dotted lines) during development. Whole mount β-galactosidase staining was performed at e10.5 (A). At later ages, embryos were frozen and sections were stained for β-galactosidase (B, C). Pictures were taken at 200X and scale bars represent 100 µm.
Pituitary Morphology
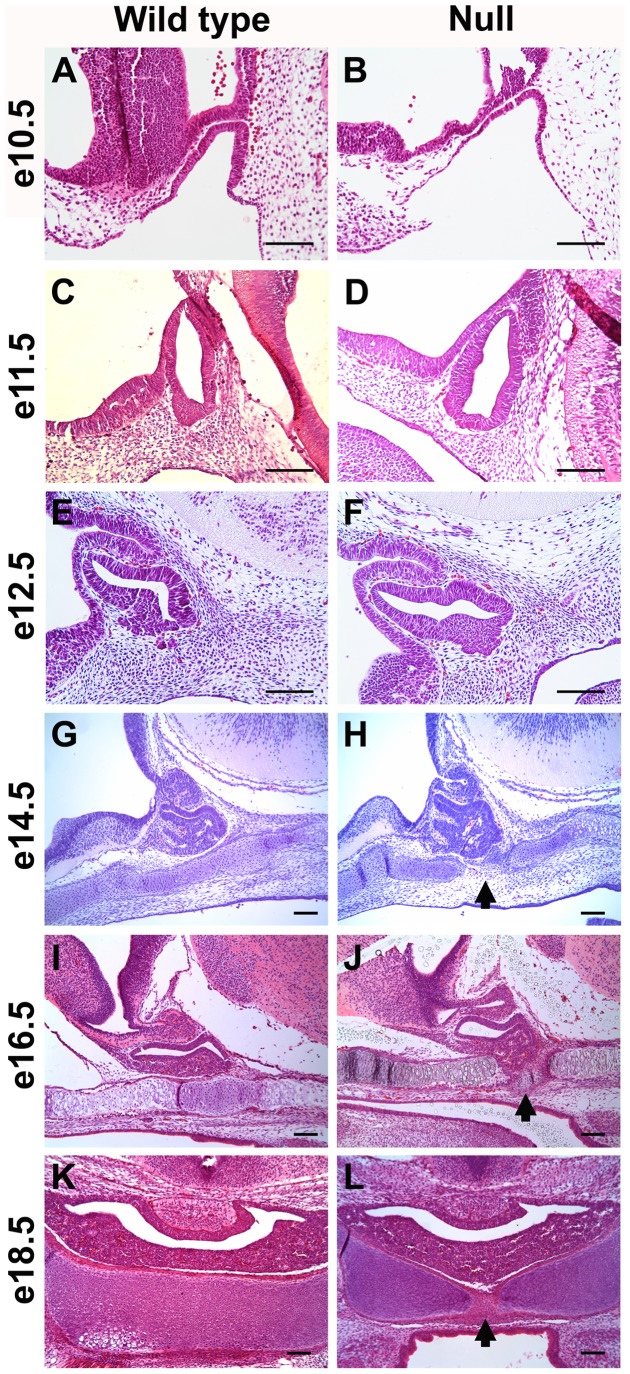
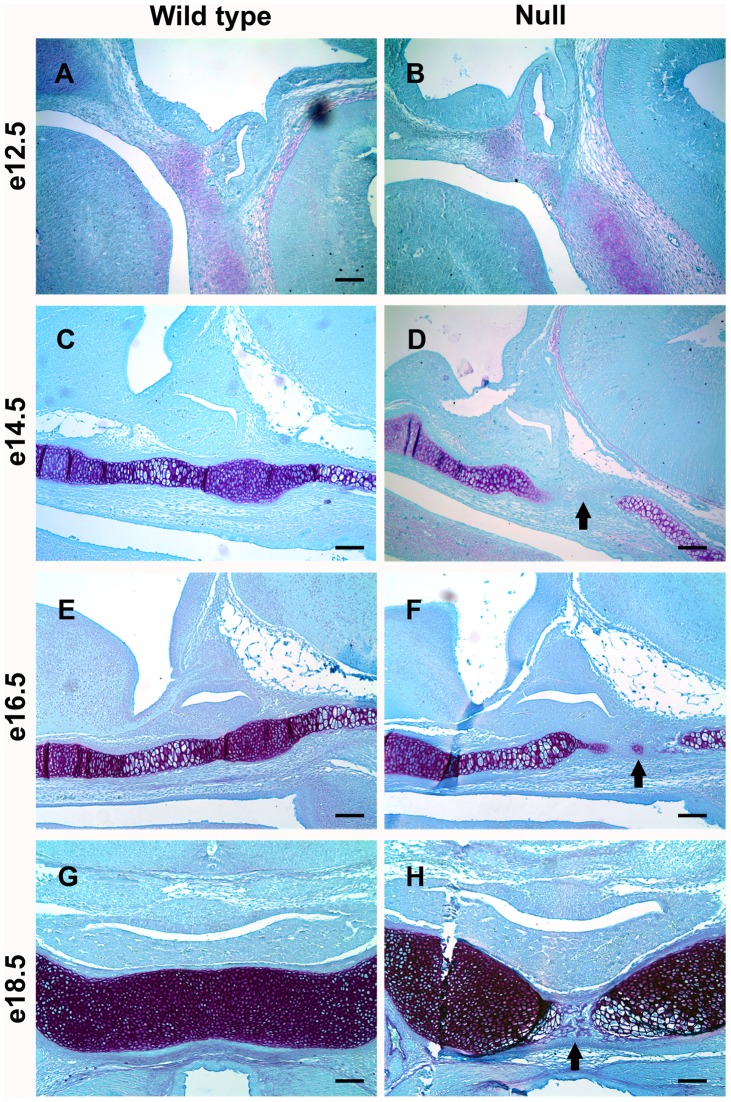
The expression of Foxd1 in the mesenchyme surrounding the developing pituitary led us to investigate the role of FOXD1 in pituitary development. Morphology of the pituitary gland was examined in embryos lacking Foxd1 expression. Hematoxylin and eosin stains of Foxd1 null and wild type embryos revealed that the pituitary gland extends through the cartilage plate ventral to the pituitary gland (Fig. 2A–L). To better visualize the cartilage plate, Gomori’s aldehyde fuchsin stains were performed. These experiments showed that the developing pituitary extends through the cartilage plate evident by e14.5 (Fig. 3A–H).
Figure 2. Loss of Foxd1 expression results in pituitary dysmorphology.
Hematoxylin and eosin staining of mid-sagittal sections (A–J) and coronal sections (K–L) revealed that the developing pituitary protrudes through the cartilage plate by e14.5 in Foxd1LacZ/LacZ embryos (arrows). Pictures were taken at 200X (A–F) or 100X (G–L). Scale bars represent 100 µm.
Figure 3. Loss of Foxd1 expression results in disruption of the developing cartilage plate.
Mid-sagittal (A–F) and coronal (G–H) sections from Foxd1LacZ/LacZ embryos and wild type littermates were stained with Gomori’s aldehyde fuchsin to visualize cartilage. (A–B) The prospective pituitary gland and surrounding tissue appears normal at e12.5. (C–H) A break in the cartilage plate ventral to the pituitary is apparent in Foxd1LacZ/LacZ embryos by e14.5 (arrows). Pictures were taken at 100X and scale bars represent 100 µm.
Pituitary Hormone Expression
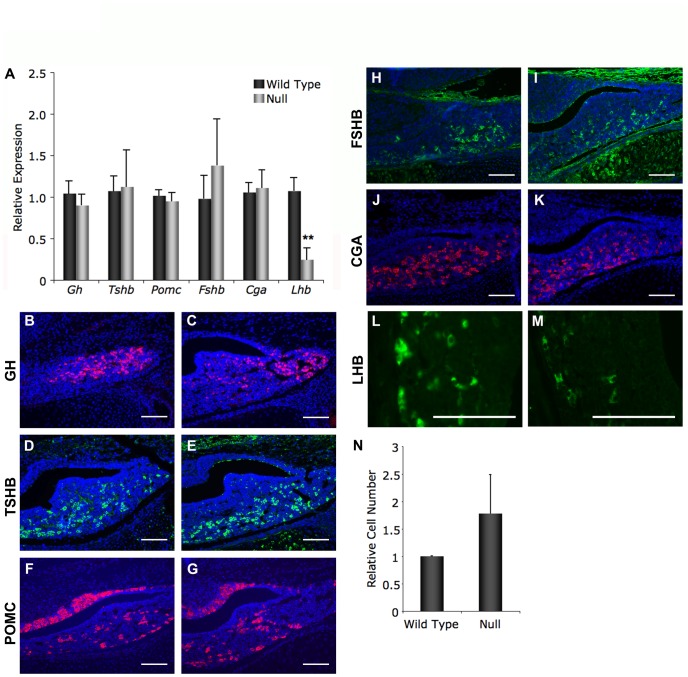
The pituitary gland is essential for orchestrating numerous physiological processes. To determine if Foxd1 expression is important for pituitary function, we performed real time RT-PCR on pituitary from e18.5 Foxd1 wild type and null embryos. This age was chosen because most pituitary hormones are detectable at this age and Foxd1 null mice die within 24 hours after birth. Prl was excluded from this analysis because CT values for Prl at e18.5 are consistently over 30 and we feel that this does not represent an accurate measurement of expression (data not shown). This is consistent with findings by Brannick et al., demonstrating that neither prolactin protein nor prolactin mRNA is detectable until after postnatal day 1 [29]. Expression of growth hormone, thyroid-stimulating hormone-β, pro-opiomelanocortin, follicle-stimulating hormone-β, and the glycoprotein hormone α-subunit is not significantly different between wild type and null embryos (Fig. 4A). However, expression of luteinizing hormone is significantly decreased in Foxd1 null embryos as compared to wild type littermates (Fig. 4A). No apparent loss of somatotrope, thyrotrope, or corticotrope cells was observed in Foxd1 null embryos (Fig. 4B–K). While the intensity of staining is decreased for LHB, the number of gonadotrope cells is not decreased in Foxd1 null embryos, suggesting that it is the level of Lhb expression that is decreased and not an inability of the gonadotropes to differentiate that causes the lower Lhb expression (Fig. 4L–N). This suggests that FOXD1 is required for normal pituitary expression of luteinizing hormone.
Figure 4. Expression of luteinizing hormone is reduced in Foxd1LacZ/LacZ embryos.
(A) Real time RT-PCR revealed that expression of Lhb is significantly reduced in embryos lacking Foxd1 expression compared to wild type littermates at e18.5 (P<0.05). Expression of other pituitary hormones was not significantly reduced. (B–K) No apparent differences in GH, TSHB, POMC, FSHB, or CGA were observed. (L–M) The intensity of LHB staining is reduced in mutant pituitary glands. (N) The number of LHB-positive cells was counted manually and set relative to wild type controls. No significant difference was detected in Foxd1LacZ/LacZ pituitaries as compared to wild type. Cell nuclei are stained with DAPI (blue). Pictures were taken at 200X (B–K) or 630X (L–M). Scale bars represent 100 µm.
Pituitary Cell Apoptosis and Proliferation
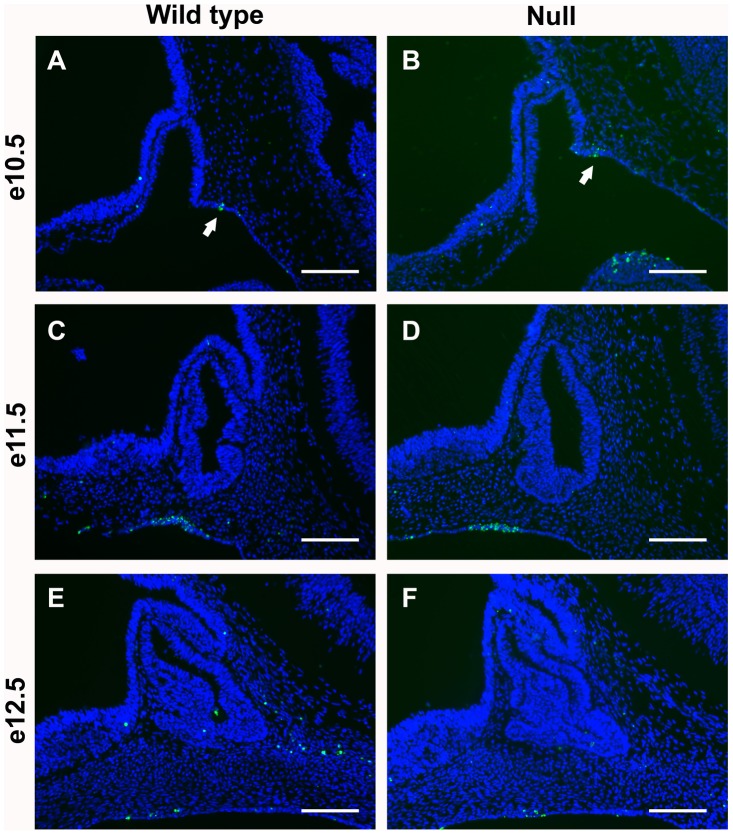
Apoptosis and proliferation are essential processes for normal pituitary development. Apoptosis occurs at e10.5 to separate Rathke’s pouch from the oral ectoderm that will form the lining of the mouth [30]. After Rathke’s pouch is separated from the rest of the oral ectoderm, a cartilage plate forms to separate the pituitary from the oral cavity. If apoptosis fails to occur, the pituitary will remain attached to the rest of the oral ectoderm and the cartilage plate will not form completely [31]. To determine if failure of apoptosis caused the pituitary of Foxd1 null embryos to protrude through the cartilage plate, apoptotic cells were labeled by TUNEL analysis. No significant difference in the number of apoptotic pituitary cells was detected in Foxd1 null embryos as compared to wild type littermates (Fig. 5A–F). This suggests that the pituitary/cartilage dysmorphology in Foxd1 null embryos is not due to a failure in apoptosis.
Figure 5. Apoptosis is not different in Foxd1LacZ/LacZ embryos.
TUNEL was performed to label apoptotic cells in mid-sagittal sections from Foxd1LacZ/LacZ embryos and wild type littermates. (A–F) No loss of apoptosis was observed. (A–B) Arrows indicate regions of pituitary cell apoptosis. Pictures were taken at 200X and scale bars represent 100 µm.
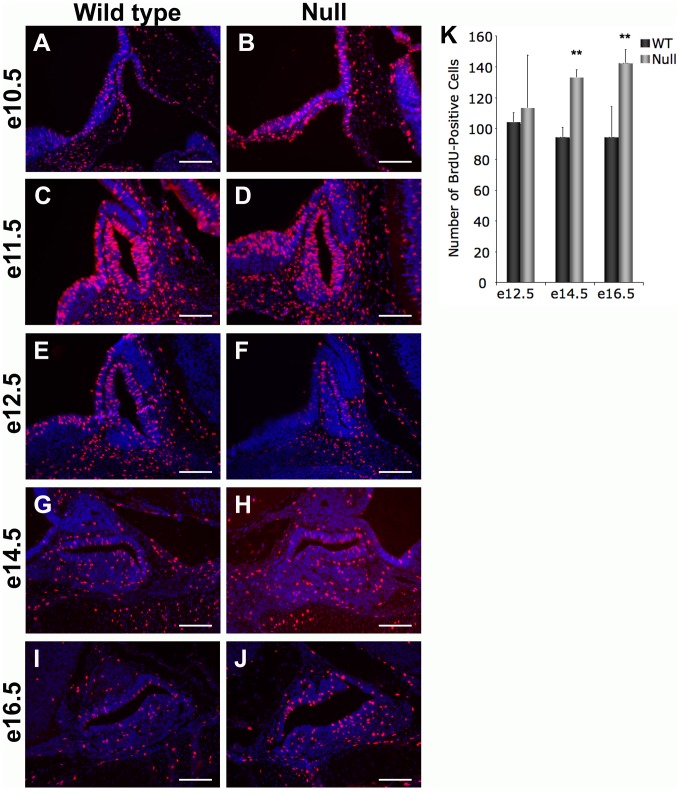
Excessive pituitary cell proliferation can cause the pituitary gland to increase in size and protrude through the cartilage plate. Actively dividing cells were labeled in Foxd1 wild type and null embryos from e10.5 through e18.5 with the thymidine analog bromodeoxyuridine (BrdU) (Fig. 6A–J). The number of BrdU-positive pituitary cells was counted manually. The number of proliferating pituitary cells is significantly increased in Foxd1LacZ/LacZ embryos at e14.5 and e16.5 (Fig. 6K).
Figure 6. Pituitary cell proliferation is increased in Foxd1LacZ/LacZ embryos.
(A–J) Actively dividing cells were labeled with bromodeoxyuridine (BrdU). Immunohistochemistry was performed to detect BrdU in mid-sagittal sections from Foxd1LacZ/LacZ embryos and wild type littermates. (K) The number of BrdU-positive cells was manually counted in pituitary sections from Foxd1LacZ/LacZ embryos and wild type littermates. The number of BrdU-positive cells is significantly higher in Foxd1LacZ/LacZ embryonic pituitaries at e14.5 and e16.5 as compared to wild type littermates (P<0.01).
LHX3
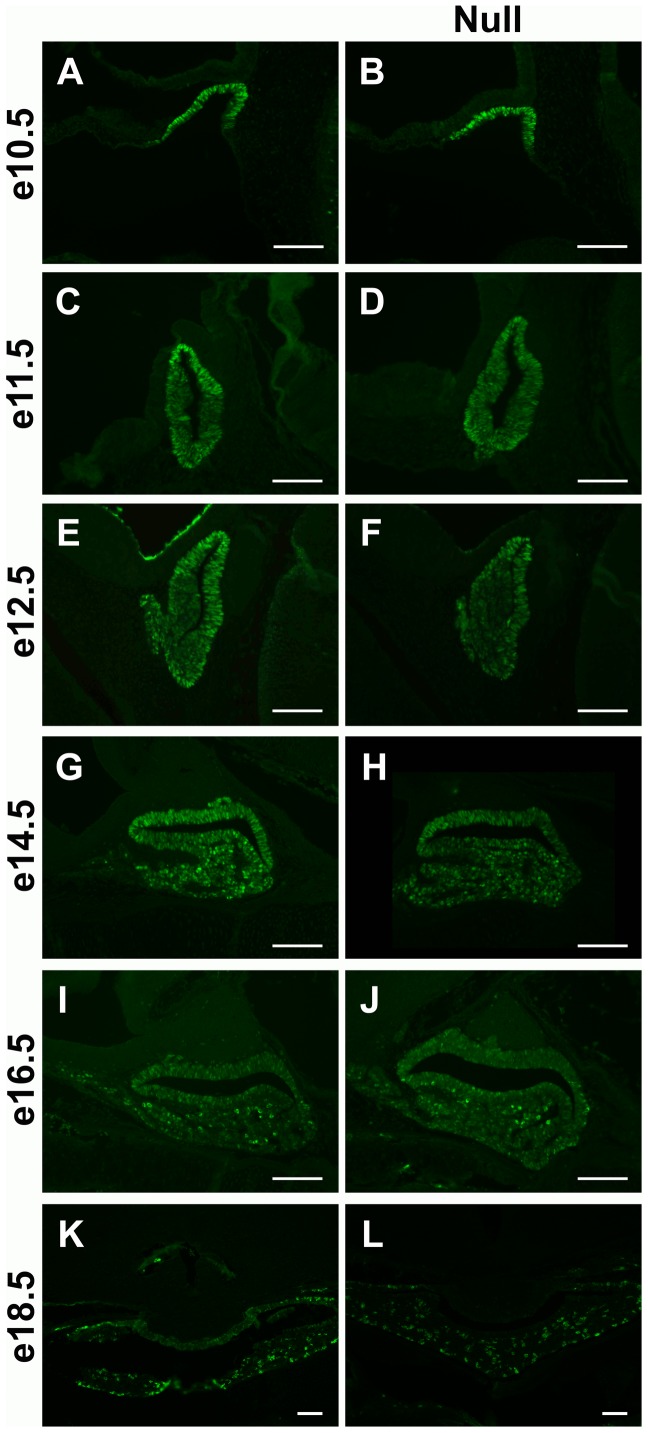
Fibroblast growth factor (FGF) signaling is essential for normal pituitary development. Pituitary expression of the LIM homeodomain factor, Lhx3, is regulated by FGF and loss of Lhx3 results in a decrease in LH production [31], [32], [33], [34]. To determine if the expression patterns for LHX3 are altered in the developing pituitary in the absence of Foxd1, immunohistochemistry for LHX3 was performed. These studies indicate that LHX3 immunoreactivity is not different in Foxd1 null embryos as compared to wild type littermates (Fig. 7A–L).
Figure 7. LHX3 patterns are unchanged in Foxd1LacZ/LacZ embryos.
Immunohistochemistry was performed to observe LHX3 patterns in mid-sagittal (A–J) and coronal (K–L) sections of embryonic pituitary glands from Foxd1LacZ/LacZ embryos and wild type littermates. No difference in LHX3 protein distribution patterns was observed. Pictures were taken at 200X (A–J) or 100X (K–L). Scale bars represent 100 µm.
Discussion
Forkhead transcription factors are important for diverse physiological functions including embryonic development of almost every type of tissue [35], [36], [37], [38]. In order to identify genes that contribute to pituitary development and function, we have turned to the forkhead family. Foxd1 was identified in an embryonic pituitary expression library [26]. However, when Foxd1 expression was analyzed by β-galactosidase staining of Foxd1+/Lacz embryos, it was discovered that Foxd1 is expressed in the mesenchyme surrounding the pituitary, but not in the pituitary itself, suggesting that when dissecting pituitaries from e14.5 mouse embryos, some mesenchyme was obtained as well. The mesenchyme surrounding the developing pituitary gland is a rich source of signaling factors that are essential for normal pituitary development. Bmp2 is expressed in the mesenchyme ventral and rostral to Rathke’s pouch at e12.5 and e14.5 and is believed to be important for inducing proliferation and differentiation of ventral cell types [5], [39]. The BMP inhibitors chordin and noggin are expressed in the mesenchyme caudally and ventrally, respectively, to the pituitary by approximately e12.5 [5], [27], [39]. FOXD1 is present in the mesenchyme ventral and caudal to the putative pituitary gland from e12.5 and continuing through development. This expression pattern places FOXD1 in a region where it could affect several signaling factors that are important for pituitary development.
Expression of Lhb was significantly reduced in Foxd1 mutant embryos as compared to wild type littermates. These data demonstrate that Foxd1 is indirectly required for Lhb expression in gonadotrope cells. Because Foxd1 is not expressed in the developing pituitary gland, but is present in the mesenchyme surrounding the developing pituitary, the reduction in Lhb expression may be due to the loss of signaling factors from the mesenchyme surrounding the pituitary gland. Signals from outside of the pituitary are important for proper specification of pituitary cells [34]. FGFs from the infundibulum can increase immunorectivity for ACTH and decrease immunoreactivity for the LIM homeodomain factor, ISL1, as well as for αGSU [34]. In contrast, BMPs from the ventral juxtapituitary mesenchyme increases immunoreactivity for αGSU and decreases immunoreactivity for ACTH [34]. In embryos null for noggin, a BMP2 and 4 antagonist, a secondary pituitary is sometimes induced, however in the primary pituitary all hormones are produced normally [27]. The BMP antagonist, chordin, is expressed in the caudal mesenchyme adjacent to Rathke’s pouch at e12.0 and may be important for counteracting BMP2 signals [5]. It may be that FOXD1 is important for production of one or more of these signaling factors and that loss of FOXD1, and ultimately of certain signals from the mesenchyme, disrupts Lhb expression.
Another possibility is that loss of Foxd1 in the mesenchyme surrounding the hypothalamus affects hypothalamic function or that GnRH neuronal migration is abnormal. In mouse embryos lacking Foxd1 retinal ganglion cell axons that form the optic chiasm aberrantly project contralaterally [22]. Thus, FOXD1 appears to be important for proper neuronal migration. Hpg mice, which have a mutation of the Gnrh gene resulting in a loss of GnRH secretion, exhibit reduced expression of Lhb and Fshb [40], [41]. In Foxd1 mice expression of the GnRH-responsive genes Fshb and Cga is normal. Thus, it seems unlikely that GnRH stimulation of gonadotrope cells is altered in Foxd1 mutants.
Apoptosis is an important process in pituitary development, allowing the oral ectoderm that will form the pituitary gland to separate from the oral ectoderm that will form the lining of the mouth with a cartilage plate forming between. Foxd1 mutants exhibit normal apoptosis, however abnormal apoptosis has been observed with other mutations [27], [31], [42], [43]. The pituitary fails to separate from the lining of the oral cavity in the absence of Lhx3 expression, however it has not been determined if a failure in apoptosis during early development is responsible [31]. Increased apoptosis later in development does contribute to the hypopituitarism observed in Lhx3 null embryos [42], [43]. In the absence of the BMP inhibitor, noggin, apoptosis in Rathke’s pouch is decreased and the pituitary fails to separate from the oral ectoderm, disrupting formation of the cartilage plate [27].
Proliferation is an essential process in organogenesis. During pituitary development, proliferation occurs in Rathke’s pouch, but not in the rostral tip of the pituitary gland [6]. Loss of TCF4, which mediates WNT signaling, causes pituitary cell hyperplasia, leading to a disruption of the cartilage plate ventral to the pituitary gland [44], [45]. The number of proliferating pituitary cells was increased at e14.5 and e16.6 in embryos lacking Foxd1, suggesting that Foxd1 is important for regulating proliferation in the developing pituitary gland. This increase in pituitary cell proliferation may contribute to the break in the cartilage plate ventral to the pituitary gland as seen in Tcf4 mutants.
There is much to be learned about how signaling factors from the juxtapitutiary mesenchyme regulate pituitary hormone production. The data described in this manuscript demonstrate that Foxd1 indirectly contributes to normal expression of Lhb, but not the gonadotropin genes Fshb and Cga. In the absence of Foxd1, pituitary cell proliferation is increased and the cartilage plate ventral to the pituitary fails to fuse. These studies provide important clues as to the role of FOXD1 and the juxtapituitary mesenchyme during pituitary development and for pituitary function.
Acknowledgments
We thank A.F. Parlow and the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Child Health and Human Development for providing antibodies for TSHB, GH, LHB, FSHB, CGA, and POMC. We thank Maureen Doran and Dawn Grisley for their assistance with histological techniques.
Funding Statement
This work was supported by a REACH award (E.M.P.), startup funds (B.S.E.), and an ORDA Faculty Seed Grant (B.S.E.) from Southern Illinois University School of Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cushman LJ, Camper SA (2001) Molecular basis of pituitary dysfunction in mouse and human. Mamm Genome 12: 485–494. [DOI] [PubMed] [Google Scholar]
- 2. Watkins-Chow DE, Camper SA (1998) How many homeobox genes does it take to make a pituitary gland? Trends Genet 14: 284–290. [DOI] [PubMed] [Google Scholar]
- 3. Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ (2003) Transcriptional control during mammalian anterior pituitary development. Gene 319: 1–19. [DOI] [PubMed] [Google Scholar]
- 4. Scully KM, Rosenfeld MG (2002) Pituitary development: regulatory codes in mammalian organogenesis. Science 295: 2231–2235. [DOI] [PubMed] [Google Scholar]
- 5. Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, et al. (1998) Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12: 1691–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, et al. (2006) FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol 20: 2796–2805. [DOI] [PubMed] [Google Scholar]
- 7. Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, et al. (2003) The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Molecular and Cellular Endocrinology 206: 93–111. [DOI] [PubMed] [Google Scholar]
- 8. Coss D, Mellon PL, Thackray VG (2010) A FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab 21: 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ (2009) A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol Endocrinol 23: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Justice NJ, Blount AL, Pelosi E, Schlessinger D, Vale W, et al. (2011) Impaired FSH{beta} Expression in the Pituitaries of Foxl2 Mutant Animals. Mol Endocrinol 25: 1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziegler SF (2006) FOXP3: of mice and men. Annu Rev Immunol 24: 209–226. [DOI] [PubMed] [Google Scholar]
- 12. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, et al. (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27: 68–73. [DOI] [PubMed] [Google Scholar]
- 13. Jung DO, Jasurda JS, Egashira N, Ellsworth BS (2012) The Forkhead Transcription Factor, FOXP3, Is Required for Normal Pituitary Gonadotropin Expression in Mice. Biol Reprod 86: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB (1991) Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A 88: 5528–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyon MF (1986) Hypogonadism in scurfy (sf) males. Mouse News Letter 74: 93. [Google Scholar]
- 16. Norquay LD, Yang X, Jin Y, Detillieux KA, Cattini PA (2006) Hepatocyte nuclear factor-3alpha binding at P sequences of the human growth hormone locus is associated with pituitary repressor function. Mol Endocrinol 20: 598–607. [DOI] [PubMed] [Google Scholar]
- 17. Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG (2012) FOXO1 inhibits transcription of luteinizing hormone beta in pituitary gonadotrope cells. J Biol Chem 287: 33424–33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalinichenko VV, Gusarova GA, Shin B, Costa RH (2003) The forkhead box F1 transcription factor is expressed in brain and head mesenchyme during mouse embryonic development. Gene Expr Patterns 3: 153–158. [DOI] [PubMed] [Google Scholar]
- 19. Zannini M, Avantaggiato V, Biffali E, Arnone MI, Sato K, et al. (1997) TTF-2, a new forkhead protein, shows a temporal expression in the developing thyroid which is consistent with a role in controlling the onset of differentiation. Embo J 16: 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E (1996) Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev 10: 1467–1478. [DOI] [PubMed] [Google Scholar]
- 21. Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, et al. (2005) Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539. [DOI] [PubMed] [Google Scholar]
- 22. Herrera E, Marcus R, Li S, Williams SE, Erskine L, et al. (2004) Foxd1 is required for proper formation of the optic chiasm. Development 131: 5727–5739. [DOI] [PubMed] [Google Scholar]
- 23. Nowakowski RS, Lewin SB, Miller MW (1989) Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol 18: 311–318. [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 25. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 26. Carninci P, Waki K, Shiraki T, Konno H, Shibata K, et al. (2003) Targeting a complex transcriptome: the construction of the mouse full-length cDNA encyclopedia. Genome Res 13: 1273–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis SW, Camper SA (2007) Noggin regulates Bmp4 activity during pituitary induction. Dev Biol 305: 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dasen JS, Rosenfeld MG (1999) Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr Opin Cell Biol 11: 669–677. [DOI] [PubMed] [Google Scholar]
- 29. Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, et al. (2012) Prenatal exposure to low doses of bisphenol a increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod 87: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, et al. (2005) PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol 19: 1893–1903. [DOI] [PubMed] [Google Scholar]
- 31. Sheng HZ, Zhadanov AB, Mosinger B Jr, Fujii T, Bertuzzi S, et al. (1996) Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272: 1004–1007. [DOI] [PubMed] [Google Scholar]
- 32. Achermann JC, Jameson JL (2001) Advances in the molecular genetics of hypogonadotropic hypogonadism. J Pediatr Endocrinol Metab 14: 3–15. [DOI] [PubMed] [Google Scholar]
- 33. Savage JJ, Mullen RD, Sloop KW, Colvin SC, Camper SA, et al. (2007) Transgenic mice expressing LHX3 transcription factor isoforms in the pituitary: effects on the gonadotrope axis and sex-specific reproductive disease. J Cell Physiol 212: 105–117. [DOI] [PubMed] [Google Scholar]
- 34. Ericson J, Norlin S, Jessell TM, Edlund T (1998) Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 35. Carlsson P, Mahlapuu M (2002) Forkhead transcription factors: key players in development and metabolism. Dev Biol 250: 1–23. [DOI] [PubMed] [Google Scholar]
- 36. Wijchers PJ, Burbach JP, Smidt MP (2006) In control of biology: of mice, men and Foxes. Biochem J 397: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cirillo LA, Barton MC (2008) Many forkheads in the road to regulation. EMBO Rep 9: 721–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hannenhalli S, Kaestner KH (2009) The evolution of Fox genes and their role in development and disease. Nat Rev Genet 10: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT (2009) Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30: 790–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, et al (1986) A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science 234: 1366–1371. [DOI] [PubMed] [Google Scholar]
- 41. Charlton HM, Halpin DM, Iddon C, Rosie R, Levy G, et al. (1983) The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology 113: 535–544. [DOI] [PubMed] [Google Scholar]
- 42. Ellsworth BS, Butts DL, Camper SA (2008) Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol 313: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, Morales DC, Hermesz E, Lee WK, Pfaff SL, et al. (2006) Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development. Mech Dev 123: 605–613. [DOI] [PubMed] [Google Scholar]
- 44. Brinkmeier ML, Potok MA, Davis SW, Camper SA (2007) TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol 311: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, et al. (2003) TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol 17: 2152–2161. [DOI] [PubMed] [Google Scholar]