Abstract
Viral infections of the CNS and their accompanying inflammation can cause long-term neurological effects, including increased risk for seizures. To examine the effects of CNS inflammation, we infused polyinosinic: polycytidylic acid, intracerebroventricularly to mimic a viral CNS infection in 14 day-old rats. This caused fever and an increase in the pro-inflammatory cytokine, interleukin (IL)-1β in the brain. As young adults, these animals were more susceptible to lithium-pilocarpine and pentylenetetrazol-induced seizures and showed memory deficits in fear conditioning. Whereas there was no alteration in adult hippocampal cytokine levels, we found a marked increase in NMDA (NR2A and C) and AMPA (GluR1) glutamate receptor subunit mRNA expression. The increase in seizure susceptibility, glutamate receptor subunits, and hippocampal IL-1β levels were suppressed by neonatal systemic minocycline. Thus, a novel model of viral CNS inflammation reveals pathophysiological relationships between brain cytokines, glutamate receptors, behaviour and seizures, which can be attenuated by anti-inflammatory agents like minocycline.
Keywords: Seizure, Epilepsy, Infection, Inflammation, Virus, Cytokine, Fever, Hippocampus, Glutamate receptor, Minocycline
Introduction
Encephalitis is normally defined as acute inflammation of the brain parenchyma caused by a CNS infection which often results in significant morbidity, long-term disability, and can be fatal (Kramer and Bleck, 2008). The majority (~60%) of acute encephalitis cases have unknown causes, however, in the known cases, viruses are the most common etiological agent (Chen et al., 2006; Davison et al., 2003; Khetsuriani et al., 2002; Kramer and Bleck, 2008; Whitley, 1990). Most concerning is that the incidence of viral encephalitis in children is almost three times that of adults suggesting that this portion of the population is particularly susceptible (Beghi et al., 1984; Davison et al., 2003; Nicolosi et al., 1986). The clinical presentation of viral encephalitis is non-specific and can include fever, neurological deficits and seizures. Although seizures can occur frequently during the acute phase (Misra et al., 2008), epidemiological analyses have demonstrated that CNS infections are a major cause of acquired epilepsy (Annegers et al., 1988; Hauser and Kurland, 1975; Marks et al., 1992; Rantakallio et al., 1986; Rocca et al., 1987), as well as neurological morbidity, including memory impairment and behavioural abnormalities (Chen et al., 2006; McGrath et al., 1997; Raschilas et al., 2002; Schmutzhard, 2001; Whitley et al., 1977), even after the infection has resolved.
The risk for unprovoked seizures increases by 16 fold after an episode of viral encephalitis, and can remain elevated for the next 20 years (Annegers et al., 1988). These unprovoked seizures are recurrent with up to 98% of patients experiencing additional seizures (epilepsy) (Annegers et al., 1988). The long-term risk for seizures and epilepsy, as well as the increased risk of developing cognitive and neurobehavioural disorders following CNS infection presents an important challenge for both researchers and clinicians interested in understanding the long-term complications associated with encephalitis (Singh and Prabhakar, 2008). At present, the factors that contribute to the increased seizure propensity in these patients remain poorly understood.
In the current study we used an animal model of brain inflammation to mimic a clinical viral encephalitic process in order to investigate mechanisms that may underlie increased seizure predisposition after viral encephalitis. Because there are dozens of viral agents that can cause encephalitis and many of the cases examined have no conclusive isolation of the exact virus (Solomon et al., 2007), we used the viral mimetic polyinosinic:polycytidylic acid (POLY I:C) which is a double-stranded RNA molecule that can evoke an immune response much like viruses do but has the advantage of having a predictable intensity and duration. Thus, we injected POLY I: C intracerebroventricularly (ICV) into young rats at 14 days of age to induce brain inflammation. We then examined the acute and persistent changes within the brain that can predispose to neurological and behavioural deficits later in life.
Materials and methods
Animals and drugs
Pregnant Sprague–Dawley rats at mid gestation were obtained from Charles River Laboratories (Quebec, CAN) and housed under specific pathogen-free environmental conditions at a constant temperature (20–21 °C) with food and water available ad libitum. The light/dark cycle was 12 h/12 h with photophase onset at 0600 h local time. Pregnant females were monitored for the parturition date that was taken as postnatal day (P)0 at which time all litters were culled to 10. Following the neonatal treatments as described below, animals were returned to their dams, weaned at P21 and housed 2 per cage and subjected to regular specific pathogen-free husbandry until further testing. All adult experimentation took place between 7 and 8 weeks of age. Male rats from more than one litter were used in each experiment to minimize any litter effects (Meaney and Szyf, 2005). All procedures were approved by the local Animal Care Committee and were compliant with the guidelines of the Canadian Council on Animal Care. All drugs were obtained from Sigma-Aldrich (MO, USA) and administered at 1 ml/kg unless otherwise stated.
Brain inflammation
CNS inflammation (encephalitis) was induced by ICV injection of POLY I:C (10 μg/rat). Administration of POLY I:C is not associated with a live viral infection and there is no risk of transmission of viral products. On P14, when rats are thought to be developmentally equivalent to a 1–2 yr old human (Avishai-Eliner et al., 2002), male rat pups were separated from the dam and placed in a small cage on a heating pad one litter at a time. Animals were anaesthetised with isoflurane (4% induction, 2–2.5% maintenance) and a small incision was made in the skin over the skull. A 10 μL Hamilton syringe with a 25 G needle was stereotaxically placed over the right lateral ventricle (1 mm posterior to bregma, 1 mm lateral to bregma) and lowered 2 mm below the ventral surface of the skull. POLY I:C (10 μg in 3 μL) or pyrogen-free saline (SAL; 3 μL) was slowly infused into the ventricle over a 1 min period. Similar doses of lipopolysaccharide (LPS) have been used to induce brain inflammation in neonatal rats (Fan et al., 2005). The incision was sutured and animals were returned to the heating pad until all male rats from that litter were injected. Each injection required less than 5 min. Approximately equal numbers of pups in each litter received POLY I:C or SAL.
Minocycline injections
Minocycline is a tetracycline derivative capable of crossing the blood brain barrier (Aronson, 1980), and can suppress inflammation, viral replication, and apoptosis (Irani and Prow, 2007; Michaelis et al., 2007; Mishra and Basu, 2008; Richardson-Burns and Tyler, 2005; Zink et al., 2005). Minocycline (90 mg/kg) was dissolved in phosphate-buffered-saline (PBS; pH=7.4) and administered intraperitoneally (Fan et al., 2005), concurrently with the ICV injections of POLY I:C at P14 in an attempt to interfere with inflammation and attenuate any long-term changes induced by POLY I:C.
Lithium-pilocarpine seizure susceptibility testing
Following P14 ICV injections of SAL, POLY I:C (10 μg/rat) or POLY I: C plus minocycline (90 mg/kg), adult rats (n=5–6/group) received subcutaneous injections of lithium (3 mEq/kg) followed 4 h later by pilocarpine (30 mg/kg; LI-PILO). The latency to the first behavioural seizure after pilocarpine administration, referred to as the seizure onset time (SOT), was defined by the occurrence of forelimb clonus, rearing and loss of balance (stage five seizure), and was recorded to the nearest second. SOT is a commonly used measure to describe seizure susceptibility to convulsant compounds like LI-PILO in rats (Galic et al., 2008).
Pentylenetetrazol seizure susceptibility testing
Seizure susceptibility was also assessed using an intravenous (IV) infusion of pentylenetetrazol (PTZ) into the jugular vein of adult rats as previously described (Galic et al., 2008; Riazi et al., 2008). Briefly, jugular vein catheters were surgically implanted under ketamine/xylazine (85:15) anaesthesia, into adult rats which previously received ICV SAL (n=10) or POLY I:C (n=6) at P14. The clonic seizure threshold was determined 3–4 days later by IV infusion of a 1% PTZ solution to the unrestrained rat through the catheter at a constant rate of 0.58 ml/min using an infusion pump. The infusion was terminated when generalized clonus was observed and the amount of PTZ (in mg/kg) required to induce a generalized clonic seizure was calculated and used as the index of seizure susceptibility. The smaller the volume of PTZ infused, the higher the seizure susceptibility was.
Water maze
A water maze was used to examine the spatial memory ability of adult rats treated with SAL or POLY I:C (n=6/group) at P14 as previously described (Harre et al., 2008). Briefly, the water maze pool contained a 20 cm diameter platform located below the surface of the water. A non-toxic blue paint was added to the pool to visually obscure the platform location. The pool was virtually divided into 4 equally sized quadrants with the platform located in the centre of one of the quadrants. Six sessions were conducted, once daily, over 6 successive days with each session consisting of 4 trials separated by 30 s. The latency to find the platform was recorded to a maximum of 120 s for each trial. Rats that did not find the platform within 120 s were assigned this value and guided onto the platform. On the following day (probe trial) after the last hidden-platform training session, the escape platform was removed from the pool and rats were released into the quadrant opposite to that previously associated with the platform. The time spent swimming in each of the 4 quadrants of the pool was manually recorded. Training day results are expressed in seconds to locate the platform, while the probe trial score is expressed as a percentage of time spent in the target quadrant.
Contextual fear conditioning
To examine the fear learning ability of adult rats treated neonatally with SAL or POLY I:C (n=9–10/group), a contextual fear conditioning task was conducted as previously described (Harre et al., 2008). Briefly, rats were placed in a conditioning chamber (Coulbourn Instruments, PA, USA) for a 3 min baseline period and were then given 3 unsignalled footshocks through a metal grid floor (2 s, 0.5 mA, 60 s inter-stimulus interval). During the baseline period, spontaneous motor activity (midline chamber crossovers) was recorded for each rat. Every 8 s after each footshock, rats were scored for defensive freezing which was defined as the cessation of all non-respiratory associated movement. Twenty-four hours later, the rats were placed again into the conditioning chamber for a retention (memory) trial and scored for defensive freezing every 8 s for an 8 min period. The amount of postshock freezing and retention trial freezing scores were calculated as a percentage of total time.
RT-PCR for glutamate receptor mRNA
In SAL, POLY I:C or POLY I:C plus minocycline (n=6/group) adult rats, we measured hippocampal mRNA levels of select subunits for N-methyl-D-aspartate (NMDA) receptors (NR1, NR2A, B, C and D) and Alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors (GluR1, 2 and 3) using real-time RT-PCR as previously described (Harre et al., 2008). Semi-quantitative analysis was performed by real-time monitoring of the increase in fluorescence of the SYBR-green dye (LightCycler FastStart DNA MasterPLUS SYBR Green I kit, Roche Diagnostics, USA) on a LightCycler (Roche Diagnostics, USA). All data were normalized against the mRNA levels of an endogenous reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and expressed relative to SAL-treated controls using the “delta–delta CT” method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008). Data are presented as relative fold change (RFC) to SAL-controls. Primers were derived from previously published reports (Barbon et al., 2006; Lai et al., 2000) and primer sequences can be found in Table 1.
Table 1.
| Primer | Sequence |
|---|---|
| GAPDH-for | AAG ATG GTG AAG GTC GGT GT |
| GAPDH-rev | TGG AAG ATG GTG ATG GGT TT |
| NR1-for | AAC CTG CAG AAC CGC AAG |
| NR1-rev | GCT TGA TGA GCAGGTCTATGC |
| NR2A-for | TCC ATTC TTC TGT CAT CCT GC |
| NR2A-rev | AAG ACC GTC TCT CAC TCT TGC |
| NR2B-for | TGC ACA ATT ACT CCT CGA CG |
| NR2B-rev | TCC GAT TCT TCT TCT GAG CC |
| NR2C-for | TTG AGG ACA ACG TGG ACA CC |
| NR2C-rev | TCC AGT CGT ATT CCT CCA GC |
| NR2D-for | GCA CTT GCA TCA GAG ACT CG |
| NR2D-rev | CTC ACC AAT CAT GCC ATT CC |
| GluR1-for | AGA GGC TGG TGG TGG TTG ACT |
| GluR1-rev | ACC CTG GTA TGG TCT CGG GA |
| GluR2-for | GGG ATA TCT ATC ATG ATC AAG AAG CC |
| GluR2-rev | CCA CAC ACC TCC AAC AAT GC |
| GluR3-for | TGT GCA GTT ATA CAA CAC CAA CCA G |
| GluR3-rev | GCA TCT GGA TGA CAA ACT GCA C |
Cytokine measurements
To quantify the amount of brain inflammation induced by POLY I:C, we measured the levels of pro-inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)α in the hippocampus either acutely (6 h) after P14 ICV injections (n=6/group) or later in adulthood in rats given either SAL, POLY I:C or POLY I:C plus minocycline (n=5/group). Briefly, rats were deeply anaesthetised with pentobarbital (60 mg/kg) and perfused with PBS. Hippocampal tissue was rapidly dissected out, frozen in liquid nitrogen and stored at −80 °C until further processing. Measurements of the cytokines TNFα and IL-1β were completed using enzyme-linked immunosorbent assay kits (Biosource, CA, USA). All hippocampal samples were adjusted according to the protein content determined using a modified Bradford method (Bradford, 1976) and expressed as the ratio to the mean of the control values (Riazi et al., 2008).
Body temperature recordings
Since fever is one of the most common signs of encephalitis, we measured body temperature. To record body temperature after P14 ICV injection of SAL, POLY I:C or POLY I:C plus minocycline, we implanted miniature temperature dataloggers (SubCue™, AB, CAN) on P12 (n=6/group). Prior to surgery, animals were separated from the dam and placed in a small cage on a heating pad. Anaesthesia was induced using isoflurane (4% induction, 2–2.5% maintenance) and dataloggers were implanted into the abdomen as previously described (Heida et al., 2004). The entire surgical procedure required about 5 min per rat. Animals were then returned to the dam after all male pups from that litter received an implant. At P14, body temperature was recorded every 5 min for 1 h before and 5 h after the ICV injection. We noted that this stereotaxic ICV procedure, carried out under anaesthesia caused wide fluctuations in body temperature over the first 90 min after injection. Thus to quantify changes in body temperature, we calculated a fever index (area under the curve) between 100 and 300 min after the injection using an average baseline temperature acquired prior to injection as baseline.
Statistical analysis
All analyses were completed using Statistical Package for the Social Sciences (SPSS; v.13) software. Between group differences in seizure threshold, mRNA levels, fear conditioning performance, water maze probe trial scores, cytokine levels and fever index were analyzed using one-way analysis of variance (ANOVA) and independent t-tests. A repeated-measures ANOVA was used to examine acquisition trial performance in the water maze. Student–Newman–Keuls post hoc tests were used to examine the main effects. Results are expressed as the mean±standard error of the mean. The criterion for statistical significance was set at p≤0.05.
Results
Brain inflammation during development increases seizure susceptibility
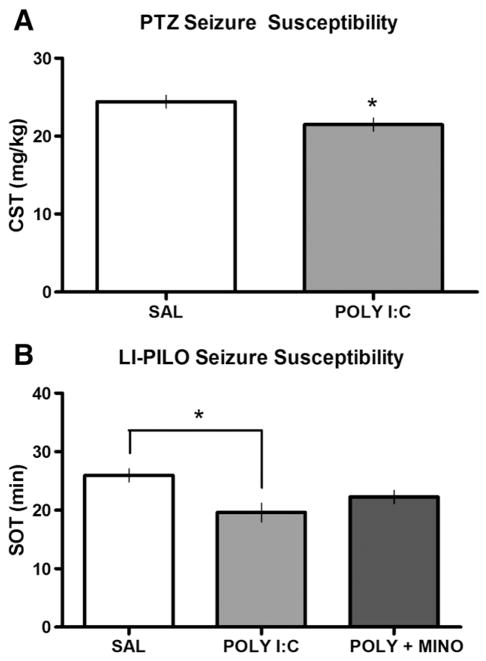
To determine if ICV POLY I:C injected at P14 altered seizure susceptibility in adulthood, rats were administered PTZ or LI-PILO. PTZ-induced clonic seizure thresholds were significantly lower in POLY I:C-treated rats compared to SAL-controls (t=2.34, p<0.05; Fig. 1A). Using a different seizure drug we also found a significant increase in seizure susceptibility in POLY I:C-treated rats. Adult rats injected with POLY I:C at P14 had significantly lower SOTs to LI-PILO than SAL-treated controls (F(2,16) =5.40, p < 0.05; Fig. 1B). This decrease in seizure threshold was attenuated by the peripheral administration of minocycline at the time of POLY I:C injection such that seizure scores of the POLY I:C plus minocycline group were not significantly different from SAL-controls.
Fig. 1.
Increased seizure susceptibility. (A) Adult clonic seizure threshold (CST; in mg/kg) to PTZ following postnatal ICV injections of either SAL (n=10) or POLY I:C (n=6) and, (B) adult seizure onset time (SOT; min) to LI-PILO following postnatal ICV injections of either SAL (n=5), POLY I:C (n=6) or POLY I:C+MINO (n=6) at P14. POLY I:C-treated rats showed significantly (p<0.05; *) lower mean CSTs and SOTs compared to controls suggesting increased seizure susceptibility. POLY I:C+MINO-treated rats were not significantly different from SAL-controls when seized with LI-PILO.
Impact of brain inflammation on learning and memory performance
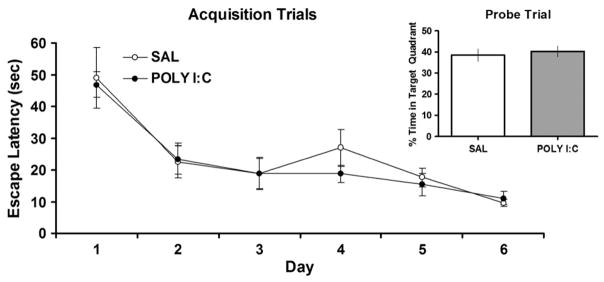
The water maze task was used to evaluate the spatial learning ability of adult rats treated with ICV SAL or POLY I:C at P14. A repeated-measures ANOVA was performed with one level repeated [days (1–6)] and one level not repeated [treatment (SAL or POLY I:C)]. The two treatment groups showed the expected training effect [F(5,50) =17.64; p < 0.001] as evidenced by a conspicuous decrease in escape latency over days (Fig. 2). There was no statistically significant interaction between days and treatment, indicating that performance of the POLY I:C-treated rats was comparable to SAL-controls during training. Similarly, in the probe trial conducted 24 h after training; no significant differences existed between the treatments with respect to time spent in the target quadrant (Fig. 2 inset).
Fig. 2.
No differences in water maze testing. Water maze acquisition and probe trial (inset) scores presented as escape latency (s), or percent time in target quadrant respectively, for animals treated with either SAL or POLY I:C at P14 (n=6/group). There were no significant differences between the groups in either the acquisition or probe trial tests.
Contextual fear conditioning is a task that requires subjects to learn and recall an association between a novel environment (context) and a negative stimulus (footshock). Analysis of fear conditioning scores determined that there were no statistically significant differences between adult rats treated at P14 with SAL or POLY I:C in the number of midline chamber crossovers (SAL 9.40±0.63 vs. 9.22±0.66), or postshock freezing scores during training (Fig. 3). However, during the retention test, rats treated with POLY I:C showed significantly less freezing behaviours than controls (t=2.25, p<0.05; Fig. 3). Together these data suggest mild impairment of conditioned fear behaviours in adult rats treated with POLY I:C at P14 relative to SAL-treated controls.
Fig. 3.
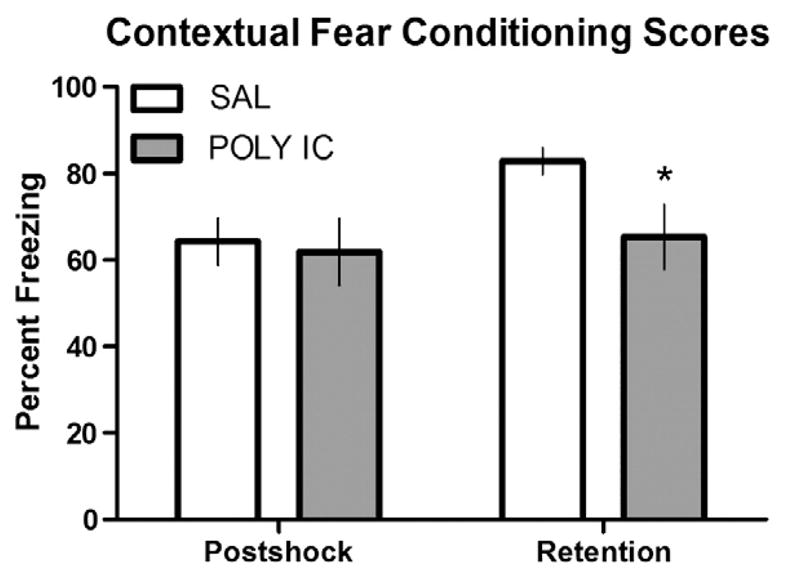
Deficits in contextual fear conditioning. Contextual fear conditioning scores presented as percent freezing during the postshock trial or the retention trial, for adult animals treated with either SAL (n=10) or POLY I:C (n=9) at P14. There was a significant (p<0.05; *) reduction in percent freezing during the retention trial in rats treated with POLY I:C.
NMDA and AMPA receptor subunit mRNA expression
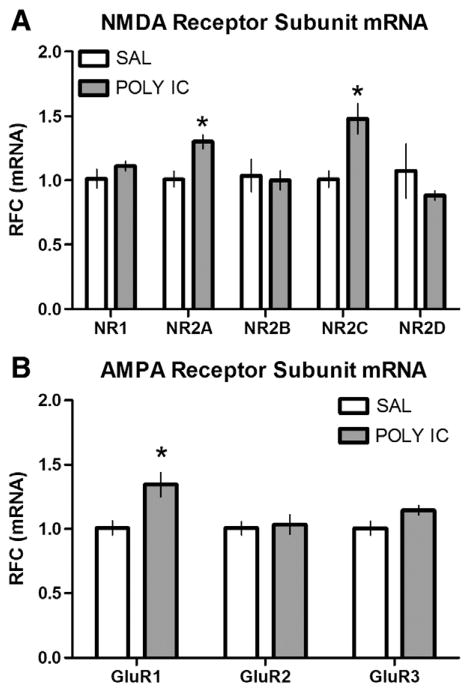
In order to examine whether a single ICV injection of POLY I:C influenced NMDA or AMPA receptor subunit expression in the hippocampus, we performed real-time RT-PCR. Adult rats given POLY I:C at P14 showed significantly higher levels of mRNA for NR2A (t=3.52, p<0.01), NR2C (t=3.49, p<0.01) and GluR1 (t=3.08, p<0.05) receptors compared to SAL-treated controls (Figs. 4A and B). We also noted a strong trend towards increased mRNA for the GluR3 (t=2.16, p=0.058) receptor in POLY I:C-treated rats compared to controls. No statistically significant differences were observed between the treatment groups for the remaining receptor subunits (NR1, NR2B, D and GluR2). Administration of minocycline at the same time as POLY I:C on P14 reversed the upregulation of NR2A, NR2C and GluR1 receptor subunits, such that mRNA expression levels (in RFC) were significantly (t≥6.11, p<0.01) lower than the SAL-treated group (NR2A 0.33±0.018; NR2C 0.55±0.037; GluR1 0.24±0.011). There were no statistically significant differences in the expression of GAPDH between the three treatments suggesting that reference (housekeeping) gene activity was not altered.
Fig. 4.
Increased NMDA and AMPA receptor subunit mRNA. Real-time RT-PCR data for (A) NMDA (NR1, NR2A, B, C and D) and (B) AMPA (GluR1, 2 and 3) receptor subunit mRNA from hippocampal (HPC) tissues taken from animals treated with either SAL or POLY I:C (10 μg/kg) at P14 (n=6/group). Data are expressed as a relative fold change (RFC). Rats given POLY I:C showed significantly (p<0.05; *) more mRNA levels of NR2A, NR2C, and GluR1 receptor subunits compared to controls.
POLY I:C induces fever at P14
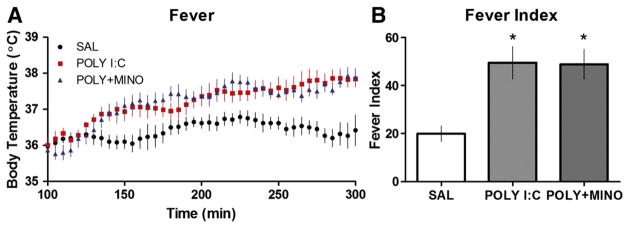
We injected either SAL, POLY I:C or POLY I:C plus minocycline and recorded body temperature for 5 h. During the first 90 min, there was marked variability in the temperature data collected from all groups recorded. This was attributed to (1) post-op recovery of the pups, including handling stress, (2) a delay in return to the dam (as per methodology), and (3) disturbing of maternal behaviours following return of the pups. Despite this, rats that received POLY I:C showed a conspicuous fever of about 1–1.5 °C which began about 2 h after injection and persisted until recordings were completed. A similar fever was noted for rats given POLY I:C plus minocycline suggesting that minocycline had no effect on fever per se (Figs. 5A, B). A one-way ANOVA demonstrated a significant interaction between treatment and fever index [F(2,16) =7.66; p < 0.01]. Post hoc analysis determined that both POLY I:C and POLY I:C plus minocycline treatment groups had higher fever index scores than SAL-treated controls, but that they did not differ significantly from one another.
Fig. 5.
ICV POLY I:C-induced fever. (A) Body temperature and (B) fever index scores of P14 rats (n=6/group) to ICV injection of SAL, POLY I:C (10 μg/rat) or POLY I:C (10 μg/rat) plus minocycline (90 mg/kg). Each data point represents the average body temperature every 5 min for a particular treatment group and plotted from 100 min to 300 min after injection. Rats treated with POLY I:C and POLY I:C plus minocycline showed fever responses (higher body temperatures) and significantly (p<0.05; *) greater fever index scores than controls.
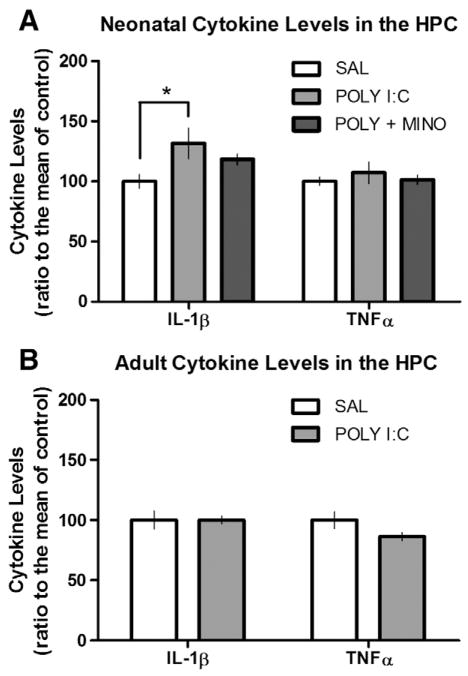
Hippocampal IL-1β levels acutely elevated during brain inflammation
We examined cytokine levels of IL-1β and TNFα from the hippocampus 6 h after injection of SAL, POLY I:C or POLY I:C plus minocycline at P14 to determine the amount of acute inflammation. The treatments resulted in significant changes in IL-1β (F(2,17) =3.52, p=0.05; Fig. 6A), but not TNFα, levels in the hippocampus. Post hoc analysis revealed that the POLY I:C-treated group had significantly higher IL-1β levels compared to controls. There was no significant difference in IL-1β levels between POLY I:C plus minocycline-treated rats and controls. In adult rats, there were no differences in the basal levels of either IL-1β or TNFα levels between SAL- and POLY I:C-treated rats (Fig. 6B).
Fig. 6.
Cytokine upregulation in the hippocampus. (A) Neonatal and (B) Adult hippocampal (HPC) cytokine (IL-1β and TNFα) levels from rats treated with SAL, POLY I:C (10 μg/rat) or POLY I:C (10 μg/rat) plus minocycline (90 mg/kg) on P14. Rats given POLY I:C showed significantly (p<0.05; *) more IL-1β in the hippocampus compared to controls 6 h later, however, this effect was attenuated by the concurrent administration of minocycline (n=6/group). There were no differences in the amounts of IL-1β or TNFα in the hippocampus between SAL or POLY I:C-treated rats in adulthood (n=5/group).
Discussion
In the present study, we show that rats given a single ICV injection of POLY I:C during development display a long-lasting increase in seizure susceptibility that can be attenuated by concurrent peripheral administration of minocycline, which may act by suppressing the pro-inflammatory cytokine IL-1β within the hippocampus. In adulthood, these changes in seizure threshold were not accompanied by long-term alterations in cytokine (IL-1β, TNFα) levels, but did result in mild deficits in contextual fear conditioning which may be associated with significant increases in mRNA levels of select excitatory glutamate receptor subunits including NR2A, NR2C and GluR1 which were reversed by administration of minocycline. To our knowledge, this is the first study to mimic a viral CNS infection using a synthetic non-replicating viral-like compound, with an emphasis on measuring seizure susceptibility (Stringer, 2006).
Other models that used active viral infections (Herpes simplex virus; HSV) have also revealed long-term differences in seizure susceptibility that were due to changes in the physiological properties of neurons within the hippocampus (Wu et al., 2003). In organotypic hippocampal cultures, HSV caused mossy-fiber sprouting, neuronal loss and spontaneous epileptiform activity (Chen et al., 2004). The major limitation of using active viral agents such as HSV to model encephalitis is the extreme variability in the immunological and neurological responses. For example, rodents with corneal or intranasal inoculations of HSV were reported to exhibit a wide continuum of neurological features from mild to severe impairment (Beers et al., 1993; Wu et al., 2003). Although increased seizure susceptibility was reported, up to 50% of the subjects died. Other models of viral encephalitis report 100% mortality (Lehrmann et al., 2008). These studies highlight the variable nature of infectious disease models and help rationalize the use of non-replicating viral mimetics like POLY I:C that act through the same toll-like-receptor 3 (Edelmann et al., 2004), as all other viral infections (Alexopoulou et al., 2001), including HSV (Zhang et al., 2007). Dozens of viral agents can cause encephalitis (Solomon et al., 2007), and many suspected cases of infection have no conclusive isolation of the virus due to the short period of viremia, difficulty in obtaining biopsy, and lack of specificity for culturing viruses. We reasoned that the results gained from using the POLY I:C compound could yield broad conclusions about the mechanisms of viral encephalitis and seizures in the absence of any mortality.
The finding that minocycline can block the increase in seizure susceptibility is interesting from many perspectives. First, although minocycline is regarded as a multifaceted anti-inflammatory compound, it did not influence the fever produced by POLY I:C (see Fig. 5). Fever is the regulated increase in body temperature driven by the cyclo-oxygenase and prostaglandin signalling pathways within the hypothalamus (Blatteis, 2000). Thus, the acute fever in the neonate is not responsible for the increased seizure susceptibility in adulthood. The anti-inflammatory properties of minocycline appears to stem from its ability to decrease cytokine release from microglial cells (Tikka et al., 2001), therefore it does not have a classic anti-pyretic role and did not influence the POLY I:C-induced fever. The magnitude of the fever evoked by the viral mimetic is similar to what has been reported by other immune stimulants like LPS, which is considered a mild immunological challenge relative to other pyretic agents. However, even such seemingly innocuous immune challenges during development have been known to produce a variety of long-term effects on physiological functions (Ellis et al., 2005; Spencer et al., 2005; Walker et al., 2004), including seizures (Galic et al., 2008).
Identifying the presence of a febrile response to POLY I:C is important as it helps validate its use as a model of viral encephalitis where fever is one of the most consistent presenting symptoms in patients with encephalitis. Other groups have identified a complementary role of neonatal hyperthermic (febrile) seizures as a precipitant of changes in neuronal excitability and behavioural dysfunction (Baram et al., 1997; Chen et al., 1999; Dube et al., 2000, 2006; Liebregts et al., 2002; Tsai and Leung, 2006). Although fever and hyperthermia are distinct physiological processes (for review see Roth et al., 2006), they do share some characteristics with regard to cytokine signalling (Bender and Baram, 2007). For instance, antagonism of cytokine pathways can interfere with acute seizure generation (Dube et al., 2005; Heida and Pittman, 2005), and attenuate the long-term consequences of inflammation on seizure susceptibility as found here, as well as in previous work (Galic et al., 2008). Therefore, the critical feature of hyperthermia and fever with respect to early-life interventions could be the mutual expression of cytokines which encourage increased neuronal excitability.
POLY I:C is thought to mediate inflammation by stimulating IL-1β production (Fortier et al., 2004). In our study, we observed a similar pattern of pro-inflammatory cytokine release within the brain. There was a marked increase in IL-1β 6 h after ICV injection of POLY I:C and no change in TNFα levels relative to SAL-controls. IL-1β has been suspected to play an important role in the epileptogenic brain as many reports have determined that amplifying or interfering with its production can markedly modulate seizure propensity or progression (Dube et al., 2005; Heida et al., 2005; Ravizza et al., 2006; Ravizza et al., 2008a,b). Most interestingly, changes in IL-1β have been shown to modulate glutamatergic receptor expression. For example, IL-1β increases NMDA receptor sensitivity and function by augmenting phosphorylation and thus exacerbates neuronal degeneration (Balosso et al., 2008; Viviani et al., 2003, 2006). Our findings of changes in excitatory receptor subunit mRNA expression after brain inflammation, if reflected by number of active receptors in the membrane, may also be one reason why seizure susceptibility in POLY I:C-treated rats increased. This is consistent with what has already been hypothesized, that HSV-mediated hippocampal injury may be precipitated by dysfunctional glutamate regulation (Theodore et al., 2008), possibly through NMDA receptors (Nair et al., 2007). In previous experiments, we have also found that NMDA receptors were strongly influenced by peripheral inflammation caused by LPS (Harre et al., 2008). Although there were no differences in NR2B mRNA, this receptor could still be involved if the phosphorylation status of the receptor has changed (Viviani et al., 2003). The relationship between cytokines and changes in brain excitability is being increasingly acknowledged and the data point towards changes in glutamatergic transmission as the source of these effects (Vezzani et al., 2008).
The present data showing that minocycline may be effective for attenuating brain inflammation could be promising for clinicians trying to treat patients suffering from viral encephalitis. Microglia are increasingly being targeted as one of the main immunotherapeutic targets for other neurological diseases (Villoslada et al., 2008), as they represent one of the most important cell types in the cascade of cytokine release within the brain (Lokensgard et al., 2002). The dose of minocycline used in the present study has been shown effective in attenuating LPS-induced white matter injury (periventricular leukomalacia) via reduction in pro-inflammatory cytokine (IL-1β and TNFα) release in young rats (Fan et al., 2005). Dosages in this range are known to be well-tolerated by patients (Kloppenburg et al., 1995), and cost effective (Michaelis et al., 2007). Drugs like minocycline that can be given systemically and can cross the blood brain barrier could prove most useful in the treatment of encephalitis (Holbrook and Gowen, 2008). Nonetheless, it is important to note that administration of minocycline concurrently with POLY I:C actually reduced glutamate receptor mRNA to levels below that seen with saline. It may be important in the future to determine if minocycline itself has any effect on development or on stability of mRNA. It is unlikely to be a non-specific toxic effect, as fever was not affected and previous studies suggest it does not affect numbers or activation of microglia in the absence of inflammation.
The enhanced cytokinergic response within the brains of rats given ICV POLY I:C is consistent with a CNS inflammatory response, however, it is the location of the inflammation that is most interesting. For example, viral encephalitis models using live inoculation with HSV in mice and rats have shown viral antigens in the hippocampus, amygdala, entorhinal, and pyriform cortex (Beers et al., 1993; Wu et al., 2003). In addition, post-mortem sections from the temporal lobes of patients who suffered seizures as a result of HSV infection show robust pro-inflammatory infiltrates relative to other brain regions (Chadwick, 2005; Esiri, 1982; Jay et al., 1998; Theodore et al., 2008; Yamada et al., 2003). These findings have led some to suspect that the epileptogenicity of viral encephalitis results from the recruitment of the hippocampus causing long-term excitability (Chen et al., 2004; Wu et al., 2003), even in the absence of acute or chronic cytological injury (Beers et al., 1993; Rempel et al., 2005); while others have proposed that marked neuronal damage contributes to the hyperexcitable state (Pearce et al., 1996).
The proclivity of viral infection sequelae for the temporal lobes may explain the robust memory deficits found in some patients with HSV encephalitis during neuropsychological testing (Gordon et al., 1990; Kapur et al., 1994). However, when we tested adult rats with a history of brain inflammation, they showed only mild deficits in behaviours mediated by mesial temporal lobe structures. Performance in the water maze was preserved, however, contextual fear conditioning was impaired in POLY I:C-treated rats during the retention task 24 h later. Although fear conditioning to context has a strong hippocampal-dependency (Sanders et al., 2003), much like water maze learning (for review see D’hooge and De Deyn, 2001), it also relies heavily on amygdalar function (Fendt and Fanselow, 1999). If the hippocampus was the most strongly affected of the mesial temporal structures, then one might expect to see deficits in both the water maze and fear conditioning tests in conjunction with the alterations in glutamate receptors which underlie acquisition of hippocampal-dependent memories (Morris et al., 1986; Quinn et al., 2005). This data suggests the potential involvement of other limbic structures, such as the amygdala, in the CNS consequences of immune challenge. In support of this, previous experiments using LPS at P14 also showed a relative sensitivity of the amygdala over the hippocampus, in relation to neuronal cell loss after cerebral ischemia (Spencer et al., 2006). In future experiments it may be advisable to investigate glutamate receptor function in the amygdala.
Although standard treatment for viral encephalitis involves acyclovir, an inhibitor of DNA polymerase (Whitley et al., 1986), poor long-term neurologic and cognitive outcomes still occur (Elbers et al., 2007; Hokkanen et al., 1996; Lahat et al., 1999). However, some have reported that although acyclovir can reduce CNS viral titres to undetectable levels, inflammatory markers remain unaffected (Lund-berg et al., 2008). These reports emphasise the urgent need for alternative anti-inflammatory adjuncts to the treatment of viral encephalitis over and above simply suppression of viral replication and viral cytotoxicity (Skoldenberg et al., 2006), especially since the prevalence for some variants of HSV infection is almost 100% in childhood (Caserta et al., 2001; Johnson, 1982).
Acknowledgments
This work was supported by funding from the Canadian Institutes of Health Research (CIHR; QJP). M. A. Galic was a Killam Scholar and supported by scholarships from CIHR, the Alberta Heritage Foundation for Medical Research (AHFMR), and the Natural Sciences and Engineering Research Council of Canada (NSERC). K. Riazi was funded by an AHFMR Post-Doctoral Fellowship and A. K. Henderson was supported by a Ruby Doctoral Scholarship from the University of Calgary. Q. J. Pittman is an AHFMR Medical Scientist. The authors would like to thank N. M. Fournier for his comments on previous versions of the manuscript.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407–1410. doi: 10.1212/wnl.38.9.1407. [DOI] [PubMed] [Google Scholar]
- Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980;176:1061–1068. [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain. 2008;131:3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Gerth A, Schultz L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Res Dev Brain Res. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, Tiraboschi E, Musazzi L, Giambelli R, Gennarelli M, Racagni G, Barlati S. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–720. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Schaefer DC, Rose JW, Stroop WG. Neuropathology of herpes simplex virus encephalitis in a rat seizure model. J Neuropathol Exp Neurol. 1993;52:241–252. doi: 10.1097/00005072-199305000-00008. [DOI] [PubMed] [Google Scholar]
- Beghi E, Nicolosi A, Kurland LT, Mulder DW, Hauser WA, Shuster L. Encephalitis and aseptic meningitis, Olmsted County, Minnesota, 1950–1981: I. Epidemiology. Ann Neurol. 1984;16:283–294. doi: 10.1002/ana.410160304. [DOI] [PubMed] [Google Scholar]
- Bender RA, Baram TZ. Epileptogenesis in the developing brain: what can we learn from animal models? Epilepsia. 2007;48 (Suppl 5):2–6. doi: 10.1111/j.1528-1167.2007.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteis CM. The afferent signalling of fever. J Physiol. 2000;526 (Pt. 3):470. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829–833. doi: 10.1086/322691. [DOI] [PubMed] [Google Scholar]
- Chadwick DR. Viral meningitis. Br Med Bull. 2005;75–76:1–14. doi: 10.1093/bmb/ldh057. [DOI] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SF, Huang CC, Wu HM, Chen SH, Liang YC, Hsu KS. Seizure, neuron loss, and mossy fiber sprouting in herpes simplex virus type 1-infected organotypic hippocampal cultures. Epilepsia. 2004;45:322–332. doi: 10.1111/j.0013-9580.2004.37403.x. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Fang PC, Chow JC. Clinical characteristics and prognostic factors of postencephalitic epilepsy in children. J Child Neurol. 2006;21:1047–1051. doi: 10.1177/7010.2006.00223. [DOI] [PubMed] [Google Scholar]
- D’hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Davison KL, Crowcroft NS, Ramsay ME, Brown DW, Andrews NJ. Viral encephalitis in England, 1989–1998: what did we miss? Emerg Infect Dis. 2003;9:234–240. doi: 10.3201/eid0902.020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Elbers JM, Bitnun A, Richardson SE, Ford-Jones EL, Tellier R, Wald RM, Petric M, Kolski H, Heurter H, MacGregor D. A 12-year prospective study of childhood herpes simplex encephalitis: is there a broader spectrum of disease? Pediatrics. 2007;119:e399–e407. doi: 10.1542/peds.2006-1494. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–1521. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- Esiri MM. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci. 1982;54:209–226. doi: 10.1016/0022-510x(82)90183-6. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci. 2008;28:6904–6913. doi: 10.1523/JNEUROSCI.1901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B, Selnes OA, Hart J, Jr, Hanley DF, Whitley RJ. Long-term cognitive sequelae of acyclovir-treated herpes simplex encephalitis. Arch Neurol. 1990;47:646–647. doi: 10.1001/archneur.1990.00530060054017. [DOI] [PubMed] [Google Scholar]
- Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27:644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16:1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46:1906–1913. doi: 10.1111/j.1528-1167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Heida JG, Boisse L, Pittman QJ. Lipopolysaccharide-induced febrile convulsions in the rat: short-term sequelae. Epilepsia. 2004;45:1317–1329. doi: 10.1111/j.0013-9580.2004.13704.x. [DOI] [PubMed] [Google Scholar]
- Heida JG, Teskey GC, Pittman QJ. Febrile convulsions induced by the combination of lipopolysaccharide and low-dose kainic acid enhance seizure susceptibility, not epileptogenesis, in rats. Epilepsia. 2005;46:1898–1905. doi: 10.1111/j.1528-1167.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- Hokkanen L, Poutiainen E, Valanne L, Salonen O, Iivanainen M, Launes J. Cognitive impairment after acute encephalitis: comparison of herpes simplex and other aetiologies. J Neurol Neurosurg Psychiatry. 1996;61:478–484. doi: 10.1136/jnnp.61.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook MR, Gowen BB. Animal models of highly pathogenic RNA viral infections: encephalitis viruses. Antiviral Res. 2008;78:69–78. doi: 10.1016/j.antiviral.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Irani DN, Prow NA. Neuroprotective interventions targeting detrimental host immune responses protect mice from fatal alphavirus encephalitis. J Neuropathol Exp Neurol. 2007;66:533–544. doi: 10.1097/01.jnen.0000263867.46070.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay V, Hwang P, Hoffman HJ, Becker LE, Zielenska M. Intractable seizure disorder associated with chronic herpes infection. HSV1 detection in tissue by the polymerase chain reaction. Childs Nerv Syst. 1998;14:15–20. doi: 10.1007/s003810050167. [DOI] [PubMed] [Google Scholar]
- Johnson RT. Viral Infections of the Nervous System. Raven Press; New York: 1982. [Google Scholar]
- Kapur N, Barker S, Burrows EH, Ellison D, Brice J, Illis LS, Scholey K, Colbourn C, Wilson B, Loates M. Herpes simplex encephalitis: long term magnetic resonance imaging and neuropsychological profile. J Neurol Neurosurg Psychiatry. 1994;57:1334–1342. doi: 10.1136/jnnp.57.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988–1997. Clin Infect Dis. 2002;35:175–182. doi: 10.1086/341301. [DOI] [PubMed] [Google Scholar]
- Kloppenburg M, Mattie H, Douwes N, Dijkmans BA, Breedveld FC. Minocycline in the treatment of rheumatoid arthritis: relationship of serum concentrations to efficacy. J Rheumatol. 1995;22:611–616. [PubMed] [Google Scholar]
- Kramer AH, Bleck TP. Neurocritical care of patients with central nervous system infections. Curr Treat Options Neurol. 2008;10:201–211. doi: 10.1007/s11940-008-0022-0. [DOI] [PubMed] [Google Scholar]
- Lahat E, Barr J, Barkai G, Paret G, Brand N, Barzilai A. Long term neurological outcome of herpes encephalitis. Arch Dis Child. 1999;80:69–71. doi: 10.1136/adc.80.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wong CK, Yang MS, Yung KK. Changes in expression of N-methyl-D-aspartate receptor subunits in the rat neostriatum after a single dose of antisense oligonucleotide specific for N-methyl-D-aspartate receptor 1 subunit. Neuroscience. 2000;98:493–500. doi: 10.1016/s0306-4522(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Guidetti P, Love A, Williamson J, Bertram EH, Schwarcz R. Glial activation precedes seizures and hippocampal neurodegeneration in measles virus-infected mice. Epilepsia. 2008;49 (Suppl 2):13–23. doi: 10.1111/j.1528-1167.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- Liebregts MT, McLachlan RS, Leung LS. Hyperthermia induces age-dependent changes in rat hippocampal excitability. Ann Neurol. 2002;52:318–326. doi: 10.1002/ana.10285. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lokensgard JR, Cheeran MC, Hu S, Gekker G, Peterson PK. Glial cell responses to herpesvirus infections: role in defense and immunopathogenesis. J Infect Dis. 2002;186 (Suppl 2):S171–S179. doi: 10.1086/344272. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DA, Kim J, Spencer DD, Spencer SS. Characteristics of intractable seizures following meningitis and encephalitis. Neurology. 1992;42:1513–1518. doi: 10.1212/wnl.42.8.1513. [DOI] [PubMed] [Google Scholar]
- McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Kleinschmidt MC, Doerr HW, Cinatl J., Jr Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981–986. doi: 10.1093/jac/dkm307. [DOI] [PubMed] [Google Scholar]
- Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. 2008;105:1582–1595. doi: 10.1111/j.1471-4159.2008.05238.x. [DOI] [PubMed] [Google Scholar]
- Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49 (Suppl 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8(+) T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav Immun. 2007;21:791–806. doi: 10.1016/j.bbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Nicolosi A, Hauser WA, Beghi E, Kurland LT. Epidemiology of central nervous system infections in Olmsted County, Minnesota, 1950–1981. J Infect Dis. 1986;154:399–408. doi: 10.1093/infdis/154.3.399. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Steffensen SC, Paoletti AD, Henriksen SJ, Buchmeier MJ. Persistent dentate granule cell hyperexcitability after neonatal infection with lymphocytic choriomeningitis virus. J Neurosci. 1996;16:220–228. doi: 10.1523/JNEUROSCI.16-01-00220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Rantakallio P, Leskinen M, von Wendt L. Incidence and prognosis of central nervous system infections in a birth cohort of 12,000 children. Scand J Infect Dis. 1986;18:287–294. doi: 10.3109/00365548609032339. [DOI] [PubMed] [Google Scholar]
- Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Lucas SM, Balosso S, Bernardino L, Ku G, Noe F, Malva J, Randle JC, Allan S, Vezzani A. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47:1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008a;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis. 2008b;31:327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Rempel JD, Quina LA, Blakely-Gonzales PK, Buchmeier MJ, Gruol DL. Viral induction of central nervous system innate immune responses. J Virol. 2005;79:4369–4381. doi: 10.1128/JVI.79.7.4369-4381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Burns SM, Tyler KL. Minocycline delays disease onset and mortality in reovirus encephalitis. Exp Neurol. 2005;192:331–339. doi: 10.1016/j.expneurol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Sharbrough FW, Hauser WA, Annegers JF, Schoenberg BS. Risk factors for complex partial seizures: a population-based case-control study. Ann Neurol. 1987;21:22–31. doi: 10.1002/ana.410210106. [DOI] [PubMed] [Google Scholar]
- Roth J, Rummel C, Barth SW, Gerstberger R, Hubschle T. Molecular aspects of fever and hyperthermia. Neurol Clin. 2006;24:421–439. doi: 10.1016/j.ncl.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schmutzhard E. Viral infections of the CNS with special emphasis on herpes simplex infections. J Neurol. 2001;248:469–477. doi: 10.1007/s004150170155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Prabhakar S. The association between central nervous system (CNS) infections and epilepsy: epidemiological approaches and microbiological and epileptological perspectives. Epilepsia. 2008;49 (Suppl 6):2–7. doi: 10.1111/j.1528-1167.2008.01749.x. [DOI] [PubMed] [Google Scholar]
- Skoldenberg B, Aurelius E, Hjalmarsson A, Sabri F, Forsgren M, Andersson B, Linde A, Strannegard O, Studahl M, Hagberg L, Rosengren L. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol. 2006;253:163–170. doi: 10.1007/s00415-005-0941-6. [DOI] [PubMed] [Google Scholar]
- Solomon T, Hart IJ, Beeching NJ. Viral encephalitis: a clinician’s guide. Pract Neurol. 2007;7:288–305. doi: 10.1136/jnnp.2007.129098. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006;26:456–467. doi: 10.1038/sj.jcbfm.9600206. [DOI] [PubMed] [Google Scholar]
- Stringer JL. Models available for infection-induced seizures. In: Pitkanen A, Schartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier, Academic Press; San Diego: 2006. pp. 521–526. [Google Scholar]
- Theodore WH, Epstein L, Gaillard WD, Shinnar S, Wainwright MS, Jacobson S. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49:1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai ML, Leung LS. Decrease of hippocampal GABA B receptor-mediated inhibition after hyperthermia-induced seizures in immature rats. Epilepsia. 2006;47:277–287. doi: 10.1111/j.1528-1167.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Villoslada P, Moreno B, Melero I, Pablos JL, Martino G, Uccelli A, Montalban X, Avila J, Rivest S, Acarin L, Appel S, Khoury SJ, McGeer P, Ferrer I, Delgado M, Obeso J, Schwartz M. Immunotherapy for neurological diseases. Clin Immunol. 2008;128:294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Viral encephalitis. N Engl J Med. 1990;323:242–250. doi: 10.1056/NEJM199007263230406. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch’ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med. 1977;297:289–294. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Alford CA, Hirsch MS, Schooley RT, Luby JP, Aoki FY, Hanley D, Nahmias AJ, Soong SJ. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- Wu HM, Huang CC, Chen SH, Liang YC, Tsai JJ, Hsieh CL, Hsu KS. Herpes simplex virus type 1 inoculation enhances hippocampal excitability and seizure susceptibility in mice. Eur J Neurosci. 2003;18:3294–3304. doi: 10.1111/j.1460-9568.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- Yamada S, Kameyama T, Nagaya S, Hashizume Y, Yoshida M. Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J Neurol Neurosurg Psychiatry. 2003;74:262–264. doi: 10.1136/jnnp.74.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]