Abstract
This study is to investigate the long-term effects of PEG-PLA nano artificial cells containing hemoglobin (NanoRBC) on renal function and renal histology after 1/3 blood volume top loading in rats. The experimental rats received one of the following infusions: NanoRBC in Ringer lactate, Ringer lactate, stroma-free hemoglobin (SFHB), polyhemoglobin (PolyHb), autologous rat whole blood (rat RBC). Blood samples were taken before infusions and on days 1, 7 and 21 after infusions for biochemistry analysis. Rats were sacrificed on day 21 after infusions and kidneys were excised for histology examination. Infusion of SFHB induced significant decrease in renal function damage evidenced by elevated serum urea, creatinine and uric acid throughout the 21 days. Kidney histology in SFHb infusion group revealed focal tubular necrosis and intraluminal cellular debris in the proximal tubules, whereas the glomeruli were not observed damaged. In all the other groups, NanoRBC, PolyHb, Ringer lactate and rat RBC, there were no abnormalities in renal biochemistry or histology. In conclusion, injection of NanoRBC did not have adverse effects on renal function nor renal histology.
Keywords: Nanotechnology, nanomedicine, nanobiotechnology, PEG-PLA nano-capsules, artificial red blood cells, renal function, renal histology, kidney, PolyHb
INTRODUCTION
The first artificial RBCs prepared by microencapsulation of hemolysate have oxygen dissociation curve similar to RBCs [1]. However, these artificial RBCs, even with diameters of down to one micron, survived for a short time in the circulation after intravenous injection [2]. Our study shows that the long circulation time of RBCs is due to the presence of neuraminic acid on the membrane [3]. This led us to study the effects of changing surface properties of the artificial RBCs [2,3]. This has resulted in significant increases in circulation time, but still not sufficient for clinical uses [3]. Djordjevich and Miller [4] increased the circulation time further by preparing smaller lipid membrane Hb artificial cells with diameter of about 0.2 micron. One of the next important developments is the incorporation of PEG onto the lipid membrane artificial cells, resulting in useful increases in the circulation time [5]. Ongoing efforts are being carried out in preclinical studies towards clinical trial [6,7]. We have used a biodegradable polymer, polylactic acid (PLA), for the microencapsulation of Hb, enzymes and other biologically active material since 1976 [8]. More recently, we started to prepare nanodimension artificial RBCs of less than 200 nanometer mean diameter using PLA membrane and other biodegradable polymers [9–11]. We have replaced most of the 6g/dl of lipid membrane in Hb lipid vesicles with 1.6g/dl of biodegradable polymeric membrane material. This marked decrease in the lipid component would lessen effects on the RES and lessen lipid peroxidation in ischemia-reperfusion. Unlike lipid membrane, biodegradable polymeric membrane is permeable to glucose. Thus, the inclusion of RBC enzymes prevents MetHb formation even at 37°C, and we can also convert MetHb to Hb at 37°C [12–14]. In addition, unlike lipid membrane, the nanocapsule membrane allows plasma factors like ascorbic acid to enter the nanocapsules to prevent MetHb formation. In order to increase the circulation time, we synthesized a new PEG-PLA copolymer to prepare NanoHb [12]. After extensive research, we now have a circulation time in rats that is double that of glutaraldehye crosslinked PolyHb [12–14].
In this report, we carried out studies in animals to investigate the effect of 1/3 volume toploading infusions on renal function and histology when followed on days 1, 7 and 21 after infusions.
MATERIALS AND METHODS
Preparation of Hb Solution
SFHb was prepared by the method as previously described [14]. Briefly, SFHb was obtained by hypotonic hemolysis of bovine RBCs and it was made stroma-free by toluene extraction and high-speed centrifugation. The resulting solution contained 10–15 g Hb/dl.
Preparation of PolyHb
This has been described in detail [14]. Briefly, 0.5 M glutaraldehyde was added and allowed to react for 24 hr at 4°C under anaerobic conditions with constant stirring. The reaction was stopped with excess 2.0 M lysine HCl at a molar ratio of 100:1 (lysine/Hb). Removal of reactants and free Hb molecules was carried out using dialysis and molecular exclusion chromatography [14].
Preparation of PEG-PLA NanoHb
This has been described in detail [14]. Briefly, PEG-PLA copolymer was prepared from 1.5 g of D,L-PLA [M.W. 15,000] and 0.75 g of methox-ypolyethylene glycol [M.W. 2000] [21]. Just before use, 150 mg of the PEG-PLA copolymer were dissolved in 8 ml acetone. Fifty mg of hydrogenated soybean phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) were dissolved in 4 ml ethanol. The two solutions were mixed and emulsified with the PolyHb solution to form NanoHb. This could be followed by glutaraldehyde crosslinking to reduce the permeability of the membrane [14]. This was then followed by vacuum solvent extraction to remove the organic solvent and high porosity ultrafiltration to remove unencapsulated Hb or PolyHb [14].
Toploading
Twenty male Sprague-Dawley rats in the weight range of 300±40g were used. They were randomly assigned into 5 groups. Each group received one of the following solutions: (1) NanoHb in Ringer lactates; (2) LactRing as control; (3) SFHb; (4) PolyHb; (5) Autologous rat RBCs.
Rats were anesthetized with sodium pentobarbitum (60 mg/kg), then unilateral femoral vein was cannulated using 3 cm length PE10 tubing (0.28 mm inner, 0.61 mm outer diameter, Clay Adams, Becton Dickinson, Sparks, MD, USA). Each rat received intravenous infusion of 33% of the total blood volume of one of the above 5 solutions. Total blood volume in ml was calculated as 6.5% of body weight in grams.
Blood Chemistry
Blood samples were taken from rats before injections, and on days 1, 7, 21 after injections. Plasma samples were stored in −76°C freezer until tested in the analytical laboratory of the McIntyre Animal Centre, McGill University. Blood levels of urea, creatinine, calcium, inorganic phosphorus and electrolytes were determined.
Histology Study
At day 21 after injection of various solutions, rats in all groups were sacrificed and kidneys were excised for histology examination. The kidney was fixed in 10% formalin solution, embedded in paraffin, then cut into serial 5-μm thick sections, mounted on slides, then sections were stained with hematoxylin and eosin (H&E) for histology examination.
Statistics
Blood chemistry assays were presented as mean±S.E. For multiple comparisons of biochemical data in all groups, we performed one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered significant.
RESULTS
Biochemistry
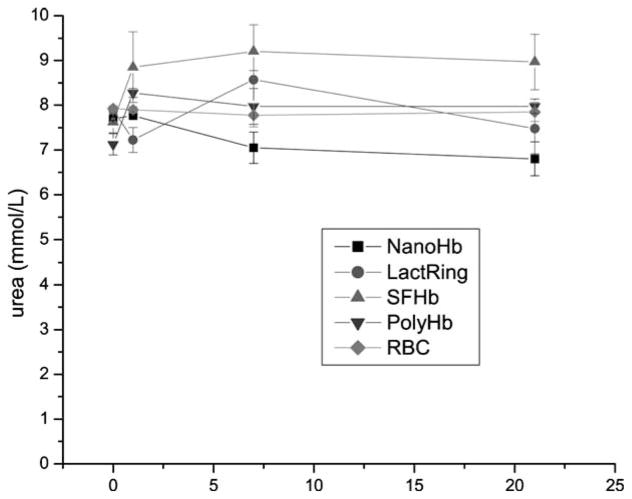
Toploading of PEG-PLA NanoRBC into rats did not result in any elevation of systemic urea (Figure 1). LactRing, PolyHb or autologous RBCs also did not result in any elevations. On the other hand, similar toploading using SFHb resulted in the elevation of urea one day after infusion and the elevated levels were maintained on day 7 and day 21 (Figure 1).
Figure 1.
Systemic levels of urea in rats infused with 33% volume of NanoRBC, LactRing, SFHb, PolyHb or autologous RBC (Mean±S.E.).
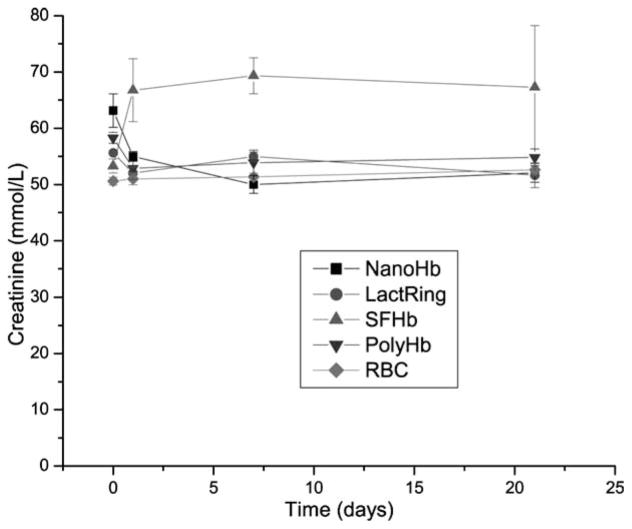
Figure 2 shows that toploading of PEG-PLA NanoHb into rats did not result in any elevation of systemic creatinine. LactRing, PolyHb or autologous RBCs also did not result in any elevations. Toploading using SFHb resulted in the elevation of creatinine one day after infusion and the elevated levels were maintained on day 7 and day 21 (Figure 2).
Figure 2.
Systemic levels of creatinine in rats infused with 33% volume of NanoRBC, LactRing, SFHb, PolyHb or autologous RBC (Mean±S.E.).
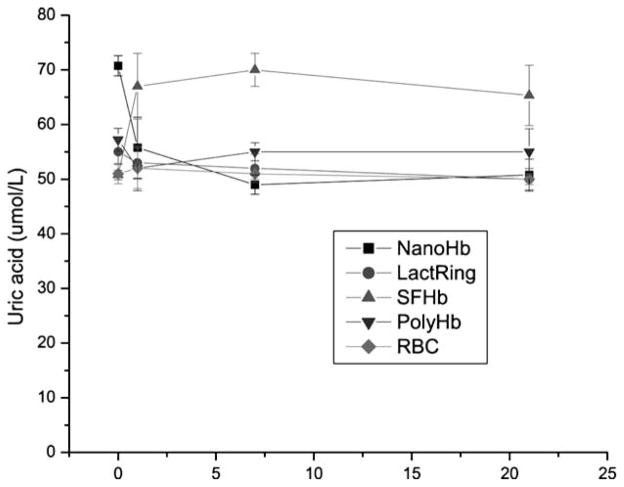
Figure 3 shows that toploading of PEG-PLA NanoRBC into rats did not result in any elevation of uric acid. LactRing, PolyHb or autologous RBCs also did not result in any elevations. But similar toploading using SFHb resulted in the elevation of uric acid one day after infusion and the elevated levels were maintained on day 7 and day 21 (Figure 3).
Figure 3.
Systemic levels of uric acid in rats infused with 33% volume of NanoHb, LactRing, SFHb, PolyHb or autologous RBC (Mean±S.E.).
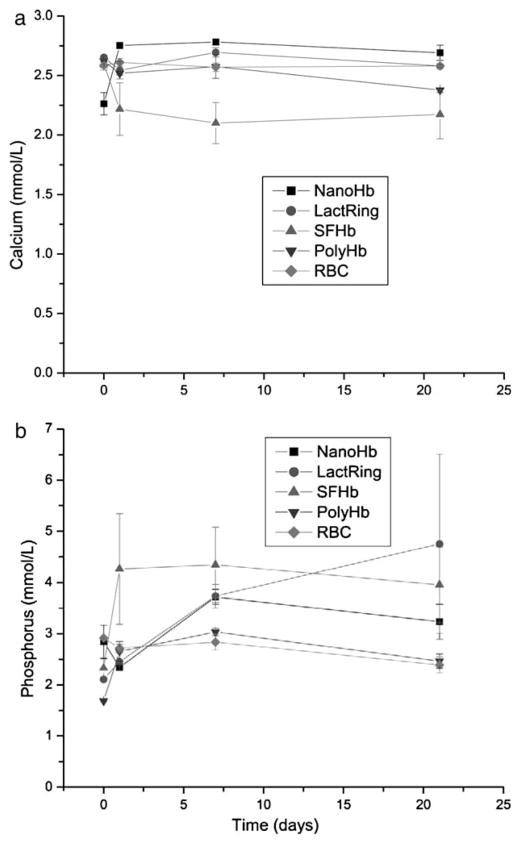
Figure 4a and Figure 4b show that there were no abnormalities in the plasma calcium and phosphate levels in rats of all infusion groups throughout the 21 days (Figure 4).
Figure 4.
Systemic levels of calcium (a), phosphorus (b) in rats infused with 33% volume of NanoRBC, LactRing, SFHb, PolyHb or RBC (Mean±S.E.).
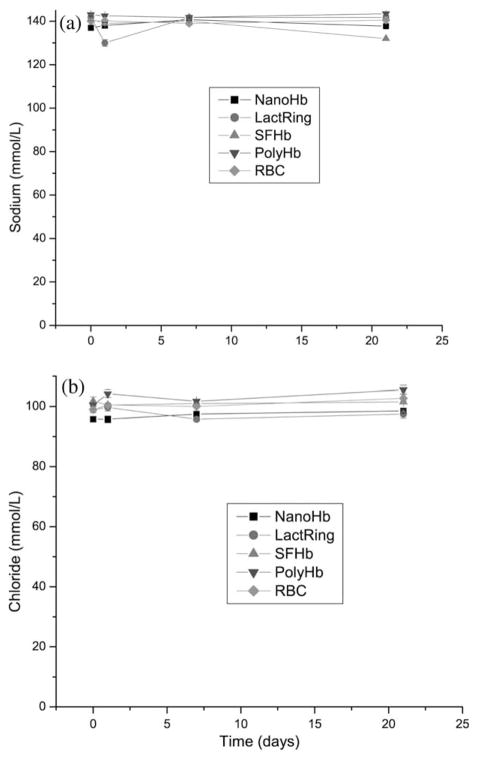
There were no abnormalities in the plasma sodium and chloride levels in rats of all infusion groups throughout the 21 days (Figure 5 a and b).
Figure 5.
Systemic levels of calcium sodium (a) and chloride (b) in rats infused with 33% volume of NanoHb, LactRing, SFHb, PolyHb or RBC (Mean±S.E.).
Renal Histology
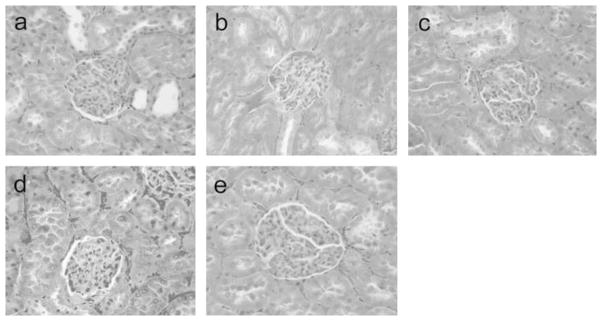
The renal glomeruli were not observed remarkably damaged in all infusion groups, with normal number of renal glomerulus, no glomerulus necrosis, distention and degeneration (Figure 6).
Figure 6.
Histology of renal glomeruli in rats toploading with NanoRBC (a), LactRing (b), SFHb (c), PolyHb (d) or RBC (e). Under light microscope, the renal glomeruli in rats of all groups did not show abnormality with normal number of renal glomeruli, no glomerular necrosis, distention or degeneration. H&E staining, ×400.
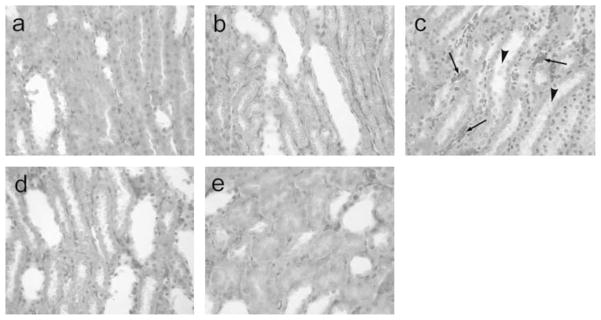
When the renal tubular system was examined in rats infused with SFHB, proximal tubular necrosis was observed, and intraluminal cellular debris was found in the proximal tubules, showing that the proximal necrosis occurrence (Figure 7c). In all the other groups, there were no tubular lesions in the kidneys, no protein or other sediments within the tubular lumens (Figure 7a, b, d, e).
Figure 7.
Histology of renal tubular system in rats toploading with NanoHb (a), LactRing (b), SFHb (c), PolyHb (d) or RBC (e). Under light microscope, in the groups a, b, d and e, the tubular system showed normal, no protein or other sediments within the tubular lumens. However, in the rats infused with SFHb (d), the proximal tubular necrosis was observed (arrows), and necrotic cell debris presented in the lumens of proximal tubules (arrow head), showing the ongoing necrosis in the kidney, although 3 weeks after SFHB injection. H&E staining, ×400.
DISCUSSION
Strom-free hemoglobins are readily filtered at the glomerulus. In the tubular lumen hemglobin causes hemoglobin-associated nephrotoxicity. In our present study we also observe that SFHb causes renal function damage and proximal tubular obstruction and necrosis. The chemical cross-linking of hemoglobin with glutaraldehyde into polyhemoglobin when infused did not have abnormal renal function or histology. This study shows that PEG-PLA Nano artificial bells containing Hb also did not induce renal function disorders, and there were no kidney histology abnormalities.
Polymers for forming nanocapsule membranes have to be nontoxic and must be degradable in the body into nontoxic degradation products. Many polymers are not biodegradable and some biodegradable ones like alkyl-cyanoacrylates form toxic degradation products. Other potentially useful biodegradable polymers may release toxic residual monomers or chemicals used in polymerization. Chang’s original solvent evaporation method used to prepare PLA microcapsules avoids the need for polymerization [8]. PLA and related polyesters like polyglycolic acids have been approved by the FDA for routine clinical uses in humans as suture materials, bone graft, drug carriers and other areas with no adverse or toxic effects. In the case of PEG, it has already been approved by the FDA in routine use as PEG-enzymes and PEG-drugs. Compared to PEG-Hb, a much smaller amount of PEG is needed for the PEG-PLA NanoHb. Thus, it is not surprising that NanoHb did not show adverse effects related to renal function and renal histology. In another study to be reported, we found that PEG-PLA nano artificial red blood cells do not have adverse effects on the reticuloendothelial system.
Acknowledgments
This research was supported by the Canadian Institutes of Health Research and the Quebec Ministry of Health’s Hemovigillance Program in the form of a FRSQ Research Group (d’equip) on Blood Substitutes in Transfusion Medicine. Earlier, it was also supported by a Bayer/Canadian Red Cross/Medical Research Council of Canada research development award.
Abbreviations
- Hb
hemoglobin
- MetHb
methemoglobin
- NanoRBC
nanodimension artificial red blood cells
- PEG
polyethylene glycol
- PLA
polylactide
- PolyHb
polyhemoglobin
- RBC
red blood cells
- SFHb
stroma-free hemoglobin
References
- 1.Chang TMS. Report of a research project of B.Sc. Honours Physiology. McGill University; Medical Library, McGill University; 1957. Hemoglobin corpuscles; pp. 1–25. (also reprinted in J. Biomaterials, Artificial Cells and Artificial Organs 16:1–9, 1988) [Google Scholar]
- 2.Chang TMS. Semipermeable microcapsules. Science. 1964;146(3643):524. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 3.Chang TMS. Monograph. Charles C. Thomas; Springfield, IL: 1972. Artificial Cells. [Google Scholar]
- 4.Djordjevich L, Miller IF. Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp Hematol. 1980;8:584. [PubMed] [Google Scholar]
- 5.Hunt AC, Burnette RR, MacGregor RD. Science. 1985;230:1165. doi: 10.1126/science.4071041. [DOI] [PubMed] [Google Scholar]
- 6.Philips WT, Klpper RW, Awasthi VD, Rudolph AS, Cliff R, Kwasi-borski VV, Goins BA. Polyethylene glyco-modified liposome-encapsulated Hb: a long circulating red cell substitute. J Pharm Exp Therapeutics. 1999;288:665–670. [PubMed] [Google Scholar]
- 7.Kobayashi K, Tsuchida E, Horinouchi H, editors. Artificial Oxygen Carriers. Springer-Verlag; 2005. [Google Scholar]
- 8.Chang TMS. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J Bioengineering. 1976;1:25–32. [PubMed] [Google Scholar]
- 9.Chang TMS, Yu WP. U.S.A. Patent 5670173. Biodegradable polymer membrane containing hemoglobin as potential blood substitutes. 1997 Sep 23;
- 10.Yu WP, Chang TMS. Submicron biodegradable polymer membrane hemoglobin nanocapsules as potential blood substitutes: a preliminary report. Artificial Cells, Blood Substitutes and Immobilization Biotechnology, An International Journal. 1994;22:889–894. doi: 10.3109/10731199409117926. [DOI] [PubMed] [Google Scholar]
- 11.Yu WP, Chang TMS. Submicron biodegradable polymer membrane hemoglobin nanocapsules as potential blood substitutes. Artificial Cells, Blood Substitutes and Immobilization Biotechnology, An International Journal. 1996;24:169–184. [Google Scholar]
- 12.Chang TMS, Powanda D, Yu WP. Ultrathin polyethylene-glycol-polylactide copolymer membrane nanocapsules containing polymerized Hb and enzymes as nano-dimension RBC substitutes. Artificial Cells, Blood Substitutes & Biotechnology, An International Journal. 2003;31:231–248. doi: 10.1081/bio-120023155. [DOI] [PubMed] [Google Scholar]
- 13.Chang TMS. Therapeutic applications of polymeric artificial cells. Nature Review: Drug Discovery. 2005;4:221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 14.Chang TMS. Monograph on Artificial Cells: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy. World Science Publishers; Singapore: 2007. [Google Scholar]