Abstract
Melanotic neuroectodermal tumour of infancy (MNTI) is a rare neoplasm of neural crest origin. It is benign but locally aggressive and tends to occur most often during the first few months of life. It has a predilection for the head and neck region, particularly for the maxilla. Presence of melanin in this tumour is said to give it distinct clinicopathological, immunohistochemical, ultrastructural and imaging features [1]. We describe five further cases of MNTI, with an emphasis on computed tomography (CT) and magnetic resonance (MR) imaging findings, which have yet to be clearly described in the available radiological literature for this tumour.
Introduction
Melanotic neuroectodermal tumour of infancy (MNTI) is a rapidly growing, melanin containing tumour, which despite its locally aggressive behaviour, is generally classified as a benign tumour. In 1918, Krompecher first described this tumour under the name of congenital melanocarcinoma, believing it to be derived from pigmented odontogenic or epithelial cells [2]. Difficulty in deciding the cellular origin has led to a variety of terminologies assigned to this tumour, including melanotic epithelial odontoma, pigmented teratoma, atypical melanoblastoma, melanotic adamantinoma and pigmented epulis. In 1966, Borello and Grolin first used the name, melanotic neuroectodermal tumour of infancy, to describe a tumour in the maxilla of a 3 month-old boy, with high urinary levels of vanillylmandelic acid (VMA) [3]. Subsequent histochemical, ultrastructural, and immunophenotypic studies have confirmed a neural crest origin of this tumour. There are only a few cases of MNTI in the radiological literature. The purpose of this case series is to document the imaging findings in MNTI to contribute to its small but accruing radiological data.
Materials & Methods
Data for patients with histologically confirmed MNTI following biopsy or surgery, between 1990 and 2008, were retrieved from departmental archives at BLIND. Clinical details were obtained from the request forms and clinical case notes. The radiological investigations available (plain radiographs, CT and MR scans) both prior to and following the diagnosis of MNTI were retrospectively reviewed in conjunction with the initial radiology reports.
Results
The imaging findings have been summarised in Table 1.
Table 1.
| Site | Ultrasound | CT | MR | |
|---|---|---|---|---|
| Case 1 | Occipital bone | x | Spiculated appearance of the occipital bone | Marked uniform contrast enhancement |
| Case 2 | Left maxilla | x | Hypodense mass Bone destruction No calcification |
↓T1 ↓T2 No contrast given |
| Case 3 | Occipital bone | Well-defined Heterogenous | Isodense lesion Indenting bone (no destruction) |
↔ T1 ↓T2 Marked uniform contrast enhancement |
| Case 4 | Right maxilla | x | Isodense lesion | x |
| Case 5 | Bilateral alveolar canine fossae | x | Hypodense mass | x |
Case One
This Afro-Caribbean male was found to have ventriculomegaly on prenatal sonography. At birth he was noted to have a “lump on the back of his skull”. Per history provided by the mother, CT of the head was performed and interpreted as normal. At six months of age, the patient was taken to a local physician because of parental concern regarding the same skull mass. A repeat CT and subsequent MR (Fig 1a & 1b) revealed a 6 × 6 × 5 cm intracranial, extra-axial, solid, intensely enhancing mass arising from the left occipital bone. No ventriculomegaly was seen. The mass was confirmed to be a MNTI following open biopsy. The patient was treated with gross total resection alone. Post operatively he developed subdural hematomas requiring shunting and a large pseudomeningocele requiring surgical drainage. He was disease-free at 10 months after primary tumour resection and remains under follow-up.
Figure 1.
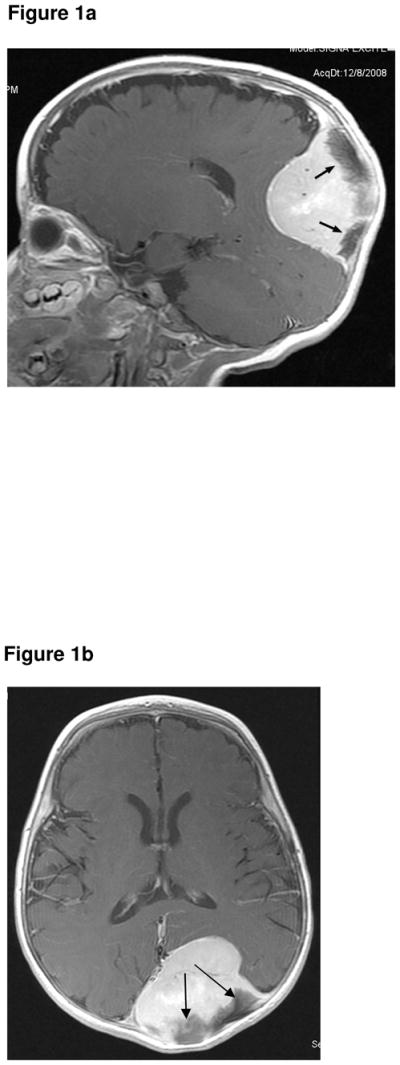
Figure 1a. Sagittal, contrast-enhanced, T1-weighted image (1.5T, TR 650, TE 15) showing a solid, intensely enhancing, large, intracranial, extra-axial, tumour. The dark structures (arrows) are spiculated bone, a known feature of MNTI.
Figure 1b. Axial, contrast-enhanced, T1-weighted MR sequence (1.5T, TR 700, TE 14) showing the large enhancing tumour compressing but not invading the adjacent brain. The dark structures (arrows) are spiculated bone, a known feature of MNTI.
Case Two
A 1 month-old white male infant was referred with a rapidly growing tumour of the left maxilla. The first symptoms of swelling in the anterior maxilla had been noticed at 2 weeks age, but had rapidly increased in size. There was a history of progressive feeding difficulty. On clinical examination, a firm, non-ulcerated, non-tender, bluish tumour of approximately 2 × 2cm was seen on the left side of the anterior maxilla. A CT scan of the head and neck region (Fig 2a) showed a hypodense tumour with bone destruction. No calcification was seen within the tumour itself. A MR scan performed 3 weeks later showed the mass had rapidly enlarged, and now extended upwards to displace the left eye and also extended across the midline. The mass was predominantly hypointense on both the T1- (Fig 2b & 2c) and T2-weighted (Fig 2d) sequences. Results of routine laboratory investigations were normal and urinary VMA levels were not elevated. A biopsy specimen from the lesion diagnosed MNTI. The tumour was removed under general anaesthesia. Histopathology of the main resection demonstrated that the tumour was up to the resection margins. Following recurrence of symptoms, a second operation was performed two months later, requiring resection of the orbital rim, prior to which chemotherapy had been given. Clinical examination and MR showed no recurrence at 3-year follow-up.
Figure 2.
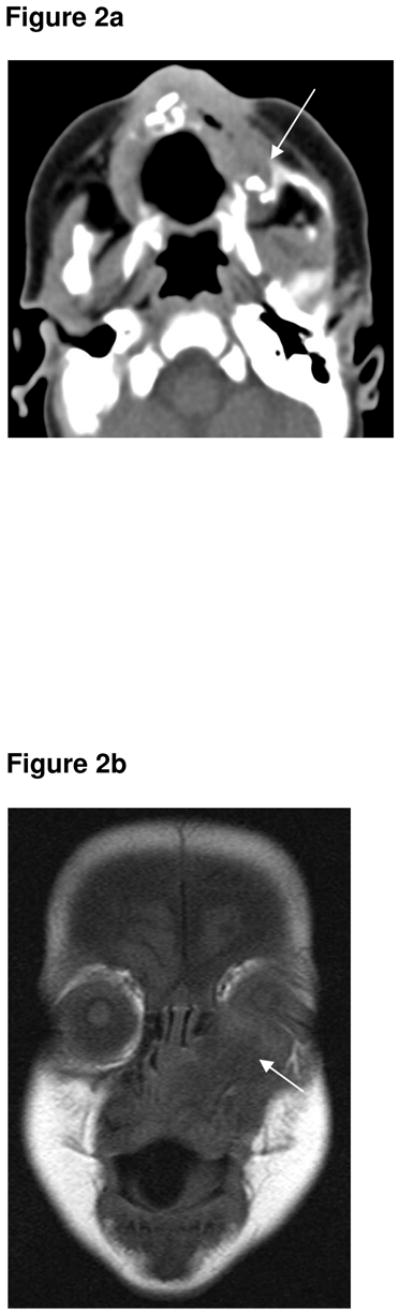
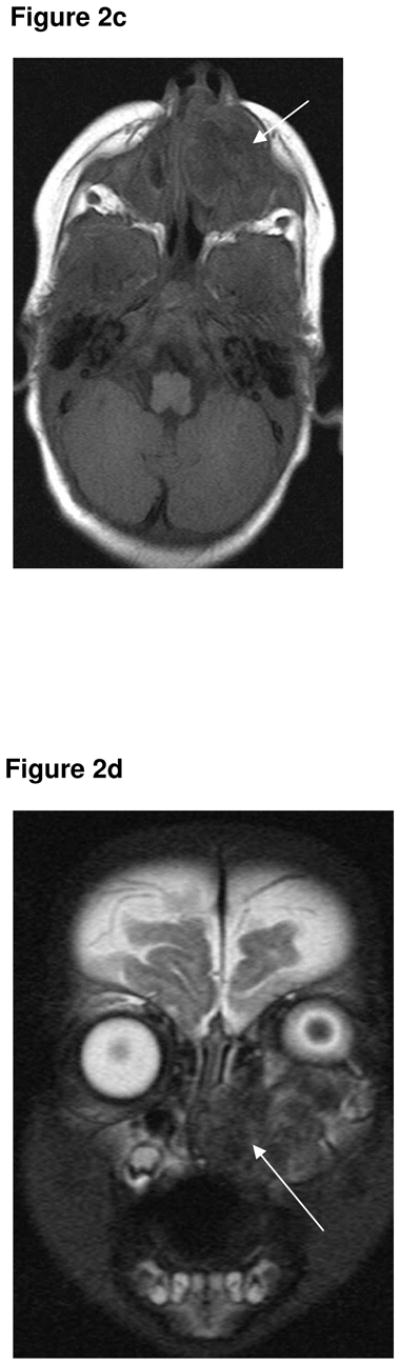
Figure 2a. Axial CT demonstrating a locally destructive tumour involving the left superior alveolar margin, extending to the left side of the nose and the left inferior orbital margin (arrow). No calcification seen on this initial CT.
Figure 2b. Coronal T1-weighted MR sequence (1.5T, TR 550, TE 14) showing the left maxillary MNTI extending upwards to displace the left eye (arrow). It is predominantly hypointense on the T1 sequence.
Figure 2c. Axial T1-weighted MR sequence (1.5T, TR 550, TE 14) showing the tumour is inhomogenously hypointense centrally (arrow).
Figure 2d. Coronal, T2-weighted MR sequence (1.5T, TR 5550, TE 119) showing a heterogenous left maxillary tumour extending upwards and displacing the left eye. The tumour also extends across the midline along the superior alveolar margin. The tumour is of low signal centrally (arrow), and this corresponds with areas of low signal on the T1-weighted sequence, and presumably represents areas of calcification within the tumour.
Case Three
A pea-sized lump was noticed posterior to the right ear, at two weeks of age, in a male infant of Turkish origin. This continued to slowly grow in size, with no overlying skin changes, tenderness or mobility. The child was otherwise asymptomatic and thriving. The child presented at the age of four months with a mass adjacent to his ear. An initial ultrasound (Fig 3a) and CT of the head and neck (Fig 3b) demonstrated an extra-axial soft tissue mass of uncertain origin, causing bony indentation of the occipital bone. A subsequent MR was performed two weeks later and showed that the mass had further increased in size. The lesion was isointense to muscle on the T1-weighted sequence (Fig 3c) and hypointense on the T2- weighted sequence (Fig 3d). The lesion demonstrated marked contrast enhancement (Fig 3e & 3f). Following fine needle biopsy, he was diagnosed as having MNTI, which was treated with surgery. He underwent surgical excision of the tumour. The resection margins were clear. He had MRI follow-up for one year, which showed no evidence of recurrence. He was then followed clinically for three years, with no tumour recurrence.
Figure 3.
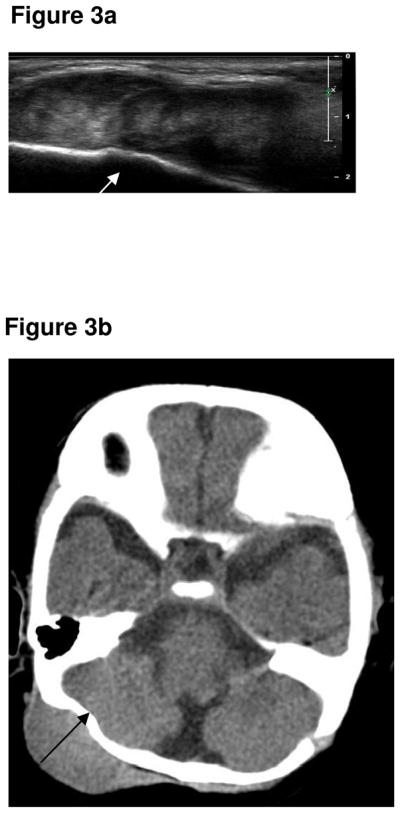
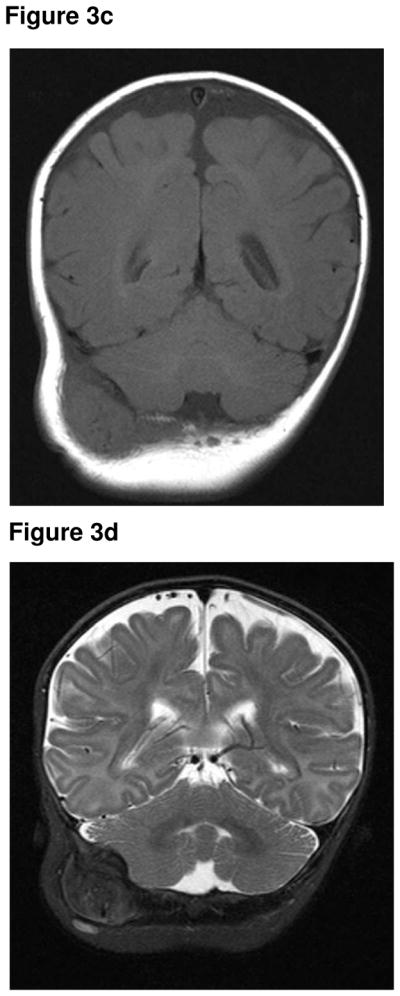
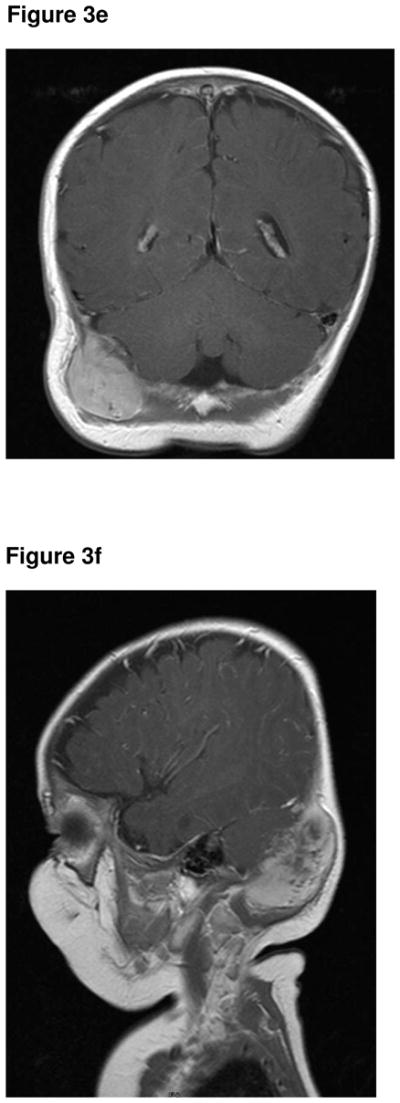
Figure 3a. Ultrasound demonstrates a well-defined, heterogenous solid lesion adjacent to the right occipital bone (arrow).
Figure 3b. Axial CT of the brain demonstrated a well-defined, isodense lesion closely applied and indenting the right side of the occipital bone (arrow). No bone destruction or intracranial extension.
Figure 3c. Coronal T1-weighted MR sequence (1.5T, TR 561, TE 13) showing an isointense lesion, which has increased in size from the CT.
Figure 3d. Coronal T2-weighted MR sequence (1.5T, TR 6180, TE 115) showing a inhomogenously hypointense lesion.
Figure 3e. Coronal post contrast T1-weighted MR sequence (1.5T, TR 561, TE 13) showing marked contrast enhancement of the lesion.
Figure 3f. Coronal post contrast T1-weighted MR sequence (1.5T, TR 561, TE 13) showing marked contrast enhancement of the lesion. No intracranial extension.
Case Four
A swelling was noted at the right anterior maxillary alveolus at 9 weeks of age in a white male patient. He underwent an excisional biopsy, and MNTI was diagnosed. A month later the lesion rapidly regrew and at 13 weeks the child was referred to the oncology team. On clinical examination, a 4cm hard spherical mass was seen arising from the alveolar ridge, causing displacement of the nose and compression of the infraorbital soft tissues. A limited right hemi-maxillectomy was performed and the child was given chemotherapy (cyclophosphamide and vincristine). A CT of the affected region (Fig 4) performed at 5 months of age showed a small amount of residual soft tissue at the site of the previous surgery, with loss of the right side of the alveolar ridge. The child was given two further courses of vincristine and cyclophosphamide. Although there was no recurrence of symptoms following chemotherapy, the patient developed facial asymmetry.
Figure 4.
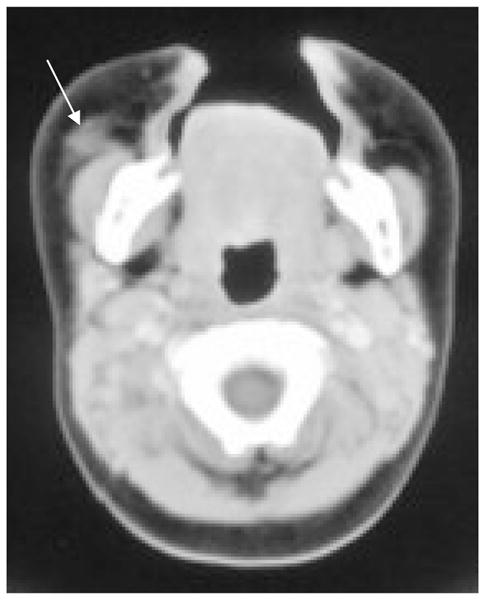
Axial CT at the level of C1. A small amount of residual soft tissue (arrow) following surgery.
Case Five
Shortly after birth, an Afro-Caribbean male infant developed a rapidly growing mass in the alveolar right canine fossa that was interfering with feeding. At 3 months of age the patient underwent total resection of the mass which was confirmed as a 3 × 4 cm melanotic neuroectodermal tumor of infancy. At that time, a resected local lymph node showed no evidence of tumour. One month later, the patient presented with two new, rapidly growing masses in the right and left alveolar canine fossae, while the surgical bed itself showed no evidence of recurrence. Because the recurrent tumours grew rapidly and there was possible involvement of the orbits, surgical resection was not considered to be a primary treatment option. He was initially treated with cytoxan and adriamycin with no response. He was switched to VP-16, cisplatinum and low dose radiation (9 Gy in 1.5 Gy fractions twice a day for 3 days) with complete response. He was disease-free four years after completion of therapy at which time he was discharged to his local physician.
Discussion
MNTI is generally found in patients younger than 1 year of age and without sex predilection. Rare cases have been reported in older children and adults. It usually presents in the head and neck region, because of its origin from the neuroectoderm. Around 70% occur in the maxilla, followed by the skull (11%) and mandible (6%) [4]. In the skull, lesions usually arise at the sutures and about half occur around the anterior fontanelle. Brain parenchymal involvement generally results from intra-axial extension of skull lesions, although reported cases of primary tumours have arisen in the cerebellar vermis and third ventricle [5]. Other uncommon sites of MNTI include the meninges, epididymis, ovary, uterus and mediastinum [6].
Patients typically present with a non-tender, non-ulcerated, rapidly growing soft tissue swelling. The tumour causes compression rather than infiltration of adjacent structures, with local invasion causing bony destruction, tooth displacement and feeding difficulties. In a minority of patients, elevated levels of urinary vanillylmandelic acid, a finding supportive of the neural crest origin of the tumour, may be of diagnostic help. Macroscopicallly, these tumours are usually firm, lobulated, well circumscribed but not encapsulated. In untreated, resected cases, microscopic examination demonstrates a typical biphasic cell population composed of collections of larger, melanin containing epithelial-like cells and smaller, round neuroblast-like cells [7], although both populations may sometimes be difficult to identify on small needle biopsies or in cases following chemotherapy.
The radiological differential diagnosis of MNTI affecting the head and neck includes developmental cysts, odontogenic lesions (e.g. ameloblastoma), infection and non-odontogenic lesions, such as, fibromatosis, fibrous dysplasia and vascular malformations. However, rapid enlargement and associated bone destruction, frequently narrows the radiological differential diagnosis to tumours encountered during infancy, such as Langerhans’ cell histiocytosis, Ewing sarcoma, rhabdomyosarcoma, lymphoma or neuroblastoma metastasis and fibromatosis.
The initial radiographic appearance of MNTI osseous lesions is typically that of a well-demarcated radiolucent lesion with bony expansion. As the tumour enlarges, it causes bony destruction and in some cases tooth displacement, sometimes within the radiolucent area of the tumour. On plain radiographs, occasionally a faint spiculated or “sunburst” appearance may be seen [8]. Limited literature is available regarding MIBG studies as an aid to diagnosis, although Chossegros et al suggested that the tumour is MIBG negative [9]. Ultrasound has been performed for peripheral lesions of MNTI, which tend to be well-defined lesions of heterogeneous echogenicity and minimal vascularity; although there are only 7 reported cases which involve the subcutaneous tissues [16]. No previous ultrasound findings have been described for a calvarial MNTI, with CT and MR correlation. In our case, (Fig 3) the lesion was well-defined and heterogenous in appearance and ultrasound demonstrated indentation of the bone, but no periosteal changes.
CT defines the extent of the lesion, clearly delineates osseous involvement and provides a good basis for surgical planning. Maxillary lesions may show the radiolucent bony lesion causing bony expansion, sometimes with “free-floating” teeth. The soft tissue component has been described as typically appearing well-defined and slightly hyperdense mass on CT, which is attributed to melanin [10]. Enhancement with intravenous contrast material is often seen. The adjacent bones can be thickened due to reactive sclerosis. Extensive tumoural calcification is uncommon, and was not seen in any of our cases [1]. In each of our cases, the CT findings of the underlying bone were variable, including a spiculated/“sunburst” appearance, indentation of the underlying bone and complete bone destruction. No sclerosis was seen in any of our cases. The soft tissue component of the lesions appeared well-defined, hypo/iso-dense in our cases, which is contrary to the appearance of increased attenuation in MNTI, which is typically described.
On MR, melanin containing tumours should follow the paramagnetic effect of melanin. Typically, these show increased signal on T1- weighted images due to melanin, which is a free radical trap that chelates paramagnetic metal ions [11]. The presence of unpaired electrons of the free radicals and of the chelated metal ions, enhances T1 relaxation and leads to an increase in signal on T1-weighted images [11]. T2 relaxation time is related to magnetic field inhomogeneity in the proton environment that leads to precessional dephasing. Melanin causes heterogeneous magnetic susceptibility of the surrounding environment, causing rapid T2 decay and enhanced T2 relaxation, which lowers the signal intensity on T2-weighted sequences [5].
Maxillary and calvarial MNTIs have been described to give T1 shortening, which can be inhomogeneous, and represent foci of extensive melanosis within the tumour [5,11, 12]. These foci of melanin concentration may reflect the lesion appearing isointense or hypointense compared to muscle on T2-weighted imaging. MR findings in MNTI do not however commonly demonstrate the signal pattern expected for melanin containing tumours [12]. MNTI has more commonly been described to be isointense on T1- weighted images [8, 13]. MNTI may appear slightly hyperintense on T2-weighted images [14]. The tumour may contain areas of reduced signal on both T1- and T2-weighted images corresponding to areas of calcification, or in tumour lying adjacent to hyperostotic bone [8]. This is seen in one of our cases, in which peripheral hypointensity is seen adjacent to the occipital bone (Fig 1), with a “sun-burst” appearance, which was also seen on CT. Contrast enhancement is usually quite marked. The cellular component of tumour is expected to give restricted diffusion on diffusion weighted images due to the abundance of small round cells and minimal cytoplasm [12]. None of the patients in our series had diffusion weighted imaging. Magnetic resonance angiography should be considered for tumours located in the midline of the skull to assess for vascular encasement.
The typical MR signal of melanin is seen in very densely pigmented tumours, and when present can make the diagnosis of MNTI [1]. None of our cases demonstrated the typical finding of hyperintensity on T1-weighted imaging; this is one of the largest case series of calvarial and maxillary MNTIs, with both CT and MR correlation of imaging findings compared to individual case reports in the literature. MNTIs in our series have been predominantly iso- or hypointense on T1-weighted images and hypointense on T2-weighted images. A previous case of MNTI arising from the right maxilla in a female infant has been reported from Great Ormond Street Hospital [8], in which the mass also appeared isointense with muscle on the T1-weighted image. This could be attributed to the variable amounts of connective tissue stroma, frequent variation in the amount of melanin deposition and the maturity of melanosomes, which cause altered paramagnetic effects. This is one of the largest case series of calvarial and maxillary MNTIs involving CT and MR correlation of findings compared to individual case reports in the literature.
Due to a marked variability in imaging findings, tissue biopsy is generally required. Surgical excision, without an incisional biopsy, can be performed based on clinical and radiological findings, for example, when urinary VMA is raised and neuroblastoma has been excluded. Surgery is the treatment of choice of MNTI and even large lesions can have a good prognosis. Pre-operative imaging assists in delineating tumour margins prior to surgery, but should be performed early as MNTI often grows rapidly, and locally invades surrounding anatomical structures. The need for clear surgical excision margins is uncertain. Some authors suggest that wide surgical margins may not be necessary because tumour islets may regress spontaneously after incomplete excision while others suggest tumour resection with wide surgical excision of at least 5mm [8]. The difficulty in achieving adequate surgical margins can lead to the use of adjuvant chemotherapy [15]. However, there are conflicting reports on the use of chemotherapy for MNTI; some report disease progression and others document disease response in both metastatic and locally aggressive tumours.
Although the majority of tumours are benign, around 35% of cases recur, based or published reports. [6,8]. Recurrence may be the consequence of incomplete excision of the primary tumour, seeding during surgery, or tumour multicentricity. Metastatic spread usually to regional lymph nodes is rare, occurring in up to 7% of patients [8]. Unfortunately, it is difficult to determine the potential for recurrence or development of metastatic disease based on clinical, imaging or histopathological features, making post-operative follow-up with imaging essential in all cases.
Conclusion
The importance of early, accurate diagnosis of MNTI cannot be overstated because it has rapid growth potential. It has distinctive clinicopathological and imaging features and it should be considered in the differential diagnosis of all patients presenting with head and neck masses, especially around the maxilla. CT and particularly MR imaging contribute in making the diagnosis of MNTI. Our findings are consistent with the previously reported cases regarding site and growth pattern. The typical CT radiological findings are of an iso/hypodense soft tissue component which demonstrates contrast enhancement. Bony involvement is variable and includes a spiculated/“sunburst” appearance, indentation of the underlying bone and complete bone destruction. MR findings in our series do not correspond with the typical T1 shortening expected with melanin deposition and tumoural calcification. Both CT and MR provide important information regarding the extent of the lesion for surgical planning. Although MNTI is considered to be a benign tumour, close clinical and radiological follow-up with MR imaging is suggested to identify recurrence or the rare development of metastatic disease.
References
- 1.Nazira B, Gupta H, Chaturvedi AK, Rao SA, et al. Melanotic neuroectodermal tumour of infancy: a discussion of a case and a review of the imaging findings. Cancer Imaging. 2009 Dec 24;9:121–5. doi: 10.1102/1470-7330.2009.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krompecher E. Zur Histogenese und Morphologie der Adamantinome und sonstiger Kiefergeschwülste. Beitr Path Anat. 1918;64:165–97. [Google Scholar]
- 3.Borello ED, Gorlin RJ. Melanotic neuroectodermal tumor of infancy--a neoplasm of neural crese origin. Report of a case associated with high urinary excretion of vanilmandelic acid. Cancer. 1966 Feb;19(2):196–206. doi: 10.1002/1097-0142(196602)19:2<196::aid-cncr2820190210>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Cutler LS, Chaudhry AP, Topazian R. Melanotic neuroectodermal Melanotic neuroectodermal tumor of infancy: An ultrastructural study, literature review, and reevaluation. Cancer. 1981;48:257–270. doi: 10.1002/1097-0142(19810715)48:2<257::aid-cncr2820480209>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.George JC, Edwards MK, Jakacki RI, et al. Melanotic neuroectodermal tumor of infancy. AJNR Am J Neuroradiol. 1995;16:1273–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Kruse-Lösler B, Gaertner C, Bürger, et al. Melanotic neuroectodermal tumor of infancy: systematic review of the literature and presentation of a case. 2006 Aug;102(2):204–16. doi: 10.1016/j.tripleo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter BF, Jimenez C, Robb IA. Melanotic neuroectodermal tumor of infancy. Pediatr Pathol. 1985;3:227–44. doi: 10.3109/15513818509078784. [DOI] [PubMed] [Google Scholar]
- 8.Fowler DJ, Chisholm J, Roebuck D, et al. Melanotic neuroectodermal tumor of infancy: clinical, radiological, and pathological features. Fetal Pediatr Pathol. 2006;25:59–72. doi: 10.1080/15513810600788715. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Sakuma J, Suzuki K, Kawakami M, Sasaki T, Kodama N. Melanotic neuroectodermal tumor of infancy in the skull: case report and review of the literature. Surg Neurol. 2005 Mar;63(3):275–80. doi: 10.1016/j.surneu.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Chossegros C, Cheynet F, Gentet F, et al. Melanotic neuroectodermal tumor in a young child. Arch Pediatr. 1995;2(6):545–47. doi: 10.1016/0929-693x(96)81198-5. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson GO, Jr, Davis PC, Patrick LE, et al. Melanotic neuroectodermal tumor of infancy: MR findings and a review of the literature. Paediatr Radiol. 1989;20:20–22. doi: 10.1007/BF02010627. [DOI] [PubMed] [Google Scholar]
- 12.Koral K, Derinkuyu B, Timmons C, et al. Melanotic neuroectodermal tumor of infancy: report of one calvarial lesion with T1 shortening and one maxillary lesion. Clinical Imaging. 2010;34:382–384. doi: 10.1016/j.clinimag.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Nishio S, Morioka T, Fukui M, et al. Melanotic neuroectodermal tumour of infancy at the anterior fontanelle. Neuroradiology. 1999;41:202–4. doi: 10.1007/s002340050735. [DOI] [PubMed] [Google Scholar]
- 14.Mirich DR, Blaser SI, Harwood-Nash DC, et al. Melanotic neuroectodermal tumor of infancy: clinical, radiologic, and pathologic findings in five cases. AJNR Am J Neuroradiol. 1991;12:689–97. [PMC free article] [PubMed] [Google Scholar]
- 15.Gaiger de Oliveira M, Thompson LD, Chaves AC, et al. Management of melanotic neuroectodermal tumor of infancy. Ann Diagn Pathol. 2004;8:207–212. doi: 10.1053/j.anndiagpath.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Lacy SR, Kuhar M. Melanotic neuroectodermal tumor of infancy presenting in the subcutaneous soft tissue of the thigh. Am J Dermatopathol. 2010;32(3):282–6. doi: 10.1097/DAD.0b013e3181b623a7. [DOI] [PubMed] [Google Scholar]


