Abstract
MDM2-A is a common splice variant of MDM2 that is frequently detected in many tumor types. Our previous work has characterized MDM2-A as an activator of p53 and therefore, in a wild-type p53 background, this splice variant would be predicted to confer p53-dependent tumor protection. To test this hypothesis, we utilized Mdm2-a transgenic mice to assess transformation and tumorigenesis in tumor susceptible murine models. A MDM2-A dependent decrease in transformation was observed in Arf-null MEFs or when wild-type MEFs were exposed to the carcinogen, ENU. However, this reduced transformation did not confer tumor protection in vivo; Mdm2-a/Arf-null mice and ENU-treated MDM2 expressing mice developed similar tumor types with equivalent latency compared to their respective controls. Interestingly, when p53 was deleted, MDM2-A expression enhanced transformation of p53 null MEFs and altered tumor spectrum in vivo. In addition, p53-heterozygous mice that expressed MDM2-A developed aggressive mammary tumors that were not observed in p53-heterozygous controls. In conclusion, we found that although MDM2-A expression enhances p53 activity and decreases transformation in vitro, it cannot confer tumor protection. In contrast, MDM2-A appears to exhibit a novel transforming potential in cells where p53 function is compromised. These data demonstrate that MDM2 splice variants, such as MDM2-A, may provide protection against transformation of normal tissues having intact p53. However, when such splice variants are expressed in tumors that have defects in the p53 pathway, these isoforms may contribute to tumor progression, which could explain why their expression is often associated with aggressive tumor types.
Keywords: MDM2-A, splice variant, p53-null, Arf-null, ENU
INTRODUCTION
Murine double minute 2 (MDM2) is a well-characterized oncoprotein that functions as a direct inhibitor of the p53 tumor suppressor (1). MDM2-mediated modulation of p53 expression and degradation is critical for proper cell growth and survival (2). Alterations in MDM2, such as gene amplification and protein overexpression, lead to reduced p53 protein and activity, the consequence of which is transformation. Elevations in MDM2 expression are frequently observed in a variety of tumors including soft-tissue sarcomas (3), osteosarcomas (4), neuroblastomas (5) and gliomas (6).
The Mdm2 transcript is frequently subjected to alternative and aberrant splicing, leading to the generation of multiple isoforms. To date, over 40 Mdm2 splice variants have been identified in both tumors (7, 8) and normal tissues (9). The presence of Mdm2 splice variants in tumors has been associated with advanced disease (7, 10) and poor prognosis (11). Several studies have implicated genotoxic stress as an inducer of MDM2 splice variants. Both UV irradiation (12, 13) and various chemotherapeutic drug treatments (12, 14) have been shown to produce specific Mdm2 splicing products. Functional analysis of these variants is limited; however, splice variants containing an intact carboxy-terminal RING finger domain have been shown to bind to full-length MDM2 and result in the activation of p53 (15, 16). Therefore, the induction of splice variants upon genotoxic stress appears to facilitate the accumulation of p53 protein under these circumstances. However, the role of MDM2 splice variants in tumors, particularly those without wild-type p53 is currently unclear.
MDM2-A is a common MDM2 splice variant that is frequently detected in rhabdomyosarcomas (17) and other tumor types (7,8). The isoform encoded by this variant lacks the p53 binding domain, but maintains the RING finger motif (7, 17). Previous studies have demonstrated contradictory results with respect to the function of MDM2-A (7, 18). One study demonstrated that MDM2-A expression enhanced transformation in NIH3T3 cells (7). In contrast, this splice variant did not accelerate the rate of lymphomagenesis in the Eµ-myc lymphoma model and was unable to enhance transformation of mouse embryonic fibroblasts (MEFs) in soft agar (18). By generating an Mdm2-a transgenic mouse model, we showed that expression of MDM2-A resulted in a p53-dependent perinatal lethality and a growth inhibitory phenotype in Mdm2-a transgenic MEFs (19). This splice variant is capable of interacting with full-length MDM2 and activating p53, although we did not observe a universal induction of p53-responsive genes (19).
MDM2-A-mediated enhancement of the tumor suppressor activity of p53 would be predicted to confer tumor protection. However, we were previously unable to test this hypothesis due to the low frequency of tumorigenesis in our transgenic mouse model (19). Therefore in this study, to enhance the rate of transformation and tumor formation, and to assess the impact of the MDM2-A splice variant on these processes, MDM2-A transgenic mice were either exposed to a carcinogen or bred with genetically susceptible tumor models.
RESULTS
Evaluation of MDM2-A protection from transformation in ARF-null mice
To determine if the previously observed MDM2-A-mediated activation of p53 could confer protection against transformation in vitro and tumorigenesis in vivo, Mdm2-a transgenic mice were crossed onto a tumor-prone Arf-null background. Deletion of Arf in wild-type mice leads to increased MDM2, decreased p53 and enhanced tumor formation (20). We hypothesized that the interaction of MDM2-A with full-length MDM2 (19) would minimize the available MDM2 protein to inhibit p53 and result in enhanced p53 activity and decreased tumor formation.
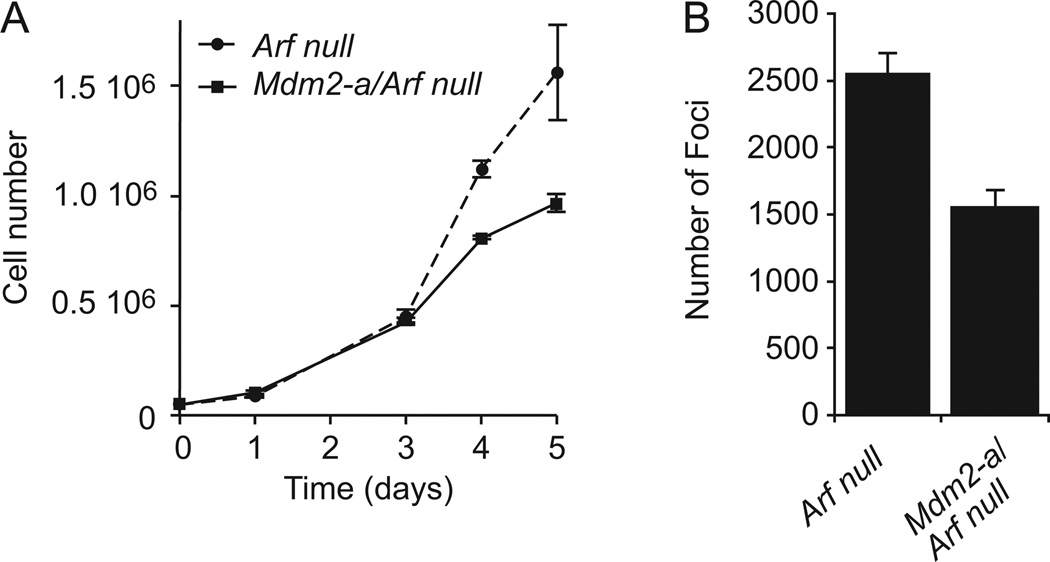
To assess the role of MDM2-A in the enhancement of transformation in vitro, MEFs were harvested from Mdm2-a/Arf-null mice and compared to those derived from Arf-null controls. Cell growth was assayed in these MEFs and Mdm2-a/Arf-null cells exhibited growth inhibition relative to Arf-null controls (Figure 1A), a phenotype similar to that observed in wild-type MEFs that express MDM2-A (19). When plated at high density, Mdm2-a/Arf-null MEFs formed significantly fewer transformed foci than Arf-null MEFs (Figure 1B). These data suggested that MDM2-A mediated activation of p53 reduced the rate of transformation in Arf-null MEFs.
Figure 1. MDM2-A mediates growth inhibition and reduces transformation in the absence of Arf.
(A) Growth curve of Arf-null (dashed line) and Mdm2-a/Arf-null (solid line) MEFs. (B) Quantitation of transformed foci/78.5 cm2 (mean of 3 with standard deviation) after MEFs were transfected with E1A and plated at high density.
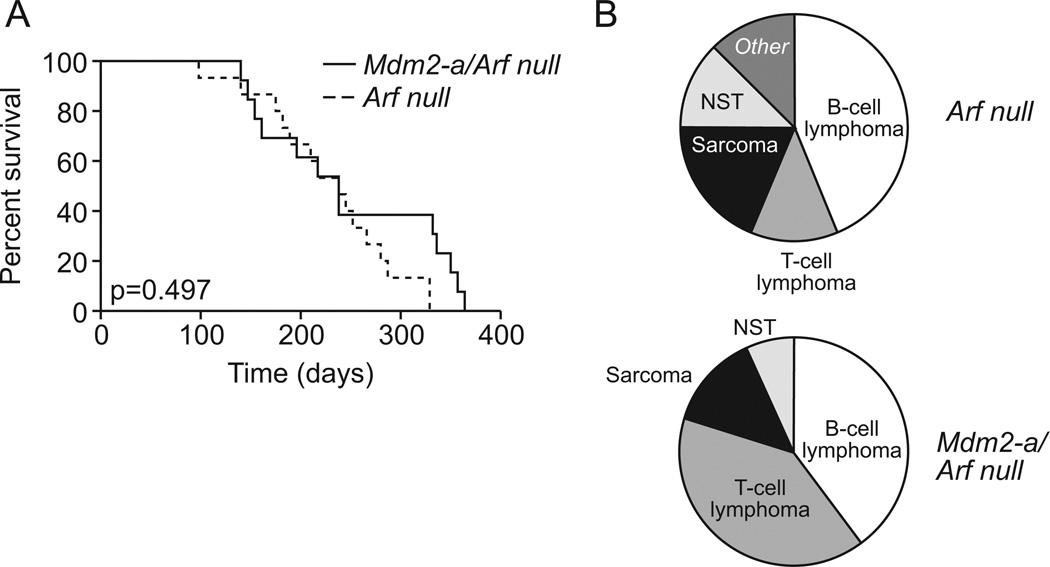
To assess the role of MDM2-A on tumorigenesis in vivo, Arf-null and Mdm2-a/Arf-null mice were observed for tumor formation. Lesions were characterized histopathologically and assigned a tumor diagnosis. There were no significant differences in survival between the Mdm2-a transgenic Arf-null and Arf-null control mice (p=0.497). Mdm2-a/Arf-null mice survived approximately 7.8 months, while Arf-null mice survived for 8.8 months (Figure 2A). However, the tumor spectrums observed for Arf-null and Mdm2-a/Arf-null were different (Figure 2B). As detailed in Supplemental Table 1, the majority of mice developed lymphoid malignancies of either B-cell or T-cell lineage and there was a marginal significant increase in the incidence of T-cell lymphomas in the presence of MDM2-A (p=0.096). These data suggested that MDM2-A does not confer tumor protection when Arf is deleted, but that it influences the types of tumors generated.
Figure 2. In the absence of Arf, MDM2-A does not alter survival, but does enhance T-cell lymphoma development.
(A) Kaplan-Meier survival curve for Arf-null (dashed line) and Mdm2-a/Arf-null (solid line). There was no significant difference in survival of these two groups of mice (p=0.497). (B) Tumor distribution in Arf-null (upper) and Mdm2-a/Arf-null (lower) displayed a marginally significant difference between the two groups (p=0.096). NST: Nerve Cell Tumor.
Evaluation of MDM2-A protection against carcinogenesis
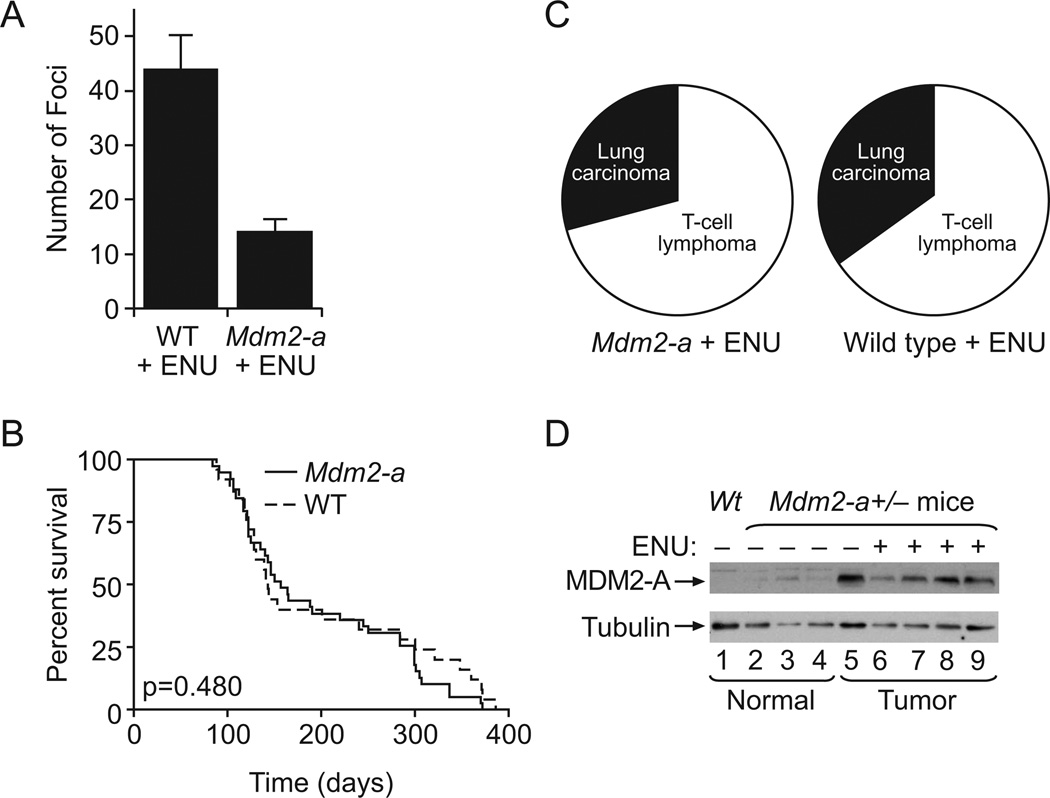
To determine if MDM2-A confers protection from carcinogen-induced transformation and tumorigenesis, control wild-type and Mdm2-a transgenic MEFs as well as newborn pups were exposed to the alkylating agent ethylnitrosourea (ENU). ENU is a potent mutagen and carcinogen that has previously been shown to enhance carcinogenesis (21). Therefore, MEFs were treated with 2mM ENU for 1 hour and incubated for 3 weeks. The Mdm2-a transgenic MEFs formed fewer transformed foci compared to wild-type MEFs (Figure 3A) demonstrating that MDM2-A expression reduces transformation induced by exposure to ENU.
Figure 3. ENU-treatment in the presence of MDM2-A leads to a reduction in transformation, does not alter survival, and its expression is stabilized in tumors.
A) Histogram of foci/78.5 cm2 detected after ENU treatment of either wild-type or Mdm2-a MEFs (mean of 3 with standard deviation). (B) Kaplan-Meier survival curve of wild-type (dashed line) and Mdm2-a transgenic (solid line) mice after ENU treatment. There was no significant difference in survival between the two groups (p=0.480). (C) Tumor spectrum in Mdm2-a transgenic mice exposed to ENU and wild-type mice exposed to ENU mice. (D) Western blot analysis of MDM2-A expression in normal tissue (lanes 1–4) and tumor tissue (lanes 5–9) obtained from the lungs of wild-type (lane 1), untreated Mdm2-a transgenic mice (lanes 2–5) and ENU-treated Mdm2-a transgenic mice (lanes 6–9). Elevated MDM2-A expression is observed in all tumor tissue irrespective of whether the tumors arose as a consequence of ENU exposure.
Earlier studies have shown that ENU treatment of mice results in the development of multiple tumor types (22); however, only a few tissues of the transgenic mice express MDM2-A, with the highest levels of expression in the salivary gland (19). Therefore, to specifically target an organ site that expressed relatively high levels of MDM2-A, newborn pups were pre-treated daily (P10–P14) with isoproterenol, a beta-adrenergic receptor agonist known to induce salivary gland hyperplasia (23), in order to predispose this tissue to carcinogenesis. The mice were then injected daily for five days (P15–P19 after birth) with 60µg/mL ENU and monitored for tumor development. The Kaplan-Meier survival curves for the isoproterenol/ENU treated mice were biphasic (Figure 3B); the majority of mice died within five months as a result of disseminated T-cell lymphoblastic lymphoma involving multiple organs, while a second group of mice succumbed to pulmonary tumors at 7 months to 1 year (Figure 3C, Supplemental Table 2). No salivary gland tumors were detected and there was no significant difference in survival or tumor type propensity of MDM2-A transgenic mice compared to wild-type (p=0.480). Western blot analyses of tumor samples from organs infiltrated with lymphoma such as spleen, kidney and thymus showed no change in MDM2-A expression compared to the same tissues in untreated mice, while lung tumors from transgenic mice with and without ENU injection showed a significant increase in MDM2-A expression compared to normal lung tissue (Figure 3D). This higher level of MDM2-A expression in the lung tumor tissue suggested that MDM2-A expression was being selected for and might in fact confer a growth advantage in this cell type. However, the tumor incidence data demonstrated that in this context, MDM2-A expression does not influence the rate of tumorigenesis (Fig 3C, Supplemental Table 2).
The influence of MDM2-A expression on transformation in p53-null mice
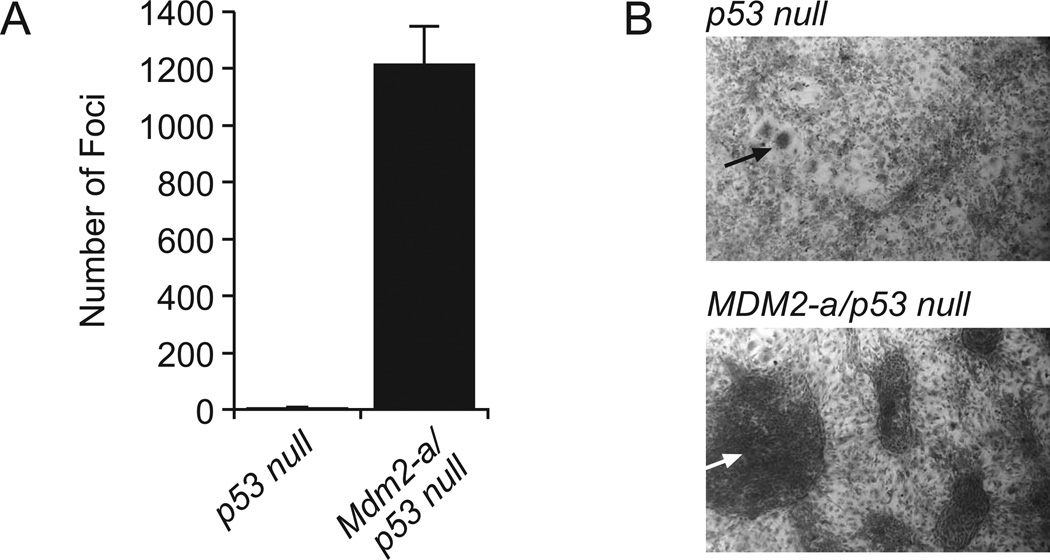
MDM2-A has previously been shown to exhibit p53-dependent growth inhibition (19); however, the selection for MDM2-A expression in tumors that developed in transgenic mice suggested a possible transforming activity which is likely independent of p53 function. Therefore, to assess the role of MDM2-A in transformation and tumorigenesis in the absence of p53 expression, transgenic mice were crossed into a p53-null background. While Mdm2-a/p53-null MEFs grew at the same rate as p53-null MEFs (19), those that expressed MDM2-A displayed a significant increase in transformed focus formation compared to MEFs derived from p53-null mice (Figure 4A, B). These data demonstrate that MDM2-A increases transformation in a p53-independent manner, a phenotype that is masked when p53 is wild-type.
Figure 4. MDM2-A enhances transformation in the absence of p53.
(A) Histogram of foci/78.5 cm2 formed in p53 null or Mdm2-a/p53-null MEFs (mean of 3 with standard deviation). (B) Micrographs of p53 null (upper) and Mdm2-a/p53-null (lower) foci at 4× magnification. Arrows point to examples of the observed foci.
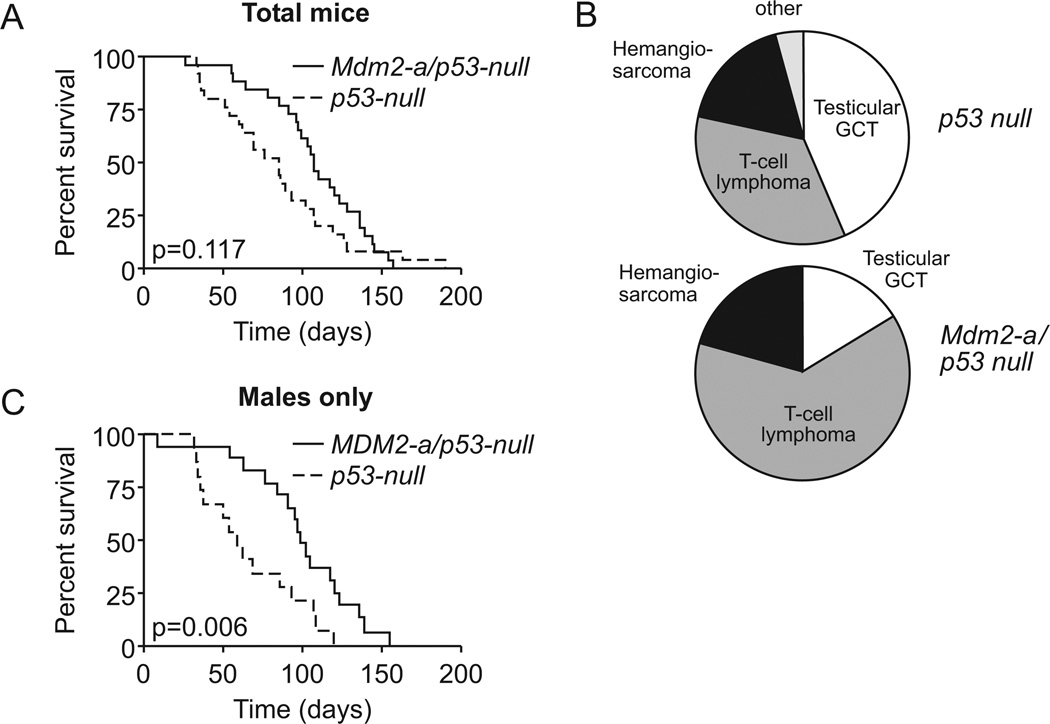
To determine if the enhanced focus forming capacity of Mdm2-a/p53-null MEFs resulted in enhanced tumorigenesis in vivo, tumor latency and spectrum was observed in these mice and compared to p53-null controls. MDM2-A expressing p53-null mice had a median survival of 107 days, while p53-null mice survived for approximately 85 days (Figure 5A); however, there was no significant difference in survival between the groups (p=0.117). Interestingly, the tumor spectrum was altered in the presence of MDM2-A. p53-null mice developed testicular germ cell tumors and T-cell lymphomas at similar frequencies, while Mdm2-a/p53-null mice were more likely to develop T-cell lymphomas and very few testicular germ cell tumors were observed (p=0.086) (Figure 5B, Supplemental Table 3). The slight shift in the survival curve appeared to be a result of the non-transgenic p53-null mice developing more testicular germ cell tumors that arose in very young mice (< 2 months). Therefore, we reevaluated the survival of male animals only and found a significant difference between the survival of the transgenic and non-transgenic mice (p=0.006, Figure 5C). These data demonstrate that MDM2-A is capable of altering the tumor spectrum in a p53-independent manner and it appears that because fewer testicular germ cell tumors were generated upon expression of MDM2-A, MDM2-A prolonged the life of these mice
Figure 5. MDM2-A does not significantly alter the survival of p53-null mice, but does shift the tumor spectrum.
(A) Kaplan-Meier survival curve of p53-null (dashed line) and Mdm2-a/p53 null (solid line). There was no significant different in survival between the two groups (p=0.117). (B) Tumor spectrum of p53-null (upper) and Mdm2-a/p53-null (lower). GCT; Germ cell tumor. There was a marginal significant difference in the tumor spectrum between the two groups (p=0.086). (C) Kaplan-Meier survival curve of males only, p53-null (dashed line) and Mdm2-a/p53-null (solid line). There was a significant difference between the two populations once the male mice were evaluated separately (p=0.006).
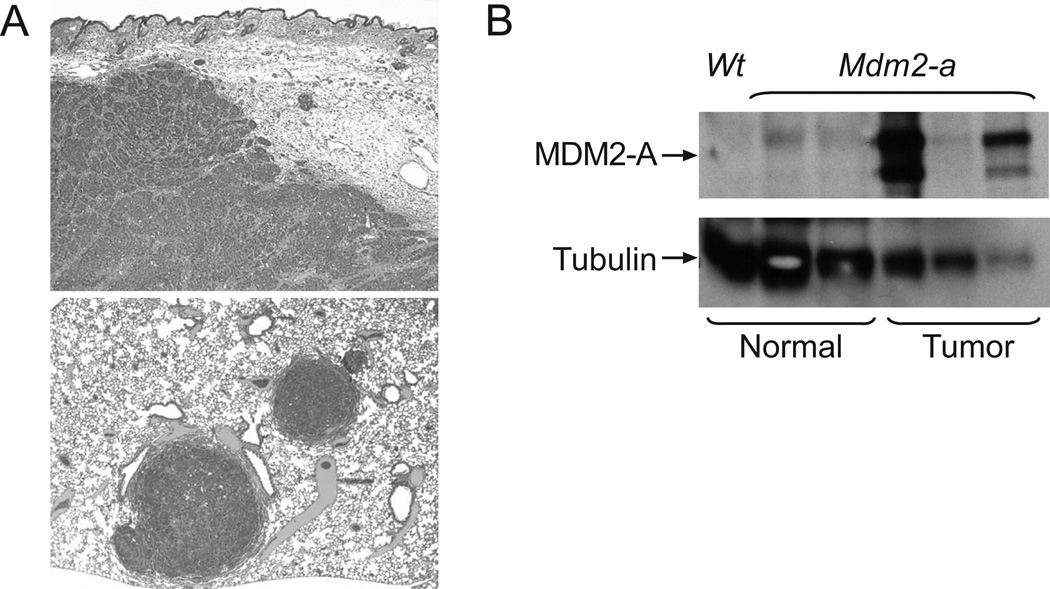
In addition to analysis of mice with homozygous p53 gene deletions, p53 heterozygous mice were also assessed for tumor formation in the presence of the Mdm2-a transgene. While no differences in overall survival were observed (p= 0.208, data not shown), several Mdm2-a/p53-heterozygous mice (6/28, 21%), but no p53-heterozygotes without Mdm2-a developed mammary carcinomas (Supplemental Table 4). Interestingly, pathological analysis indicated that these tumors displayed characteristics of aggressive mammary carcinomas that were distinct from those typically observed in p53-heterozygous mice (Figure 6A). Western blot analysis showed that 2 of 3 of the mammary tumors evaluated had high levels of MDM2-A expression, while MDM2-A could not be detected in normal control mammary tissue (Figure 6B). These data provide additional evidence that MDM2-A expression may confer a growth advantage in specific tumors or cell types.
Figure 6. Mdm2-a/p53-heterozygous mice develop aggressive mammary tumors, which have stabilized MDM2-A expression.
(A) Micrographs of Mdm2-a/p53-heterozygous mammary carcinomas. The upper panel shows a mammary carcinoma with soft tissue invasion, 4× magnification. The lower panel shows mammary carcinoma metastases in the lung parenchyma, 3.2× magnification. (B) Western blot analysis of MDM2-A expression in normal mammary tissue (lanes 1–3) and mammary tumor tissue (lanes 4–6) isolated from p53-heterozygous (lane 1) and Mdm2-a/p53-heterozygous transgenic mice (lanes 2–6).
DISCUSSION
Mdm2 splice variants are frequently overexpressed in various tumors and their expression there has been correlated with advanced disease (7, 10) and poor prognosis (11). Determination of splice variant function with respect to tumor development has yielded contradictory results. Our previous work demonstrated that expression of the MDM2-A splice variant activated wild-type p53 and inhibited growth in a p53-dependent manner (19), phenotypes predicted to confer p53-dependent tumor protection, rather than enhance tumor growth. Here, we examined the consequence of MDM2-A expression in several tumor prone mouse models to determine whether this splice variant maintains its growth inhibitory properties and reduces transformation and tumorigenesis.
As predicted, a decrease in transformation was observed in the presence of MDM2-A protein expression; both Mdm2-a/Arf-null MEFs and ENU-treated Mdm2-a MEFs developed fewer transformed foci than control cells. Surprisingly, however, this in vitro observation did not translate into a reduction in tumorigenesis in either the Mdm2-a/Arf-null or ENU-treated Mdm2-a transgenic mice. Two possible explanations for this lack of correlation between transformation and tumorigenesis are either that the activity of MDM2-A was not robust enough to result in in vivo changes or that a proportion of the observed tumors arose in tissues that did not express MDM2-A thereby masking the effect of MDM2-A expression on tumorigenesis. As previously described, MDM2-A has a limited tissue distribution in the transgenic animals (19). Consequently, many Mdm2-a/Arf-null and ENU-treated mice developed lymphomas, which did not express detectable levels of MDM2-A. Therefore, it was unlikely that MDM2-A would influence development of this tumor type unless low undetectable levels of MDM2-A were able to influence lymphoma progression. The significant increase in the incidence of T-cell lymphomas that developed in the Mdm2-a transgenic mice may have arisen as a consequence of other tumor types that express MDM2-A decreasing in frequency.
Lung tumors that developed in the ENU treated mice expressed MDM2-A, but showed no differences in tumor onset or progression. It is possible that these tumors developed containing alterations in the p53 pathway that would have precluded MDM2-A mediated activation of this tumor suppressor and abolished any subsequent tumor protective effects.
To address the aforementioned concern of tumors arising in tissues that lacked transgene expression, we attempted to enhance tumor development in the salivary gland, a MDM2-A expressing tissue. Because salivary gland hyperplasia has previously been shown to predispose the salivary gland to carcinogenesis (23), transgenic mice and wild-type controls were treated with isoproteronal to induce proliferation of the salivary gland tissue prior to ENU mutagenesis. However, despite this specific targeting, we were unable to detect any changes in this tissue and no salivary gland tumors were observed. All mice in the ENU treatment groups succumbed to either lymphoma or pulmonary tumors within the first year of life. It is possible that the salivary gland may be inherently less susceptible to ENU mutagenesis, or that tumors arising in this tissue have a longer latency than either lymphomas or lung tumors. In summary, MDM2-A expression can reduce the rate of transformation in MEFs in vitro, but we have no evidence that the phenotype can result in protection against tumorigenesis in vivo under the conditions employed.
Because Mdm2-a is frequently detected in tumors, and because the p53 pathway is frequently disrupted in tumors, it was important to assess the p53-independent role of MDM2-A. We previously showed that MDM2-A expressing p53-null MEFs had an identical growth rate compared to non-transgenic p53-null MEFs (19). Here we demonstrated that Mdm2-a transgenic p53-null MEFs more readily lose their contact inhibition and form significantly more transformed foci than p53-null controls (Figure 4). In addition, MDM2-A was found to alter the tumor spectrum in the absence of p53 in vivo, an observation consistent with a transforming potential for MDM2A. It is important to note that since no change in tumor spectrum was observed in the ENU treated mice with and without Mdm2-a expression, the difference observed in the p53 null mice was dependent upon the loss of p53.
The activity of MDM2-A to alter tumor spectrum likely requires the cooperation of another as yet unknown protein that may only be present in certain tissues, resulting in tissue specific transformation. There are a variety of different signaling pathways that could be influenced either directly by MDM2-A, or as a consequence of a reduction in availability of full-length MDM2. In addition to p53, MDM2 has been shown to bind to a number of proteins that could play a role in transformation or tumorigenesis such as, p73 (24), E2F1 (25), and MDM4 (26). A change in their abundance or regulation could result in the changes observed here. Microarray studies are currently in progress to address gene expression changes in response to MDM2-A in a p53-null context.
p53-heterozygous mice that expressed the Mdm2-a transgene developed highly undifferentiated, aggressive mammary tumors that were not detected in p53-heterozygous controls. Interestingly, Mdm2 splice variants have been detected in normal breast epithelium (9) and breast carcinomas (11). In addition, several of the observed mammary tumors displayed elevated MDM2-A expression relative to control tissues. Enhanced MDM2-A expression in high-grade tumors suggests that MDM2-A confers a growth advantage to these tumors resulting in a higher proportion of tumor cells expressing MDM2-A. Why this specific tumor type would arise in mice of this genotype is unclear but it is likely influenced by the longer tumor latency in the p53 heterozygote mice compared to those that are p53 null.
Despite its role in activation of wild-type p53, MDM2-A was unable to mediate any tumor protective activities in either Arf-null or ENU carcinogen treated tumor-prone models. However, MDM2-A expression clearly reduced the rate of transformation in vitro suggesting there may be a cellular protective effect against transformation for tissues that endogenously express MDM2 splice variants. In contrast, when p53 is deleted, MDM2-A enhances transformation, alters tumor spectrum and yields more highly undifferentiated tumors, suggesting a context-specific p53-independent oncogenic activity. These novel data help to explain the apparently contradictory observations that MDM2 splice variants are growth inhibitory yet are often expressed in aggressive tumors. Once p53 is deregulated, the oncogenic phenotype of MDM2 splice variants such as MDM2-A can be unmasked.
MATERIALS AND METHODS
Generation of Mdm2-a tumor models
The Institutional Animal Care and Use Committee (IACUC) approved all animal work and all experiments conformed to the applicable regulatory standards. Mdm2-a transgenic mice (FVB/NJ) were bred onto either the Arf null (C57Bl/6) or p53 null (C57Bl/6×129Sv) backgrounds (both generously provided by Dr. Martine Roussel, St. Jude Children’s Research Hospital). The mice were bred for two to three generations in order to obtain hemizygous and homozygous mice, and their litter mates were used as controls. For carcinogen treatment, mice were first pre-treated with isoproteronal (Sigma, St. Louis, MO), which was injected i.p. in sterile water at a dose of 25µg/g daily from P10 through P14. Pups were then treated with 60 µg/g ENU (Sigma) via daily i.p. injection on P15 through P19. Transgenic mice and their control wild-type littermates were monitored for tumor development and disease. Animals showing signs of pain or distress were euthanized according to IACUC guidelines. Euthanized mice were examined for lesions and tissue abnormalities.
Tumor diagnoses
Animals that developed tumors or displayed obvious signs of morbidity were euthanized, necropsied and tissue specimens were fixed in 10% neutral buffered formalin. Formalin fixed tissues of interest were embedded in paraffin, sectioned at 4–5µ, and stained with hematoxylin and eosin using standard methods. Tissue sections were assessed by light microscopy and immunocytochemistry was performed to determine tumor cellular lineage.
Statistical analysis
Fisher’s exact test was used to compare the proportion of animals that developed tumors among genotypes. Logrank test was used to assess differences in survival for the following groups: Mdm2-a/Arf-null (n=13) and Arf-null (n=15), Mdm2-a with ENU exposure (n=24) and wild-type littermates with ENU exposure (n=37), Mdm2-a/p53-null (n=23) and p53-null (n=24), Mdm2-a/p53-heterozygous (n=28) and p53-heterozygous (n=16).
MEF preparation and growth curve
MEFs were generated by standard methods and as described previously (27). Growth curves were performed on MEFs at P3–P5. Cells were plated in 6-well dishes in triplicate at 5×104 cells/well and counted daily for 5 days. Data are presented as the mean and standard deviation for three identical wells.
Protein extraction and western blot analysis
Protein extraction and western blot analysis was performed as previously described (28). Proteins were visualized with using anti-MDM2 antibody (R&D Systems Inc., Minneapolis, MN) and anti-tubulin antibody (clone 2.1, Sigma).
Transformation Assay
Focus forming assays were performed according to the following modifications of standard protocols (29) on Arf-null, p53-null and ENU treated MEFs in the presence and absence of Mdm2-a. Briefly, early passage MEFs were transfected with enhancing oncogenes using Fugene 6 and stably selected with 3µg/mL puromycin. Specifically, Arf-null MEFs were transfected with pBABE-puro-Ras (generously provided by Dr. Gerard Zambetti, St. Jude). The p53-null MEFs did not require any additional oncogenes. Transfected cells were plated at high density (1×106 cells/10cm dish) and observed for foci formation. Plates were stained with crystal violet and colonies were quantitated. For ENU treatment, wild-type and Mdm2-a MEFs were transfected with both pBABE-Ras and pBABE-E1A (modification of pWZL-hygro-E1A plasmid provided by Dr. Gerard Zambetti) and selected in puromycin. Cells were plated at high density, treated with 2mM ENU for 1 hour, washed and observed for foci formation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the St. Jude Animal Resource Center (ARC), June Bursi, Misty Cheney, and Justin Marlar for their technical assistance. We also thank the St. Jude ARC Diagnostic lab for histological assistance, Dorothy Bush for immunocytochemical, technical assistance, Catherine Billups for her biostatistics expertise, Julie Groff for generating the figures and Dr. Robert Cardiff (Comparative Medicine Center, University of California, Davis) for his review of the mammary gland tumors. This work was supported by NIH grants CA92401, CA21765 and the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991;10(6):1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The MDM2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 3.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 4.Ladanyi M, Cha C, Lewis R, Jhanwar SC, Huvos AG, Healey JH. MDM2 gene amplification in metastatic osteosarcoma. Cancer Res. 1993;53(1):16–18. [PubMed] [Google Scholar]
- 5.Corvi R, Savelyeva L, Breit S, et al. Non-syntenic amplification of MDM2 and MYCN in human neuroblastoma. Oncogene. 1995;10(6):1081–1086. [PubMed] [Google Scholar]
- 6.Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53(12):2736–2739. [PubMed] [Google Scholar]
- 7.Sigalas I, Calvert AH, Anderson JJ, Neal DE, Lunec J. Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: transforming ability and frequent detection in human cancer. Nat Med. 1996;2:912–917. doi: 10.1038/nm0896-912. [DOI] [PubMed] [Google Scholar]
- 8.Bartel F, Taubert H, Harris LC. Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer Cell. 2002;2:9–15. doi: 10.1016/s1535-6108(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 9.Bartel F, Pinkert CA, Fiedler W, Wurl P, Schmidt H, Taubert H. Expression of alternatively and aberrantly spliced transcripts of the MDM2 mRNA is not tumor-specific. International Journal of Cancer. 2004;24:143–151. doi: 10.3892/ijo.24.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto R, Tada M, Nozaki M, Zhang CL, Sawamura Y, Abe H. Short alternative splice transcripts of the mdm2 oncogene correlate to malignancy in human astrocytic neoplasms. Cancer Res. 1998;58(4):609–613. [PubMed] [Google Scholar]
- 11.Lukas J, Gao D-Q, Keshmeshian M, et al. Alternative and Aberrant Messenger RNA Splicing of the mdm2 Oncogene in Invasive Breast Cancer. Cancer Res. 2001;61:3212–3219. [PubMed] [Google Scholar]
- 12.Dias CS, Liu Y, Yau A, Westrick L, Evans SC. Regulation of hdm2 by stress-induced hdm2alt1 in tumor and nontumorigenic cell lines correlating with p53 stability. Cancer Research. 2006;66:9467–9473. doi: 10.1158/0008-5472.CAN-05-3013. [DOI] [PubMed] [Google Scholar]
- 13.Chandler DS, Singh RK, Caldwell LC, Bitler JL, Lozano G. Genotoxic stress induces coordinately regulated alternative splicing of the modulators MDM2 and MDM4. Cancer Research. 2006;66:9502–9508. doi: 10.1158/0008-5472.CAN-05-4271. [DOI] [PubMed] [Google Scholar]
- 14.Lents NH, Wheeler LW, Baldassare JJ, Dynlacht BD. Identification and characherization of a novel Mdm2 splice variant acutely induced by the chemotherapeutic agents adriamycin and actinomycin D. Cell Cycle. 2008;7(11):1580–1586. doi: 10.4161/cc.7.11.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans SC, Viswanathan M, Grier JD, Narayana M, El-Naggar AK, Lozano G. An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene. 2001;20:4041–4049. doi: 10.1038/sj.onc.1204533. [DOI] [PubMed] [Google Scholar]
- 16.Dang J, Kuo M-L, Eischen C, Stepanova L, Sherr C, Roussel M. The RING domain of Mdm2 can inhibit cell proliferation. Cancer Research. 2002;62:1222–1230. [PubMed] [Google Scholar]
- 17.Bartel F, Taylor AC, Taubert H, Harris LC. Novel mdm2 splice variants identified in pediatric rhabdomyosarcoma tumors and cell lines. Oncology Research. 2002;12:451–457. doi: 10.3727/096504001108747459. [DOI] [PubMed] [Google Scholar]
- 18.Fridman JS, Hernando E, Hemann MT, de Stanchina E, Cordon Cardo C, Lowe SW. Tumor promotion by Mdm2 splice variants unable to bind p53. Cancer Research. 2003;63(18):5703–5706. [PubMed] [Google Scholar]
- 19.Volk EL, Schuster K, Nemeth KM, Fan L, Harris LC. MDM2-A, a common Mdm2 splice variant, causes perinatal lethality, reduced longevity and enhanced senescence. Disease Models and Mechanisms. 2009;2:47–55. doi: 10.1242/dmm.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59(9):2217–2222. [PubMed] [Google Scholar]
- 21.de Kok A, Sips H, den Engelse L, Simons J. Transformation of C3H10T1/2 cells by N-ethyl-N-nitrosourea occurs as a single, low frequency, mutation-like event. Carcinogenesis. 1986;7(8):1387–1392. doi: 10.1093/carcin/7.8.1387. [DOI] [PubMed] [Google Scholar]
- 22.Weber J, Salinger A, Justice M. Optimal N-Ethyl-N-nitrosourea (ENU) Doses for Inbred Mouse Strains. Genesis. 2000;26:230–233. [PubMed] [Google Scholar]
- 23.Selye H, Veilleux R, Cantin M. Excessive stimulation of salivary gland growth by isoproterenol. Science. 1961;133:44–45. doi: 10.1126/science.133.3445.44. [DOI] [PubMed] [Google Scholar]
- 24.Zeng X, Chen L, Jost CA, et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19(5):3257–3266. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Wang H, Li M, Rayburn ER, Agrawal S, Zhang R. Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway. Oncogene. 2005;24(48):7238–7247. doi: 10.1038/sj.onc.1208814. [DOI] [PubMed] [Google Scholar]
- 26.Sharp DA, Kratowicz SA, Sank MJ, George DL. Stabilization of the MDM2 oncoprotein by interaction with the structurally related MDMX protein. J Biol Chem. 1999;274(53):38189–38196. doi: 10.1074/jbc.274.53.38189. [DOI] [PubMed] [Google Scholar]
- 27.Hogan B, Beddington R, Costnatini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 28.McKenzie PP, McPake CR, Ashford AA, Vanin EF, Harris LC. MDM2 does not influence p53-mediated sensitivity to DNA-damaging drugs. Mol Cancer Ther. 2002;1(12):1097–1104. [PubMed] [Google Scholar]
- 29.Sun H, Taneja R. Analysis of transformation and tumorigenicity using mouse embryonic fibroblast cells. Cancer Genomics and Proteomics: Humana Press; 2007. pp. 303–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.