Abstract
Background
Aberrant attentional processes in individuals with mood disorders – bipolar disorder (BD) and major depressive disorder (MDD) – have been well documented. This study examined whether unaffected youth at familial risk for mood disorders would exhibit poor alerting, orienting, and executive attention relative to age-matched controls.
Methods
A sample of youth (8–17 years old) having one parent with either BD or MDD (Mood-Risk, n=29) and youth having healthy parents (HC, n=27) completed the Attention Network Test-Short version (ANT-S), which assesses alerting, orienting, and executive attention.
Results
Relative to HCs, the Mood-Risk group had significantly slower reaction times on an index of executive attention, but no differences on indices of alerting or orienting. There were no differences between the two at-risk groups (i.e., youth with BD parent vs. youth with MDD parent) on any ANT-S measure.
Limitations
The current study is limited by its cross-sectional design, small sample size, and failure to control for familial environmental factors.
Conclusions
The findings extend previous results indicating that altered executive attention may represent an endophenotype for mood disorders in at-risk youth.
Keywords: mood disorders, attention, endophenotype, executive attention, sustained attention
Introduction
Bipolar disorder (BD) and major depressive disorder (MDD) share some genetic and familial risk factors (McMahon et al., 2010). Despite evidence demonstrating the heritability of these disorders with minimal contribution from shared environmental factors (e.g., McGuffin et al., 2003), understanding their genetic underpinnings remains tenuous due to their complexity. Because of this challenge, researchers have begun to explore easy to assess and heritable neurocognitive endophenotypes that may mediate underlying genetic mechanisms and the expression of the disorders (Glahn et al., 2004, 2010, 2012).
There is mounting evidence suggesting that deficits in attention may be a neuropsychological vulnerability marker for mood disorders (e.g., Harmer et al., 2002; Zakzanis et al., 1999). Although many studies have examined attention as a single construct, there is a growing consensus to consider attention as a multidimensional system (Posner and Peterson, 1990). This system is thought to encompass three specialized networks that subserve specific attentional functions: alerting (i.e., sustained attention and vigilance), orienting (i.e., selective attention to task informative visual cues), and executive attention (i.e., conflict processing) (Posner and Fan, 2004).
Some previous studies have shown deficits in indices of alerting and executive attention in individuals suffering from mood disorders (e.g., Bora et al., 2009; Habermann et al., 2005; Holmes and Pizzagalli, 2008). Additionally, poor performance on tasks indexing orienting abilities has been linked to unipolar depression (Haberman et al., 2005) and depressive symptomology in individuals with bipolar disorder (Jongen et al., 2007). However, in order to demonstrate that a trait is an endophenotype, it also has to be shown that the trait is mood state independent and heritable (Gottesman and Gould, 2003). Thus, studies have examined attention in unaffected relatives of individuals with mood disorders. Some studies (Bora et al. 2009; Brotman et al., 2009; Grunebaum et. al., 1978; Klimes-Dougan et al., 2006; Zalla et al., 2004) but not all (Clarke et al., 2005; Klimes-Dougan et al., 2006 ), suggest poor performance on tasks assessing executive attention and alerting. However, to the best of our knowledge no study has examined orienting problems in this population.
Of the few studies that have examined attentional processes in at-risk relatives, many have suffered from potential confounds such as medication effects and the presence of current/lifetime psychiatric disorders in some of the participants. Thus, the study of unaffected relatives, particularly youth, having a parent with either BD or MDD, represents an excellent opportunity to study premorbid vulnerability markers of mood disorders. The current study uses the Attention Network Test-Short version (ANT-S) to examine whether youth at familial risk for a mood disorder show aberrant alerting, orienting and executive attention, compared to aged-matched healthy controls.
Methods
Participants
Fifty-six youth (8–17 years old) participated. Of these, 29 (15 female; mean age = 14.10; SD=2.06) were at risk for mood disorders (Mood-Risk), by virtue of having a parent diagnosed with either BD (type I or II) (n=18; 9 males; mean age=14.77; SD=2.21) or MDD (n=11; 5 males; mean age=13.00; SD=1.20) and 27 (21 female; mean age = 14.20; SD=2.55) were healthy control (HC) participants, by virtue of having parents and first-degree relatives with no history of psychiatric diagnoses. Participants were recruited from ongoing longitudinal studies (NIMH R01MH060952, B.B.; K01MH073077, J.S.; PO1MH041712, N.D.R.).
The Structural Clinical Interview for DSM-IV (First et al., 1997) was used to ascertain current and lifetime psychopathology for both parents of all participants. The Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (Kaufman et al., 1997) was used to interview parents about their children and the children about themselves. Clinically trained interviewers under the supervision of a child and adolescent psychiatrist conducted the interviews; both were blind to the status of participants. All interviews were conducted at most 18 months (mean=5.67 months, SD=6.83) prior to the participants’ completion of the ANT-S. In addition, participants were screened on the day of the task for current psychopathology using the Stony Brook Symptom Inventory (Gadow & Sprafkin, 1998). Exclusion criteria included IQ<70, history of head trauma, neurological disorder, unstable medical illness, and use of drugs and alcohol. Socioeconomic status was measured using the Hollingshead Four-Factor Index (Hollingshead, 1975). The University of Pittsburgh Institutional Review Board approved the study. Parents signed consent forms, and youths signed assent forms.
Attention Network Test-Short Version
The ANT-S was presented using E-prime. Stimuli consisted of a central target, an arrow pointing left or right, flanked on each side by two arrows pointing in the same (congruent condition) or opposite (incongruent condition) direction. Participants indicated whether the central arrow pointed left or right by clicking on the left or right button on a mouse. Additionally, some trials contained a cue, which consisted of an asterisk that appeared before the central target arrow. There were four cue conditions: a) no cue (only a central fixation cross was presented) b) central cue (central asterisk cue was presented) c) spatial cue, (the asterisk cue was placed in the location where the central target arrow would be presented, either above or below the central fixation cross). The experiment consisted of 144 trials, divided into three blocks of 48 trials. Within each block, cue (no cue, center cue, spatial cue) and flanker (incongruent, congruent) varied in a pseudorandom order (see Fan et al., 2002).
The ANT-S yields three dependent variables that reflect the three attention networks. An index for the alerting network is derived by subtracting the median reaction time of trials with a center cue from the median reaction time of trials without a cue. An index for the orienting network is calculated by subtracting the median reaction time of trials with a spatial cue from the median reaction time of trials with a center cue. Finally, an index for the executive attention network or conflict score is derived by subtracting the median reaction time of trials with congruent flankers from trials with only incongruent flankers.
Data Analysis
ANCOVAs with Group as a between-subject factor (Mood-Risk and HC) and Age as a covariate were performed separately for mean error rates, the median reaction times for correct trials and the three attention network indices.
Results
Participant Characteristics
The two groups did not significantly differ with regard to age (t=.17, df= 54; p=.87) and socioeconomic status (t=1.03, df=47; p=.31), but differed in sex distribution (χ2 =4.13, df=1, 54; p=.04). The Mood-Risk group had a more balanced number of males and females and the control group had a higher number of females than males. Preliminary analyses did not yield significant effects of sex, thus this variable was not included in the statistical models.
Attention Network Test
There was no significant main effect of group in overall reaction time (RT) (F (1, 53) =.06, p=.82, ηp2=.001) and error rate (F (1, 53) =.04, p=.83; ηp2=.001). There was however a main effect of age for overall RT (F (1, 53) =24.84, p<.001; ηp2=.32), with reaction times becoming faster with increasing age. The attention index analyses resulted in a significant main effect of Group in executive attention performance (F (1, 53) =6.50, p=.01, ηp2=.11), reflected by higher conflict scores in the Mood-Risk than the HC group. There were no significant group differences for the alerting (F (1, 53) =.21, p=.65, ηp2=.004) or orienting (F (1, 53) =.14, p=.71, ηp2=.003) indices (see Table 1 and Figure 1). Additionally, there was a main effect of Age for alerting (F (1, 53) =7.03, p=.01, ηp2=.12), with younger participants showing less efficient alerting abilities (higher scores) than older participants.
Table 1.
Attention Network Test-Short version scores (RT and ratio), overall RT, and error rates (mean percentage) of the mood-risk and healthy control groups
| Mood-Risk Group | Healthy Control Group | |
|---|---|---|
| M (SE) | M (SE) | |
| Alerting | ||
| RT (ms) | 24.50 (4.45) | 21.56 (4.61) |
| Ratio | 0.041 (0.008) | 0.036 (0.008) |
| Orienting | ||
| RT (ms) | 58.55 (5.48)3 | 61.52 (5.67) |
| Ratio | 0.104 (0.009) | 0.106 (0.010) |
| Executive Attention | ||
| RT (ms) | 115.68 (5.94)* | 93.85 (6.16) |
| Ratio | 0.203 (0.010)* | 0.164 (0.010) |
| Overall | ||
| RT (ms) | 572.30 (13.07) | 576.72 (13.55) |
| Error (%) | 5.24 (0.74) | 5.02 (0.76) |
p<0.05,
M=Mean, SE=Standard Error, RT=Reaction Time, ms=Milliseconds
Note. Mood-Risk Group: Psychiatrically healthy youth at risk for bipolar disorder or major depressive disorder by virtue of having a parent with one of the disorders. Healthy Control Group: Psychiatrically healthy youth with parents that are free of current or lifetime psychiatric disorders.
Figure 1.
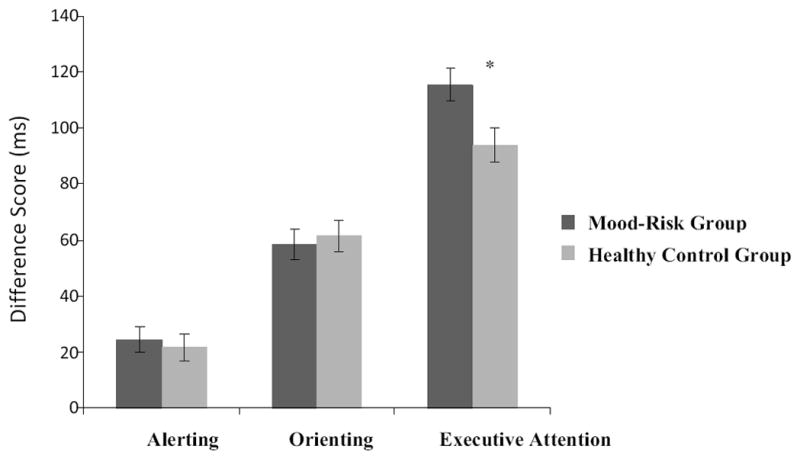
Alerting, Orienting, and Executive Attention Difference Scores (in milliseconds) for the Mood-Risk and Healthy Control groups. Note. Mood-Risk Group: Psychiatrically healthy youth at risk for bipolar disorder or major depressive disorder by virtue of having a parent with one of the disorders. Healthy Control Group: Psychiatrically healthy youth with parents that are free of current or lifetime psychiatric disorders. *p<0.05
Because attention network scores are influenced by overall RT, we eliminated this confound by developing ratio scores, which involved dividing each participant’s attention scores by their overall RT. Consistent with the original difference scores analyses, the Mood-Risk group had a higher conflict score (F (1, 53) =7.64, p=.008ηp2=.13), but the groups did not differ in alerting (F (1, 53) =.30, p=.59, ηp2=.006) or orienting (F (1, 53) =.02, p=.90, ηp2=.001) scores (see Table 1 and Figure 1). Secondary analyses were conducted comparing the HC group to participants at risk for BD, and participants at risk for MDD. The results trended toward an identical pattern to the analyses between the HC group and combined Mood-risk group, with MDD and BD demonstrating poorer conflict performance compared to HCs, with no significant differences between the BD and MDD subgroups on any ANT measures, all p>.05.
Discussion
Our results demonstrated that relative to healthy controls, unaffected youth at familial risk for mood disorders exhibited poor executive attention, but normal alerting and orienting. These findings suggest that executive attention may be a potential vulnerability marker for mood disorders. A large body of literature has linked executive attention to emotion regulation (e.g., Petersen and Posner, 2012), which in turn is thought to play a role in mood disturbance. It is therefore possible that executive attention represents a vulnerability marker for mood disorders by undermining emotion regulation abilities. Studies showing abnormal neural correlates of emotion regulation in unaffected youth at familial risk for mood disorders (Joorman et al., 2012; Mourao-Miranda et al., 2012) support this view.
We did not find differences between the groups on the alerting and orienting indices. To the best of our knowledge, no study has examined orienting processes in individuals at risk for mood disorders, but a few studies have found poor alerting performance in this population (e.g., Bora et al., 2009; Grunebaum et al., 1978). However, these studies used different tasks, such as the continuous performance task, that may tap other processes in addition to alerting. It should also be noted that evidence suggests that the alerting and orienting indices of the ANT have low reliability, which may explain lack of group differences in the current study. (The executive attention index demonstrated acceptable psychometric properties) (MacLeod et al., 2010).
Although our study has some notable strengths including a well characterized sample of unaffected youth, there are a number of limitations that merit consideration. The cross-sectional design limited our ability to investigate whether altered executive attention was associated with future onset of mood disorders. Additionally our small sample, due to the use of stringent eligibility criteria, may have limited our ability to detect statistically significant differences between each of the mood-risk groups (i.e., MDD-risk group and the BD-risk group) and the HCs. We also did not control for family environmental factors that could potentially contribute to poorer executive attention performance. However, even though these environmental factors could influence the future development of a mood disorder in these youth, the only study that examined such influence on attention and executive functioning in unaffected youth at risk for mood disorders, failed to find any associations (Klimes-Dougan et al., 2006).
In sum, our study lends support to research establishing executive attention as a potential endophenotype for mood disorders. Longitudinal studies with larger samples of offspring at familial risk for BD and MDD are needed to investigate the role of executive attention in the future onset of mood disorders.
Acknowledgments
The authors gratefully acknowledge the children and their families for participating in this study. The authors also thank Jacqueline Rosenstern (research data coordinator), Sharon Nau and Claire Dempsey (research assistants), UPMC employees on this project, for their assistance in research procedures.
Funding Sources
This study was supported in part by the National Institute of Mental Health (NIMH) (Ladouceur: K01 MH083001; Birmaher: R01 MH60952) and the National Alliance for Research on Schizophrenia and Depression (NARSAD): Blowitz-Ridgeway Young Investigator Award to Dr. Ladouceur and Nellie Blumenthal Independent Investigator Award to Dr. Phillips.
Footnotes
Conflict of Interest
Dr. Birmaher has received royalties for publications from Random House, Inc. (New Hope for Children and Teens with Bipolar Disorder) and Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). The other authors declare that they have no competing financial interests, or other interests that might be perceived to influence the results and discussion reported in the paper.
Contributors
First Author: Emily L. Belleau was primarily responsible for analyzing the study data and writing the manuscript.
Second Author: Mary L. Phillips was involved in conceptual aspects of the study and editing of the manuscript.
Third Author: Boris Birmaher was involved in conceptual aspects of the study and editing of the manuscript.
Senior Author: Cecile D. Ladouceur was primarily responsible for conceptual aspects of the study and editing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rooney MH, Skup M, Pine DS, Leibenluft E. Increased intrasubject variability in response time in youths with bipolar disorder and at-risk family members. J Am Acad Child Adolesc Psychiatry. 2009;48:628–635. doi: 10.1097/CHI.0b013e3181a27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Kempton MJ, Scarna A, Grasby PM, Goodwin GM. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic depression. Biol Psychiatry. 2005;57:183–187. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders. American Psychiatric Press, Inc; Washington, D.C: 1997. [Google Scholar]
- Gadow KD, Sprafkin J. Adolescent Symptom Inventory-4: Screening Manual. Checkmate Plus; Stony Brook, N.Y: 1998. [Google Scholar]
- Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW, Jr, Dassori A, Contreras J, Pacheco A, Lanzagorta N, Nicolini H, Raventos H, Escamilla MA. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry. 2010;67:168–177. doi: 10.1001/archgenpsychiatry.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Curran JE, Winkler AM, Carless MA, Kent JW, Jr, Charlesworth JC, Johnson MP, Goring HHH, Cole SA, Dyer TD, Moses EK, Olvera RL, Kochunov P, Duggirala R, Fox PT, Almasy L, Blangero J. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14. doi: 10.1016/j.biopsych.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grunebaum HU, Cohler BJ, Kauffman C, Gallant D. Children of depressed and schizophrenic mothers. Child Psychiatry Hum Dev. 1978;8:219–228. doi: 10.1007/BF01463553. [DOI] [PubMed] [Google Scholar]
- Habermann-Paelecke Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89:125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Clark L, Grayson L, Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia. 2002;40:1586–1590. doi: 10.1016/s0028-3932(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46:2904–2913. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen EMM, Smulders FTY, Ranson SMG, Baer MGA. Attentional bias and general orienting processes in bipolar disorder. J Behav Ther Exp Psychiatry. 2007;38:168–183. doi: 10.1016/j.jbtep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Joorman J, Coonery RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. J Abnorm Psychol. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Ronsaville D, Wiggs EA, Martinez PE. Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry. 2006;60:957–965. doi: 10.1016/j.biopsych.2006.03.031. [DOI] [PubMed] [Google Scholar]
- MacLeod JW, Lawrence MA, McConnell MM, Eskes GA, Klein RM, Shore DI. Appraising the ANT: Psychometric and theoretical considerations of the Attention Network Test. Neuropsychology. 2010;24:637–651. doi: 10.1037/a0019803. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Akula N, Schulze TG, Muglia P, Tozzi F, Detera-Wadleigh SD, Steele CJ, Breuer R, Strohmaier J, Wendland JR, Mattheisen M, Muhleisen TW, Maier W, Nothen MM, Cichon S, Farmer A, Vincent JB, Holsboer F, Preisiq M, Rietschel M. Meta-analysis of genome-wide association data identifies a risk locus for major mood disorders on 3p21.1. Nature Genet. 2010;42:128–131. doi: 10.1038/ng.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourao-Miranda J, Oliveira L, Ladouceur CD, Marquand A, Brammer M, Birmaher B, Axelson D, Phillips ML. Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders. PLoS ONE. 2012;7:e29482. doi: 10.1371/journal.pone.0029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Fan J. Attention as a organ system. In: Pomerantz JR, Crair MC, editors. Topics in integrative neuroscience: From cells to cognition. Cambridge University Press; Cambridge, UK: 2004. [Google Scholar]
- Posner MI, Peterson SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Peterson SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. Neuropsychological differential diagnosis. Swets & Zeitlinger; Amsterdam: 1999. [Google Scholar]
- Zalla T, Joyce C, Szöke A, Schürhoff F, Pillon B, Komano O, Perez-Diaz F, Bellivier F, Alter C, Dubois B, Rouillon F, Houde O, Leboyer M. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res. 2004;121:207–217. doi: 10.1016/s0165-1781(03)00252-x. [DOI] [PubMed] [Google Scholar]


