Abstract
The Wnt/β-catenin signaling pathway is well characterized in stem cell biology and plays a critical role in liver development, regeneration, and homeostasis. We hypothesized that pharmacological activation of Wnt signaling protects against hepatic ischemia/reperfusion (I/R) injury through its known proliferative and anti-apoptotic properties. Sprague-Dawley rats underwent 70% hepatic ischemia by microvascular clamping of the hilum of the left and median lobes of the liver for 90 min, followed by reperfusion. Wnt agonist (2-amino-4-[3,4-(methylenedioxy)benzylamino]-6-(3-methoxyphenyl)pyrimidine, 5 mg/kg BW) or vehicle (20% DMSO in saline) in 0.5 ml was injected intraperitoneally (i.p.) 1 h prior to ischemia or infused intravenously over 30 min right after ischemia. Blood and tissue samples from the pre-treated groups were collected 24 h after reperfusion, and a survival study was performed. Hepatic expression of β-catenin and its downstream target gene Axin2 were decreased after I/R while Wnt agonist restored their expression to sham levels. Wnt agonist blunted I/R-induced elevations of AST, ALT, and LDH and significantly improved the microarchitecture of the liver. The cell proliferation determined by Ki67 immunostaining significantly increased with Wnt agonist treatment and inflammatory cascades were dampened in Wnt agonist-treated animals, as demonstrated by attenuations in IL-6, myeloperoxdase, iNOS and nitrotyrosine. Wnt agonist also significantly decreased the amount of apoptosis, as evidenced by decreases in both TUNEL staining as well as caspase-3 activity levels. Finally, the 10-day survival rate was increased from 27% in the vehicle group to 73% in the pre-treated Wnt agonist group and 55% in the Wnt agonist post-ischemia treatment group. Thus, we propose that direct Wnt/β-catenin stimulation may represent a novel therapeutic approach in the treatment of hepatic I/R.
Keywords: Wnt/β-catenin, hepatic ischemia/reperfusion, proliferation, inflammation, nitrosative stress, apoptosis
INTRODUCTION
Liver damage induced by hepatic ischemia/reperfusion (I/R) is a key contributing factor to the morbidity associated with several surgical conditions and interventions including orthotopic liver transplantation, oncologic resection, trauma, and prolonged shock states. In these clinical settings, hepatocellular injury is in part due to the anoxic cell death incurred during the ischemic period. However, with the return of blood flow comes an accumulation of inflammatory cells and mediators, reactive oxygen species (ROS) and reactive nitrogen species (RNS), and the subsequent biochemical derangements in intracellular homeostasis that all work to induce further cell death from inflammation, apoptosis and necrosis (1). These events may lead to delayed graft function in the case of transplant recipients, or increase in complications, length of hospital stay, and cost of care for those experiencing I/R injury. Currently, ischemic preconditioning is the only technique proven to provide a benefit (2), and this can only be used as an intraoperative preventative measure. Accordingly, many attempts have been made to discover pharmacologic treatments to alleviate hepatic I/R injury, however none have yet proven successful, stressing the importance of developing modalities that limit I/R injury and improve patient outcomes.
The liver differs from other visceral organs in its innate ability for short-term regeneration (3). Therefore, therapy aimed at stimulating pathways involved in the proliferation of new hepatocytes to replace dead/damaged cells, or at conditioning cells to respond differently to an ischemic insult, might prove particularly beneficial in cases of hepatic I/R. One crucial pathway in cell replication and regeneration is the Wnt/β-catenin signaling axis. Canonical Wnt signaling has been identified as central to embryonic development, progenitor cell differentiation, and proliferation of cells arising from all three germ layers (4-6). Moreover, Wnt/β-catenin signaling has been shown to play a key role specifically in liver development, prevention of apoptosis, and protection from metabolic stress (7). In utero deletion of hepatoblast β-catenin has been shown to lead to underdeveloped livers and embryonic lethality (8). In an animal model of chemical injury with partial hepatectomy, adult hepatic stem cells, or oval cells, were also found to replicate following insult in a Wnt/β-catenin-dependent manner (9). These findings suggest that perhaps pharmacologic manipulation of Wnt signaling plays an important role in the treatment of liver injury.
The modified pyrimidine compound 2-amino-4-[3,4-(methylenedioxy)benzylamino]-6-(3-methoxyphenyl)pyrimidine (Wnt agonist) was recently identified as a small-molecule agonist of the Wnt signaling pathway (10). Its use has to date been limited to in vitro and embryologic studies, where it has demonstrated the ability to upregulate the β-catenin/T-cell factor (Tcf) dependent target genes that drive mitosis (11). The aim of the present study was therefore to test the hypothesis that administration of Wnt agonist reduces tissue damage and apoptosis, and promotes hepatocyte regeneration and proliferation in an established animal model of hepatic I/R.
MATERIALS AND METHODS
Experimental animals
Adult male Sprague-Dawley rats (250-275 g; Charles River Laboratories, Wilmington, MA) were housed in a temperature-controlled room on a 12-hour light/dark cycle and fed a standard Purina rat chow diet. Animals were fasted overnight before undergoing surgery but allowed water ad libitum. All experiments were performed in accordance with the National Institutes of Health guidelines for use of experimental animals, and this study was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Animal model of hepatic I/R
On the day of surgery, rats were premedicated with intraperitoneal injection of either 0.5 ml of Wnt agonist (5mg/kg BW, EMD Biosciences, San Diego, CA) or vehicle (20% dimethyl sulfoxide in normal saline). One hour later, animals underwent induction of anesthesia with inhalational isolfurane, after which the ventral abdomen was shaved and cleansed with 10% povidone-iodine wash. A 3-cm midline incision was performed and the hilum of the liver was exposed, allowing for identification of the hepatic artery and portal vein. A microvascular clip was placed across the hilum of the left-lateral and median lobes in order to produce 70% hepatic ischemia. The clip was removed after 90 min to allow reperfusion, the abdomen closed, and the anesthesia withdrawn. Sham-operated animals underwent midline laparotomy alone, without hepatic ischemia or administration of treatment. Core body temperature was maintained between 35.5-37°C throughout the entirety of the operation by use of an indwelling rectal thermometer and a heating pad placed below the animals. Blood and liver samples were collected 24 h following clip removal and stored at −80°C prior to use.
Survival study
Following the process of hepatic I/R described in the animal model, the non-ischematized 30% of the liver was resected at the onset of perfusion. The animals were monitored for 10 days to record survival. Three experimental groups were created. In group 1, the rats were administered intraperitoneal Wnt agonist (5mg/kg BW) 1 h prior to hepatic ischemia. In group 2, the rats were administered intravenous Wnt agonist (5mg/kg BW) over 30 min, beginning at the onset of reperfusion. In group 3, rats were administered intraperitoneal vehicle (20% dimethyl sulfoxide in normal saline) 1 h prior to hepatic ischemia.
Western blotting analysis
Liver samples (100 mg) were lysed and homogenized in 300 μl lysis buffer (10 mM Tris-HCl pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) using a sonic dismembrator on ice. Samples were centrifuged at 14,000 rpm for 15 min at 4°C, and the supernatant collected. Following measurement of sample protein concentration by Pierce BCA protein assay kit (Pierce Biotechnology, Rockford, IL), 50 μg samples were separated on 4-12% Bis-Tris gels and transferred to nitrocellulose membranes. Membranes were incubated with primary antibody against β-catenin, iNOS, nitrotyrosine, or β-actin (Santa Cruz BioTechnologies, Santa Cruz, CA). All protein bands were detected by species-specific infrared fluorescence secondary antibiodies (1:10,000) and analyzed by the LI-COR Odyssey Fc Imager (LI-COR, Lincoln, NE).
Determination of serum liver enzymes and IL-6
Whole blood samples were centrifuged at 4,000 rpm for 12 min to collect serum, which was then stored at −80°C prior to use. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were determined by commercial assay kits from Pointe Scientific (Lincoln Park, MI). Serum IL-6 levels were determined by an enzyme-linked immunosorbent assay kit specific for rat IL-6 (BD Biosciences, San Diego, CA). The assays were carried out according to the instructions provided by the manufacturer.
Real-time RT-PCR analysis
Total RNA was extracted from the liver by “TRIzol reagent” (Invitrogen, Carlsbad, CA). Real-time PCR was carried out on cDNA samples which were reversely transcribed from 2 μg RNA using murine leukemia virus reverse-transcriptase (Applied Biosystems, Foster City, CA). A PCR reaction was carried out in a 24 μl final volume containing 0.08 μmol of each forward and reverse primer, 2 μl cDNA, 9.2 μl H2O and 12 μl SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA). Amplification was conducted in an Applied Biosystems 7300 real-time PCR machine under the thermal profile of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The level of mouse β-actin mRNA was used for normalization and each specific mRNA was conducted in duplicate. Relative expression of mRNA was calculated by the 2−ΔΔCt method and results were expressed as fold change in comparison to control group. The sequence of primers for this study is listed as follow. Rat Axin2, 5′-GAC CGA CGA TTC CAT GTC C-3′ (forward) and 5′-CCA GCT CCA GTT TCA GCT TC-3′ (reverse). Rat IL-6, 5′-AGG GAG ATC TTG GAA ATG AGA AAA-3′ (forward) and 5′-CAT CAT CGC TGT TCA TAC AAT CAG-3′ (reverse). Rat β-actin, 5′-CGT GAA AAG ATG ACC CAG ACT A-3′ (forward) and 5′-TGG TAC GAC CAG AGG CAT ACA G-3′ (reverse).
Hepatic myeloperoxidase assessment
Liver tissue (100 mg) was homogenized in 1 ml of KPO4 buffer containing 0.5% hexa-decyltrimethyl-ammonium bromide by sonication and incubated at 60°C for 2 h. Samples were centrifuged to collect the supernatant, and then measured for protein concentration. The reaction was carried out in a 96-well plate by adding samples into phosphate buffer containing o-dianisidine hydrochloride and H2O2. Light absorbance was read at 460 nm over a period of 5 minutes. MPO activity (1 unit was equal to the change in absorbance per min) was expressed as units per gram of protein.
Histologic evaluation of liver injury
Liver biopsies were taken from the median lobe following 24 h of reperfusion and stored in 10% formalin before being fixed in paraffin. Biopsies were then sectioned to 4 μm cuts and stained with hematoxylin-eosin. Liver parenchymal injury was then assessed in a blinded fashion using a semi-quantitative light microscopy evaluation. The histologic injury score for each sample was expressed as the sum of the individual scores given for 6 different parameters: cytoplasmic color fading, vacuolization, nuclear condensation, nuclear fragmentation, nuclear fading, and erythrocyte stasis (12). Scores for each finding ranged from 0 (0%), to 1 (1-10%), 2 (10-50%), or 3 (>50%), with a highest possible score of 18. Each sample score was then averaged over 10 microscopic fields.
Immunostaining of Ki67 and TUNEL
To determine the proliferative status of hepatocytes in our study, we stained liver tissues for Ki67, which is a nuclear protein strictly present in proliferating cells and absent from resting/Go cells (13). Paraffin-embedded sections were dewaxed in xylene and rehydrated in a graded series of ethanol. For Ki67 staining, slides were incubated in 0.92% citric acid buffer (Vector Laboratories, Burlingame, CA) at 95°C for 15 min. After cooling to room temperature, the slides were incubated with 2% H202 in 60% methanol and blocked in 2% normal rabbit serum/TBS, after which they were incubated with goat anti-Ki67 antibody (1:50, Santa Cruz Biotechnologies) in 1% normal rabbit serum/TBS with 0.02% Triton X-100 at 4°C overnight. The detection was carried out as per the instructions provided by a commercially available immunohistochemistry kit with NovaRED substrate (Vector Laboratories). For TUNEL staining, fluorescence staining was performed using a commercially available In Situ Cell Death Detection Kit (Roche). The assay was conducted according to the manufacturer’s instructions. The nucleus was stained with propidium iodide. Results were expressed as the average number of Ki67 or TUNEL positive staining cells per 10 microscopic fields.
Determination of tissue caspase-3 activity
Liver tissue was homogenized in a lysis buffer consisting of 10 mM Hepes (pH 7.4), 5 mM MgCl, 1 mM DTT, 1% Triton-X 100, 2 mM EGTA, 2mM EDTA, and a protease inhibitor cocktail by sonication. After centrifugation, the supernatant was collected and measured for protein concentration. Cell lysate and a caspase-3 substrate peptide, Ac-DEVD-AMC (BD Biosciences, San Diego, CA), was added to the assay buffer (20 mM Hepes (pH 7.4), 5 mM DTT, 2 mM EDTA, and 0.1% CHAPS) and incubated in the dark at 37°C for 75 min, after which a fluorometric reader was used to determine the cleaved product at the excitation (370 nm) and emission (450 nm). Results were divided by corresponding protein concentrations, and caspase-3 activity was expressed as relative fold change to the sham group.
Statistical analysis
All data are expressed as a mean ± SE (n=4-6/group) and compared by one-way analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) test. Survival rate was determined by the Kaplan-Meier estimator and compared by a log-rank test (n=15-20/group). Differences in values were considered significant if P < 0.05.
RESULTS
Wnt agonist upregulates β-catenin signaling after hepatic I/R
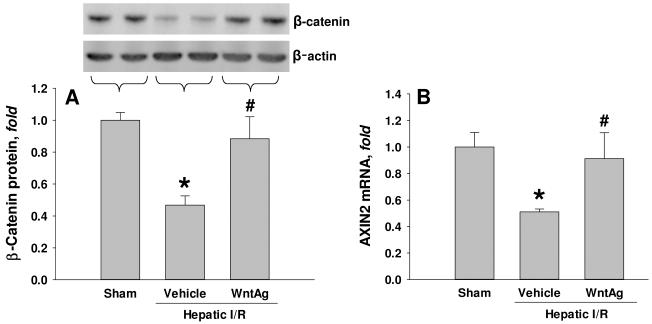
To investigate whether in vivo Wnt agonist administration had any effect on β-catenin signaling, we measured hepatic protein levels of β-catenin and its downstream target gene 24 h after hepatic I/R. Compared to sham-operated animals, the vehicle-treated group had a 53% decrease of β-catenin protein levels in liver tissue (Fig. 1A). Hepatic β-catenin protein levels were restored to 88% of sham levels with administration of Wnt agonist (Fig. 1A). In a similar fashion, hepatic I/R resulted in a 49% decrease in gene transcription of Axin2, a known target gene of Wnt/β-catenin signaling (14), compared to sham-operated animals, which was restored to 91% in the Wnt agonist-treated group (Fig. 1B). These findings suggest that administration of Wnt agonist effectively upregulates Wnt/ β-catenin signaling in the liver which is suppressed under I/R stress.
Fig. 1. Effect of Wnt agonist on β-catenin and its downstream gene expression after hepatic I/R.
Liver tissues were harvested 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (WntAg) groups. (A) Total cell lysate were subjected to Western blotting. Representative blots against β-catenin and β-actin are shown. Blots were scanned and quantified with densitometry. Band intensity of β-catenin was normalized to the corresponding band intensity of β-actin. (B) Total RNA was isolated for determining Axin2 mRNA levels by real-time RT-PCR analysis. Axin2 expression levels were normalized to β-actin. The value in sham group is designated as 1 for comparison. Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
Wnt agonist attenuates liver tissue injury after hepatic I/R
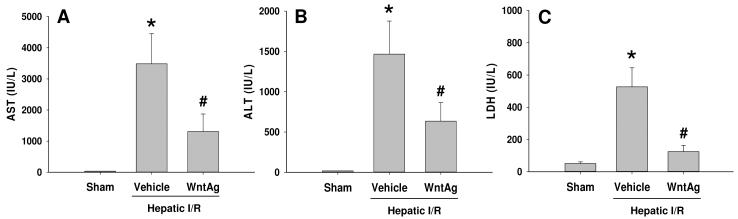
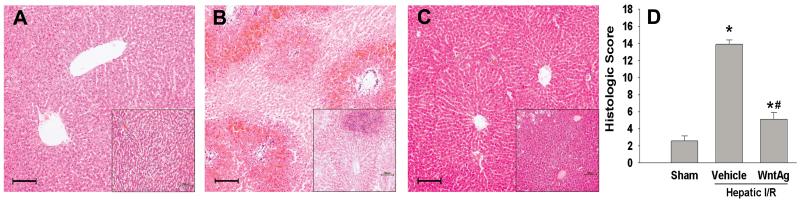
Hepatocellular damage was surveyed by measuring serum AST, ALT, and LDH levels, which increased by 97-, 83-, and 10-fold, respectively, 24 h after hepatic I/R (Fig. 2). Contrastingly, treatment with Wnt agonist prior to I/R significantly reduced injury levels by 63%, 57%, and 76%, respectively (Fig. 2). This data correlated with the alterations in tissue architecture observed histologically. At 24 h after reperfusion, vehicle-treated livers demonstrated severe architerctural abnormalities, most notably diffuse cellular edema and necrosis, micro-hemorrhage, and leukocyte infiltration (Fig. 3B). In contrast, liver tissue architecture was remarkably preserved in Wnt agonist treated animals, with moderate hepatocellular edema being the predominant difference when compared to the livers of sham-operated animals (Figs. 3A and C). As quantified in Fig. 3D, animals undergoing I/R with vehicle treatment exhibited a significant increase in the liver histologic injury score when comapared to sham-operated animals, which was reduced by 63% with administration of Wnt agonist. Together, these results demonstrate that Wnt agonist administration confers a significant protection against liver injury from hepatic I/R.
Fig. 2. Effect of Wnt agonist on liver tissue injury after hepatic I/R.
Serum samples were collected 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (WntAg) groups for measuring AST (A), ALT (B), and LDH (C). Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
Fig. 3. Effect of Wnt agonist on tissue damage and cellular architecture in the liver after hepatic I/R.
Histological findings of the liver in the sham (A), vehicle (B), and Wnt agonist-treated (C, WntAg) groups. Liver tissues were harvested 24 h after reperfusion, processed, and stained with hematoxylin-eosin. Representative photomicrographs at 100× magnification with 200x magnification insets. Bar length equal to 100 micrometers. (D) Semi-quantitative histologic injury score measuring differences in cytoplasmic vacuolization, cytoplasmic faiding, nuclear condensation, nuclear faiding, nuclear fragmentation, and erythrocyte stasis examined on standard hematoxylin-eosin staining as described in Materials and Methods. Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
Wnt agonist promotes hepatocyte proliferation after hepatic I/R
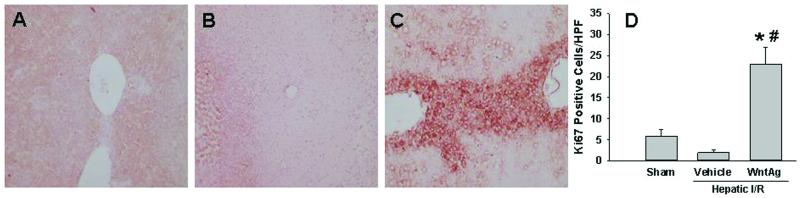
In order to determine the effect of Wnt agonist administration on hepatocyte proliferation after I/R injury, we performed immunohistochemical staining against Ki67. As shown in Figs. 4A-C, minimal immunostaining of Ki67 was seen in liver tissue sections of either the sham or vehicle groups, but was markedly increased after hepatic I/R with Wnt agonist treatment. With quantification by counting, I/R with vehicle administration resulted in a 66% reduction in Ki67 positive staining cells, compared to sham-operated animals (Fig. 4D). However, the number of Ki67 positive staining cells was increased 11.5-fold when Wnt agonist was given (Fig. 4D). These results indicate that upregulation of Wnt/β-catenin signaling was able to promote cellular proliferation despite the presence of physiologic insult.
Fig. 4. Effect of Wnt agonist on hepatocyte proliferation after hepatic I/R.
Liver tissues were harvested 24 h after reperfusion, processed, and subjected to immunohistochemical analysis against Ki67 with red staining. Representative images for sham (A), vehicle (B), and Wnt agonist-treated (C, WntAg) groups are shown at 100× magnification. (D) A graphical representation of Ki67 positive staining cells averaged over 10 microscopic fields/animal. Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
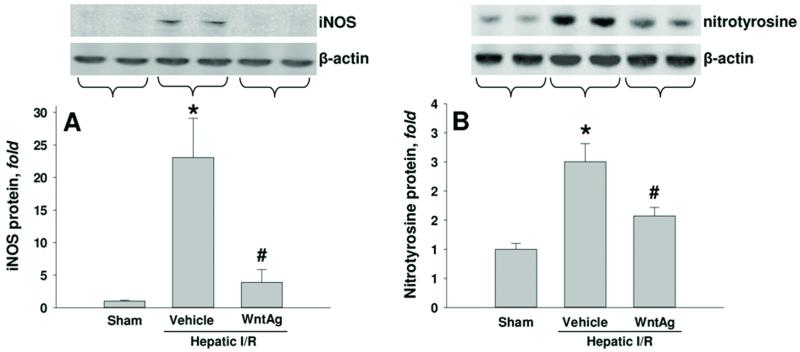
Wnt agonist lowers nitrosative stress after hepatic I/R
In order to determine the effect of Wnt agonist on nitrosative burden following I/R, hepatic tissue levels of iNOS and nitrotyrosine were evaulated 24 h after I/R. Compared to the sham group, we observed a 23-fold increase in iNOS protein expression in vehicle-treated I/R animals, which was reduced by 83% with administration of Wnt agonist (Fig. 5A). There was an associated 2.5 fold increase in nitrotyrosine levels in vehicle-treated I/R animals when compared to sham-operated animals. This was decreased by 37% in animals treated with Wnt agonist (Fig. 5B).
Fig. 5. Effect of Wnt agonist on iNOS and nitrotyrosine expression after hepatic I/R.
Liver tissues were harvested 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (WntAg) groups. Total cell lysate were subjected to Western blotting. Representative blots against (A) iNOS and corresponding β-actin (B) nitrotyrosine and corresponding β-actin are shown. Blots were scanned and quantified with densitometry. Band intensity of iNOS or nitrotyrosine was normalized to the corresponding band intensity of β-actin. Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
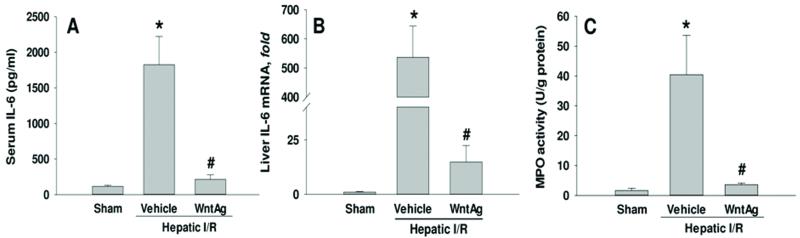
Wnt agonist reduces the inflammatory response and neutrophil infiltration into the liver after hepatic I/R
IL-6 levels in both serum and the liver were measured to ascertain the ability of Wnt/β-catenin activation the systemic and local inflammatory responses to hepatic I/R, respectively. At 24 h after reperfusion, vehicle-treated animals had circulating levels of IL-6 that were 16-fold greater than their sham-operated counterparts (Fig. 6A). This increase in serum IL-6 levels was decreased by 88% when animals were administered Wnt agonist (Fig. 6A). In the liver, hepatic I/R resulted in a 449-fold increase of IL-6 mRNA expression in comparison to sham, which was decreased by 97% when Wnt agonist was administered (Fig. 6B). An additional means by which we assessed the inflammation associated with I/R was measuring alterations in hepatic neutrophil infiltration 24 h after reperfusion. MPO activity was used as an indicator of neutrophil migration and subsequent proteolytic inflammation. When compared to the sham group, vehicle-treated animals showed a 25-fold increase in hepatic tissue levels of MPO (Fig. 6C). This was reduced by 91% with Wnt agonist administration (Fig. 6C). Together, these data suggest that Wnt agonist administration led to a downregulation of proinflammatory markers and the associated neutrophil recruitment.
Fig. 6. Effect of Wnt agonist on the proinflammatory cytokine expression and neutrophil infiltration into the liver after hepatic I/R.
Serum and liver tissues were harvested 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (WntAg) groups. (A) Serum IL-6 levels were determined by ELISA. (B) Liver tissue IL-6 mRNA expression was determined by real time RT-PCR analysis. IL-6 expression levels were normalized to β-actin. The value in sham group is designated as 1 for comparison. (C) Liver tissue myeloperoxidase (MPO) activity, a marker for neutrophil infiltration, was determined spectrophotometrically. Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
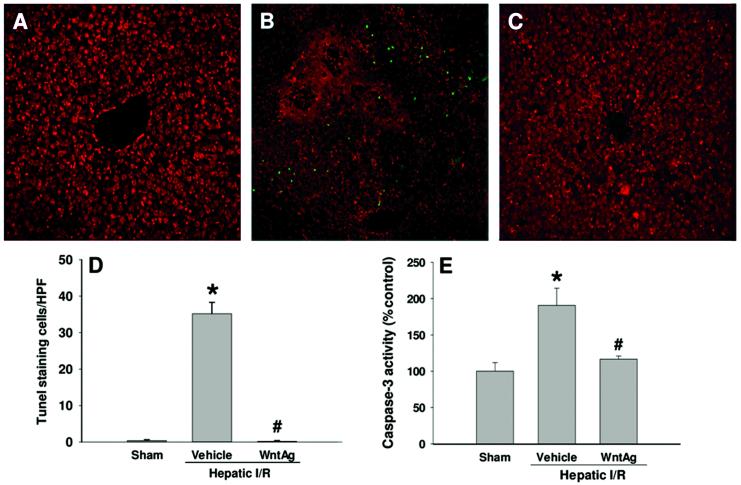
Wnt agonist reduces apoptosis after hepatic I/R
To investigate whether Wnt agonist administration had any effect on apoptosis following hepatic I/R, we first conducted the TUNEL assay to detect the DNA fragmentation in the liver tissues of each group 24 h after reperfusion. The TUNEL positive cells (green spot) were sparsely observed in the sham animals, whereas there were many green spots in the vehicle-treated animals (Fig. 7A and B). With Wnt agonist administration, the TUNEL positive cells became hardly detectable in the liver (Fig. 7C). The number of countable apoptotic cells increased dramatically in hepatic I/R with vehicle in comparison to sham, and was returned to sham levels with administration of Wnt agonist (Fig. 7D). Furthermore, we measured the activity of caspase-3 in the liver. We observed a 91% increase in caspase-3 activity in the vehicle-treated I/R group in comparison to the sham-operated group, which was reduced by 39% with administration of Wnt agonist (Fig. 7E). Although this significant attenuation of apoptosis by Wnt agonist was observed, the number of apoptotic cells and magnitude of caspase-3 activation were not markedly elevated in the vehicle group. From the analysis of hematoxylin-eosin staining, the majority of hepatocytes died from necrosis. Thus, protection of hepatocytes from apoptosis may not be the primary activity of Wnt agonist in reducing hepatic I/R injury.
Fig. 7. Effect of Wnt agonist on induction of apoptosis after hepatic I/R.
Liver tissues were harvested 24 h after reperfusion. (A-C) Liver sections were subjected to TUNEL assay to detect DNA fragmentation. Representative images for sham (A), vehicle (B), and Wnt agonist-treated (C, WntAg) groups. Green, TUNEL positive and red, propidium iodide staining for nucleus. (D) A graphical representation of TUNEL positive staining cells averaged over 10 microscopic fields/animal. (E) Liver tissue caspase-3 activity was measured by a fluorometric assay. Data presented as means ± SE (n=4-6/group) and compared by one-way ANOVA and SNK method;*P > 0.05 vs. Sham; #P > 0.05 vs. Vehicle.
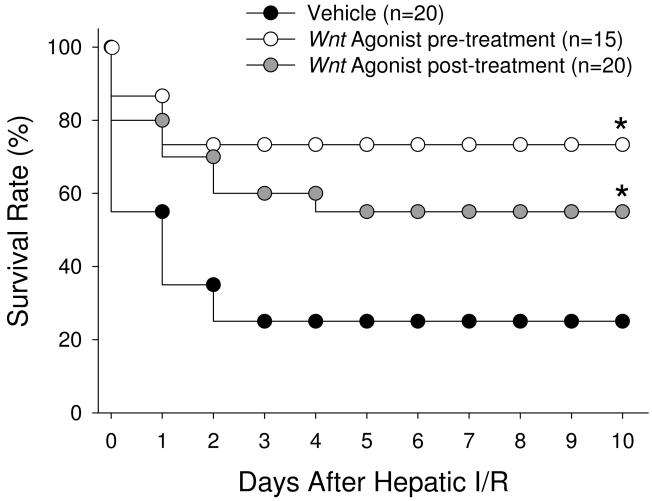
Wnt agonist improves survival following a lethal model of hepatic I/R
To determine the potential survival benefit of Wnt agonist treatment, we used a total hepatic I/R model as previously described (15). We observed that only 27% of vehicle-treated animals survived 10 days after undergoing I/R with partial hepatectomy. Contrastingly, the 10-day survival rate increased significantly to 73% in the group that was treated with Wnt agonist 1 h prior to the onset of ischemia and to 55% in those animals receiving Wnt agonist at the onset of reperfusion (Fig. 8), with no statistical difference between these two treatment groups (P = 0.34).
Fig. 8. Effect of pre- and post-administration of Wnt agonist on survival of a lethal model of hepatic I/R.
Survival rates over a 10-day period following 70% hepatic I/R with resection of the remaining 30% of liver tissue are demonstrated for vehicle (black), pre-treatment (grey), and post-treatment (white) groups. Survival rates were analyzed by the Kaplan-Meier estimator using a log-rank test. *P > 0.05 vs. Vehicle.
DISCUSSION
As advances in surgery and critical care allow for more aggressive interventions and successful resuscitations, and as the annual number of orthotopic liver transplants performed increases, so too does the number of patients at risk for hepatic I/R injury. For this reason, research aimed at the prevention and treatment of this pathology has garnered considerable attention. One molecular pathway that has demonstrated promise in animal models is Wnt/β-catenin signaling. We herein report the effect of canonical Wnt/β-catenin activation through a novel Wnt agonist on liver injury and survival in a rat model of hepatic I/R. In untreated animals, 90 min of 70% hepatic I/R resulted in dramatic increases in pro-inflammatory cytokines, apoptosis, and associated hepatocellular injury. Conversely, through pharmacologic manipulation of Wnt activation these damage indices were significantly attenuated and the mortality rate was significantly reduced in a lethal model of hepatic I/R.
In the Wnt signaling cascade, when endogenous Wnt ligands bind their G protein-coupled cell surface receptors, frizzled, cytoplasmic dishevelled is able to inactivate a β-catenin destruction complex that includes APC, Axin, and GSK-3β. This complex works to phosphorylate β-catenin, effectively tagging it for proteasomal destruction. Wnt ligand signaling thus leads to stabilized cytoplasmic β-catenin that is free to translocate to the nucleus, where it communicates with the transcription factors T-cell factor/lymphoid enhancer factor (Tcf/Lef) to drive downstream target gene expression (16). These genes include c-myc, cyclin-d1, and axin2, which regulate cell cycle progression, amongst other actions (17). Our study proved consistent with these findings, as we observed that the Wnt agonist compound was able to reverse the downregulation in β-catenin protein and axin2 mRNA that occurred as a result of I/R injury, indeed restoring them to normal levels. The direct association between β-catenin and axin2 is supported by the findings in other studies that increased axin2 levels indicate an upregulation of Wnt/β-catenin signaling (18). Axin2, also referred to as axil or conductin, acts as safety valve in Wnt/β-catenin signaling through its ability to phosphorylate/ubiquinate cytosolic β-catenin and thereby act as a negative-feedback regulator in the prevention of unregulated β-catenin gene transcription (19).
One of the striking phenomena observed in the present study is that despite the severe cellular insult caused by 90 min of warm hepatic I/R, animals treated with Wnt agonist had an abundance of hepatocytes staining with Ki67, indicating that these cells were proliferating instead of suffering from apoptosis or necrosis. Although the liver is unique amongst visceral organs in its ability to rapidly regenerate almost all of its mass after partial hepatectomy or metabolic stress (3), we did not observe this in vehicle-treated animals following I/R, which was likely due to the severity of our model. The presence of proliferating hepatocytes in our Wnt treatment group was consistent with other studies, in which activation of Wnt/β-catenin signaling has demonstrated to be associated with proliferative and regenerative activities (20). For example, Terada and colleagues demonstrated that Wnt/β-catenin signaling plays a key role in cellular regeneration after renal I/R injury by stimulating renal tubule cell cycle progression (21). Nejak-Bowen, et al, found that transgenic mice with overexpression of hepatic β-catenin levels showed more hepatocytes in the S-phase following partial hepatectomy than in wild-type mice (22). They further confirmed that this phenomenon was depended on β-catenin by activating β-catenin signaling with administration of Wnt-1 naked DNA in wild-type mice. Similarly, Lehwald, et al, observed that liver-specific β-catenin knock-down mice had increased hepatic injury following I/R, whereas transgenic Wnt-1 overexpressing mice were resistant to hepatic I/R injury (23). The authors attributed this to an increase in hypoxia inducible factor-1α (HIF-1α) activation by β-catenin. The role of HIF-1α in mediating Wnt agonist activity during hepatic I/R requires further investigation.
In addition to conditioning hepatocytes to proliferate in the presence of I/R injury, Wnt agonist administration also decreased the cellular stress caused by I/R. Nitrosative stress is increasingly being recognized as playing an important role in the cellular damage associated with I/R injury (24). When iNOS is upregulated in I/R injury the excessive nitric oxide (NO) produced is free to react with superoxide anion (O2−), creating peroxynitrite (ONOO−). Peroxynitrite then contributes to injury through lipid peroxidation, apoptosis, necrosis, and neutrophil recruitment by nitration of tyrosine residues on tissue proteins (25). Therapies aimed at iNOS inhibition have been suggested as therapeutic targets in hypoxia-induced injury settings in part because iNOS inhibition leads to decreased tissue caspase-3 activity, and therefore decreased apoptosis (26). Indeed, we observed that Wnt stimulation in hepatic I/R lead to decreased iNOS levels, which was associated with a decrease in caspase-3 activity and number of apoptotic cells. We believe this to be significant as previous studies have demonstrated that apoptosis is a key contributor to injury following hepatic I/R, and that therapies aimed at reducing apoptosis have proven to be hepatoprotective (27).
Inflammatory cascades also contribute to the tissue damage during I/R. We demonstrated that Wnt agonist administration significantly inhibited the production of proinflammatory cytokines at local and systemic levels after hepatic I/R. Activation of β-catenin signaling by other molecules has also demonstrated the anti-inflammatory properties of this pathway. R-spondin 1, a naturally secreted protein that shares similar receptor-binding properties with Wnt ligands, has demonstrated anti-inflammatory and osteoblastogenic properties in bone and joint disease and has been observed to decrease IL-6 and inflammation in an animal model of colitis (28). Moreover, inhibition of dickkopf1 protein, a Wnt signaling antagonist, has been associated with decreased inflammation, decreased apoptosis, and earlier recovery of gut mucosa in a separate animal model of colitis (29). This decrease in inflammation coincided with a greatly reduced level of MPO in liver tissue, suggesting the prevention of neutrophil infiltration into the liver following I/R with Wnt agonist treatment.
By dampening the severe tissue damage associated with hepatic I/R, Wnt agonist provided a significant survival advantage to treated animals. We found that pharmacologic activation of Wnt signaling lead to increased survival rates in animals treated either before or after the ischemic insult when partial hepatectomy was performed following hepatic I/R. To our knowledge, this represents the first study in which upregulation of Wnt/β-catenin signaling has demonstrated an in vivo survival benefit when administered during the reperfusion phase of I/R. However, the activation of Wnt/β-catenin signaling has been linked to tumorogenesis (30). Thus, it may generate a concern of promoting cancer when applying the Wnt agonist for long-term treatment. In this study with a single dose administration, we have observed a beneficial effect of Wnt agonist on the 10-day survival. Whether one-time activation of Wnt/β-catenin signaling can lead to cancer requires a long-term follow-up.
In addition, while it is always a plus to have a detailed time-course study to follow through all the changes after I/R, in animal models of hepatic I/R injury, the tissue damage, serum levels of organ injury markers and inflammation can be well detected 24 hours after I/R. So we used this time point to do all the correlated measurements in this study. We observed a significant increase of Wnt target gene expression and Ki67 staining, and a reduction of nitrosative stress and inflammation by the Wnt agonist treatment at 24 h, so we did not further pursue the effect of Wnt agonist on all these parameters at earlier time points, such as 6 hours.
There are several approaches to manipulating the canonical Wnt signaling pathway, including frizzled surface receptor binding, targeting the Wnt-axin relationship, GSK-3β inhibition, and Wnt target gene regulation. In particular, pharmacologic inhibition of GSK-3β to stabilize β-catenin has been applied to animal models of hepatic I/R, renal I/R, hemorrhage, and sepsis, and has shown protective effects (31-34). However, the serine/threonine protein kinase GSK-3β has been implicated in multiple signaling pathways apart from Wnt/β-catenin, ranging from metabolic pathways to structural proteins and cell-cycle transcription factors (35). Such non-specific effects of targeting GSK-3β in order to regulate Wnt signaling will limit this approach on clinical use. The Wnt agonist that we used in this study has been well demonstrated to have no effect on GSK-3β activity. Therefore, it reduces the off-target effect of activating Wnt signaling through GSK-3β.
In summary, administration of Wnt agonist to animals undergoing hepatic I/R was able to increase hepatocyte proliferation, decrease inflammation, and decrease apoptosis and necrosis. In addition, it offered a distinct survival advantage in a lethal hepatic I/R model when it was administered before or after the ischemic insult. It did so through upregulating β-catenin gene transcription, decreasing inflammatory cascades, and decreasing iNOS-dependent nitrosative tissue damage. We therefore propose that the findings in our study using a novel compound as a Wnt-signaling agonist provide a new strategy in the prevention and treatment in hepatic I/R injury incurred in a variety of clinical settings.
Acknowledgments
This study was supported by National Institutes of Health grants, R01HL076179, R01GM057468, and R01GM053008 (PW).
This work was awarded First Place for The Shock Society New Investigator Award at the 35th Annual Conference on Shock, Miami Beach, Florida, June 9-13, 2012.
Footnotes
Disclosure of Financial Interests and Potential Conflicts of Interest. All authors reported no financial interests or potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERRENCES
- 1.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–9. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–50. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 4.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–51. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- 6.Masckauchán TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 7.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–53. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–79. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–95. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl. 2005;44:1987–90. doi: 10.1002/anie.200462552. [DOI] [PubMed] [Google Scholar]
- 11.Kaldis P, Pagano M. Wnt signaling in mitosis. Dev Cell. 2009;17:749–50. doi: 10.1016/j.devcel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Heijnen BH, Straatsburg IH, Gouma DJ, van Gulik TM. Decrease in core liver temperature with 10 degrees C by in situ hypothermic perfusion under total hepatic vascular exclusion reduces liver ischemia and reperfusion injury during partial hepatectomy in pigs. Surgery. 2003;134:806–17. doi: 10.1016/s0039-6060(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 14.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav SS, Gao W, Harland RC, Clavien PA. A new and simple technique of total hepatic ischemia in the mouse. Transplantation. 1998;65:1433–6. doi: 10.1097/00007890-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–9. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 17.Cai C, Zhu X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol Med Report. 2012;5:1191–6. doi: 10.3892/mmr.2012.802. [DOI] [PubMed] [Google Scholar]
- 18.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–8. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–82. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 21.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14:1223–33. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 22.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Dar MJ, Khillan J, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–13. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehwald N, Tao GZ, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt-β-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology. 2011;141:707–18. doi: 10.1053/j.gastro.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varadarajan R, Golden-Mason L, Young L, McLoughlin P, Nolan N, McEntee G, et al. Nitric oxide in early ischaemia reperfusion injury during human orthotopic liver transplantation. Transplantation. 2004;78:250–6. doi: 10.1097/01.tp.0000128188.45553.8c. [DOI] [PubMed] [Google Scholar]
- 25.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiang JG, Tsen KT. Biology of hypoxia. Chin J Physiol. 2006;49:223–33. [PubMed] [Google Scholar]
- 27.Lin FS, Shen SQ, Chen ZB, Yan RC. 17β-estradiol attenuates reduced-size hepatic ischemia/reperfusion injury by inhibition apoptosis via mitochondrial pathway in rats. Shock. 2012;37:183–90. doi: 10.1097/SHK.0b013e31823f1918. [DOI] [PubMed] [Google Scholar]
- 28.Krönke G, Uderhardt S, Kim KA, Stock M, Scholtysek C, Zaiss MM, et al. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 2010;62:2303–12. doi: 10.1002/art.27496. [DOI] [PubMed] [Google Scholar]
- 29.Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, et al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–68. doi: 10.1053/j.gastro.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ, et al. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest. 2011;121:1935–45. doi: 10.1172/JCI44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren F, Duan Z, Cheng Q, Shen X, Gao F, Bai L, et al. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54:687–96. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasileva AK, Plotnikov EY, Kazachenko AV, Kirpatovsky VI, Zorov DB. Inhibition of GSK-3β decreases the ischemia-induced death of renal cells. Bull Exp Biol Med. 2010;149:303–7. doi: 10.1007/s10517-010-0932-1. [DOI] [PubMed] [Google Scholar]
- 33.Dugo L, Abdelrahman M, Murch O, Mazzon E, Cuzzocrea S, Thiemermann C. Glycogen synthase kinase-3beta inhibitors protect against the organ injury and dysfunction caused by hemorrhage and resuscitation. Shock. 2006;25:485–91. doi: 10.1097/01.shk.0000209545.29671.31. [DOI] [PubMed] [Google Scholar]
- 34.Bertsch S, Lang CH, Vary TC. Inhibition of glycogen synthase kinase 3[beta] activity with lithium in vitro attenuates sepsis-induced changes in muscle protein turnover. Shock. 2011;35:266–74. doi: 10.1097/SHK.0b013e3181fd068c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]