Abstract
Interleukin (IL)–22 maintains gut epithelial integrity and expression of antimicrobial peptides (AMPs) Reg3β and Reg3γ. Our laboratory has shown that acute alcohol/ethanol (EtOH) exposure prior to burn injury results in increased gut permeability, intestinal T cell suppression and enhanced bacterial translocation. Herein, we determined the effect of combined EtOH intoxication and burn injury on intestinal levels of IL-22 as well as Reg3β and Reg3γ expression. We further examined whether in vivo restitution of IL-22 restores gut permeability, Reg3β and Reg3γ levels, and bacterial load (e.g. gut bacterial growth) within the intestine following EtOH and burn injury. Male mice, ~25g, were gavaged with EtOH (2.9 mg/kg) prior to receiving a ~12.5% total body surface area full thickness burn. Mice were immediately treated with saline control or IL-22 (1 mg/kg) by i.p. injection. One day post injury, there was a significant decrease in intestinal IL-22, Reg3β and Reg3γ expression along with an increase in intestinal permeability and gut bacterial load following EtOH combined with burn injury, as compared to sham injury. Treatment with IL-22 normalized Reg3β and Reg3γ expression, and attenuated the increase in intestinal permeability following EtOH and burn injury. Qualitatively, IL-22 treatment reduced the bacterial load in nearly half of mice receiving EtOH combined with burn injury. Our data indicate that IL-22 maintains gut epithelial and immune barrier integrity following EtOH and burn injury; thus, the IL-22/AMP pathway may provide a therapeutic target for the treatment of patients who sustain burn injury under the influence of EtOH.
Keywords: Ethanol, Permeability, Antimicrobial Peptides, Epithelium, Mice, Thermal Injury
INTRODUCTION
Interleukin (IL)-22 maintains gut epithelial integrity and mucosal immunity by acting on epithelial cells, and is unique as it does not directly function to modulate immune cells. The major sources of IL-22 include cells of the innate and adaptive immune systems. Specifically, in humans and mice, innate sources of IL-22 include natural killer (NK) cells and innate lymphoid cells(1). In regards to T cells, the major producers of IL-22 in humans include T helper (Th)1, Th17 and Th22 cells; other T cell sources include cytotoxic (Tc)22 and Tc17 cells, as well as γδ T cells. In mice, IL-22 is observed in Th17 and Tc17 cells, as well as in the innate-like NKT cells and γδ T cells(1). Functionally, IL-22 is involved in chemotaxis, antimicrobial peptide (AMP) expression, tissue repair and epithelial cell survival, proliferation and differentiation(1–3). Recent evidence further implicates IL-22 and IL-22-induced AMPs Reg3β and Reg3γ, two C-type lectins, in the containment of Gram-negative and Gram-positive gut pathogens(1–4).
In the United States, more than one million burn injuries are reported yearly (5). Nearly one-half of these injuries occur under the influence of alcohol/ethanol (EtOH) intoxication (6–9). Burn victims who sustain injury post EtOH exposure exhibit increased clinical complications, including higher susceptibility to infection resulting in more surgical interventions, significantly longer hospital stays and higher morbidity (6, 8–11). Moreover, patients with EtOH in their blood at the time of hospital admission are more likely to die of smaller burns than non-EtOH exposed patients (6). Though, the mechanism(s) by which EtOH confounds clinical outcomes following burn injury remain(s) unknown, previous data suggest that gut pathogens and their products may play a pivotal role in the progression of disease and development of sepsis and multiple organ failure reported in alcoholics as well as burn and trauma patients (11–15). In line with these studies, our laboratory has demonstrated increased intestinal tissue damage, leakiness, and bacterial translocation as well as T lymphocyte suppression following EtOH intoxication and burn injury (12, 16–20). Therefore, further studies aimed at targeting the modulation of gut immune barrier function and immunity are required to improve clinical outcomes of patients who sustain burn injury under the influence of EtOH. Since IL-22 has been implicated in the maintenance of gut epithelial barrier, perturbation of IL-22 or AMP expression following EtOH and burn injury may severely impair the immune defense and barrier integrity.
Our present study evaluated the effects of EtOH and burn injury on gut IL-22 levels, leakiness, bacterial growth, and Reg3β and Reg3γ expression in our recently established murine model (21). Additionally, we examined whether in vivo treatment with rIL-22 prevents combined injury-associated elevations in gut leakiness and bacterial growth, as well as restores Reg3 expression following EtOH exposure and burn injury. We found that when combined, EtOH and burn injury resulted in decreased levels of IL-22 as well as decreased expression of Reg3β and Reg3γ in the small intestine. We further confirmed that dual insult results in increased gut leakiness and gut bacterial growth. IL-22 treatment successfully restored expression of Reg3 and prevented increased gut permeability following combined EtOH exposure and burn injury. Qualitatively, IL-22 treatment prevented increased gut bacterial load (e.g. bacterial growth) in half of IL-22 treated animals. Our results provide a novel role for rIL-22 in the modulation of gut barrier function, bacterial containment and AMP expression. Thus, the IL-22/AMP pathway may provide a novel therapeutic target for the treatment of patients who sustain burn injury under the influence of EtOH.
MATERIALS and METHODS
Animals and reagents
Male C57BL/6 mice, 8–9 wks old, were obtained from Harlan Laboratories. Recombinant IL-22 was obtained from GenScript (Piscataway, NJ). IL-22 ELISA kit was obtained from R&D Systems (Minneapolis, MN). Protein Assay was obtained from Bio-Rad (Hercules, CA). RNeasy Mini Kit was obtained from Qiagen (Valencia, CA). TaqMan Expression Assays for Reg3γ, Regβ and GAPDH were obtained from Applied Biosystems (Carlsbad, California). Fluorescein isothiocyanate (FITC)-conjugated dextran was obtained from Sigma Aldrich (St. Louis, MO)
Mouse model of acute EtOH intoxication and burn injury
As previously described (21), adult C57BL/6 male mice were randomly divided to receive sham or burn injury and either EtOH or vehicle (water) to yield four experimental groups: sham vehicle, sham EtOH, burn vehicle and burn EtOH. Mice were gavaged with either 0.4 ml of 25% EtOH in water (~2.9 g/kg) or water. Four hours after gavage, mice were anesthetized by intraperitoneal (i.p.) injection of ketamine hydrochloride/xylazine cocktail (~80 mg/kg and 1.2 mg/kg, respectively). Dorsal surfaces were shaved, and animals were transferred into a template fabricated to expose ~12.5% of the total body surface area (TBSA). TBSA was calculated by using Meeh’s formula as described by Walker and Mason (22). Burn-injured mice were immersed into a water bath maintained at 85–87°C for 7 s, which results in a full thickness scald injury. Sham-injured mice were subjected to identical anesthesia and treatment, but immersed into isothermic water (37°C) for 7 s. Immediately after burn or sham procedure, animals were dried and resuscitated with 1.0 ml physiological saline by i.p. injection. Animals were allowed food and water ad libitum. All experiments were conducted in accordance with the National Institutes of Health guidelines set forth in the Animal Welfare Act and were approved by the Institutional Animal Care and Use Committee at the Loyola University Health Sciences Division.
Treatment of mice with rIL-22
Immediately after sham or burn injury, a group of sham vehicle and burn EtOH injured mice were treated with rIL-22 (1 mg/kg body weight in sterile PBS, GenScript) by i.p. injection (23).
Measurement of intestinal tissue IL-22 levels
One day post injury, mice were anesthetized and the abdominal cavity exposed via midline laparotomy and a 3-cm-long segment of small intestine was removed, cleaned, snap-frozen in liquid nitrogen and stored at −70°C (20). Equal weights of tissue (100 mg) from each experimental group were suspended in 1 mL of PBS containing protease inhibitor cocktail (one tablet of protease inhibitor cocktail from Roche Diagnostics was dissolved in 10 mL PBS) and sonicated at 30 cycles twice for 30 s on ice. Homogenates were cleared by centrifuging at 12,000 rpm at 4°C. The supernatants were stored at −70°C. Protein levels in the homogenates were determined using the Bio-Rad Protein Assay. IL-22 levels in the intestinal tissue homogenates were measured by enzyme-linked immunosorbent assay (ELISA) following manufacturer’s instructions.
Measurement of Reg3β and Reg3γ expression
One day post injury, mice were anesthetized and the abdominal cavity exposed via midline laparotomy and a 1-cm-long segment of small intestine was removed, cleaned, snap-frozen in liquid nitrogen and stored at −70°C (20). Equal weights of tissue (25 mg) from each experimental group were used for RNA extraction using the Qiagen RNeasy Mini Kit. RNA was quantified using a Nanodrop Spectrophotometer ND-1000 and cDNA synthesized using the High Capacity Reverse Transcriptase Kit (Applied Biosystems). Quantitative RT-PCR was performed with the Applied Biosystems 7500 Fast Real-Time PCR system. Values were then normalized to GAPDH expression via the ΔΔCT method, as previously described (17).
In vivo gut permeability assay
A separate group of animals were used for the measurement of intestinal permeability, with minor modifications to previously described method (20). Briefly, as opposed to rats, we used a relatively shorter segment of the small intestine and less volume of FITC dextran injection. One day post sham or burn procedure and treatment with IL-22, animals were anesthetized and via a midline laparotomy, the abdominal cavity was opened and renal artery and renal vein in both kidneys were ligated. A 10 cm long segment of distal small intestine (lower jejunum and ileum) ligated at both ends without damaging intestinal and mesenteric structures. An 18 gauge BD Insyte Autoguard Shielded IV catheter (Monsey, NY) was inserted into the ligated intestinal segment from the proximal end and 0.10 ml of PBS containing 25 mg/ml of FITC-dextran was infused into the intestinal lumen via catheter. Blood was drawn via cardiac puncture at 90 min post FITC-dextran injection, and plasma was separated. Because multiple blood draws cannot be performed in mice we selected a 90 min time point, as maximum separation between sham and EtOH burn group was observed at 90 min after FITC infusion in rats. Animals remained anesthetized for this entire period, from the laparotomy to the blood draw for FITC dextran measurement. The intestine was kept under wet gauze to prevent drying of tissue and damage of mesenteric structures. Plasma samples were analyzed for FITC-dextran concentration by using a fluorescence spectrophotometer at an excitation wavelength of 480 nm and the emission wavelength of 520 nm using Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT).
Measurement of intestinal bacterial forming units
One day post injury, mice were anesthetized and the abdominal cavity exposed via midline laparotomy and a 1-cm-long segment of small intestine, ileum, was aseptically harvested and rinsed in sterile 2 ml PBS one time to remove any attached fecal/luminal content from the tissue. Tissues were weighed and homogenized in sterile PBS (10 μl/mg tissue weight), one cycle for 30 s. Separately, fecal luminal content was harvested from terminal small intestine, weighed and resuspended in PBS (40 μl/mg fecal luminal content weight) by vortexing suspension on high speed for 30 s. 25 μl of the suspension, containing 2.5 mg intestinal tissue or 0.625 mg fecal luminal content, were plated and cultured on tryptic soy agar (TSA) or MacConkey agar for the analysis of culturable total and Gram negative bacteria, respectively, for 24 h at 37°C. Following incubation, TSA and MacConkey agar plates were assessed for bacterial colony forming units (CFUs) and photographed. Results from these experiments are included in Supplemental Tables 1 and 2.
Statistical analysis
The data, wherever applicable, are presented as mean + SEM and were analyzed using ANOVA Tukey post-hoc test or Student’s t-test (GraphPad InStat). p<0.05 was considered statistically significant.
RESULTS
Intestinal tissue IL-22 is decreased following EtOH and burn injury
To determine whether EtOH and burn injury perturbs IL-22 in the intestinal tissue, we measured IL-22 in intestinal tissue homogenates one day post EtOH exposure and burn injury. As shown in Fig. 1, there was no difference in IL-22 in the intestinal tissue of sham-injured animals, regardless of EtOH. However, while burn injury alone trended towards an increase in intestinal IL-22, this was not found to be significantly different from sham animals. The intestinal tissue from the EtOH burn group demonstrated significantly decreased levels of IL-22, as compared to sham vehicle and burn vehicle groups.
Figure 1. Intestinal tissue IL-22 is decreased following EtOH and burn injury.
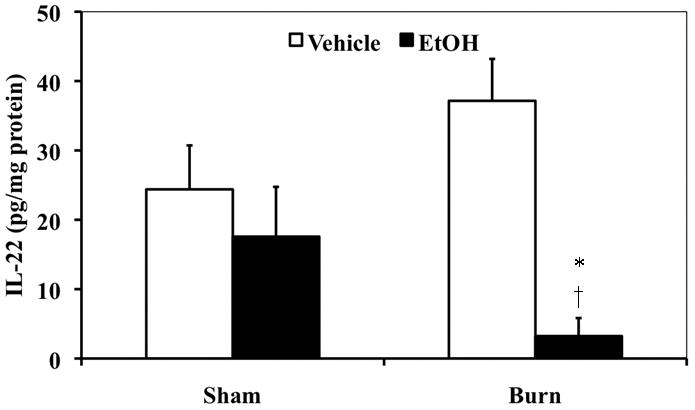
One day post insult, terminal small intestines were collected and equal weights of tissue (100 mg) from each experimental group were suspended in 1 mL of a buffer (PBS containing protease inhibitor cocktail) and sonicated. Homogenates were cleared by centrifugation and supernatants were analyzed for IL-22 by ELISA (IL-22 ELISA Kits, R&D Systems). IL-22 levels were normalized to protein levels. Values shown as mean + SEM, n=6–9 animals/group from two independent experiments. *, p=0.0381 as compared with sham vehicle by Student’s t test with Welch correction;†, p<0.01 as compared with burn vehicle by ANOVA with Tukey’s post-hoc test.
Increased gut permeability one day post EtOH exposure and burn injury
Our laboratory has previously demonstrated increased gut permeability in a rat model of EtOH exposure and burn injury (20). To confirm these findings in our recently established mouse model (21), we measured intestinal permeability one day after injury. The results presented in Fig. 2 indicate that there was no difference in the plasma FITC-dextran levels in either sham group. Similarly, plasma FITC-dextran levels in burn vehicle group were also not found to be different from shams controls. However, FITC-dextran levels were higher in burn EtOH group relative to other groups. These results suggest that the combined insult of EtOH and burn increases intestinal permeability when compared to either EtOH intoxication or burn injury alone. Moreover, our findings confirm that similar to rats, mice also exhibit a disruption in the intestinal barrier following EtOH and burn injury.
Figure 2. Increased gut permeability one day post EtOH exposure and burn injury.
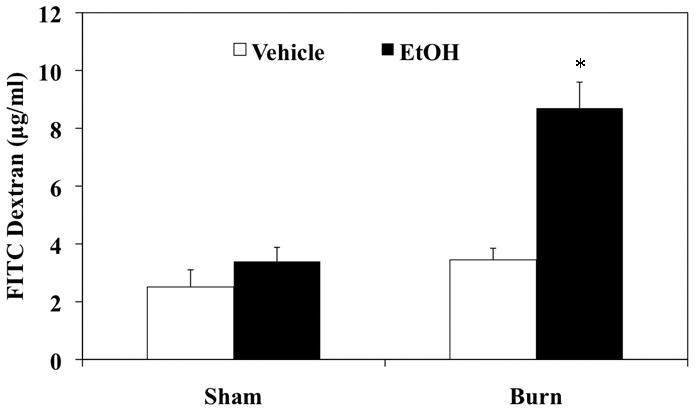
Intestinal permeability was determined by monitoring the transfer of FITC-dextran from the isolated intestinal lumen to the systemic circulation, blood was drawn by cardiac puncture 90 min after FITC-dextran injection. Values shown as mean + SEM, n=4–5 animals/group. *, p<0.01 as compared to all groups by ANOVA with Tukey’s post-hoc test.
Combined EtOH intoxication and burn injury suppress intestinal AMP expression
Among the various AMPs regulated by IL-22, Reg3β and Reg3γ are key components of gut mucosal immunity (3, 4, 24). To determine whether EtOH exposure and burn injury affects expression of Reg3β and Reg3γ, we measured mRNA levels of Reg3β and Reg3γ in small intestinal tissue one day following EtOH exposure and burn injury. As demonstrated in Fig. 3, neither EtOH alone, nor burn injury alone affect Reg3β and Reg3γ expression, as compared to sham injury. However, combined EtOH exposure and burn injury resulted in a significant suppression of Reg3β and Reg3γ expression, as compared with sham vehicle. Thus, these data suggest that the combination of EtOH and burn injury results in decreased intestinal AMP expression.
Figure 3. Decreased intestinal AMP expression one day post EtOH and burn injury.
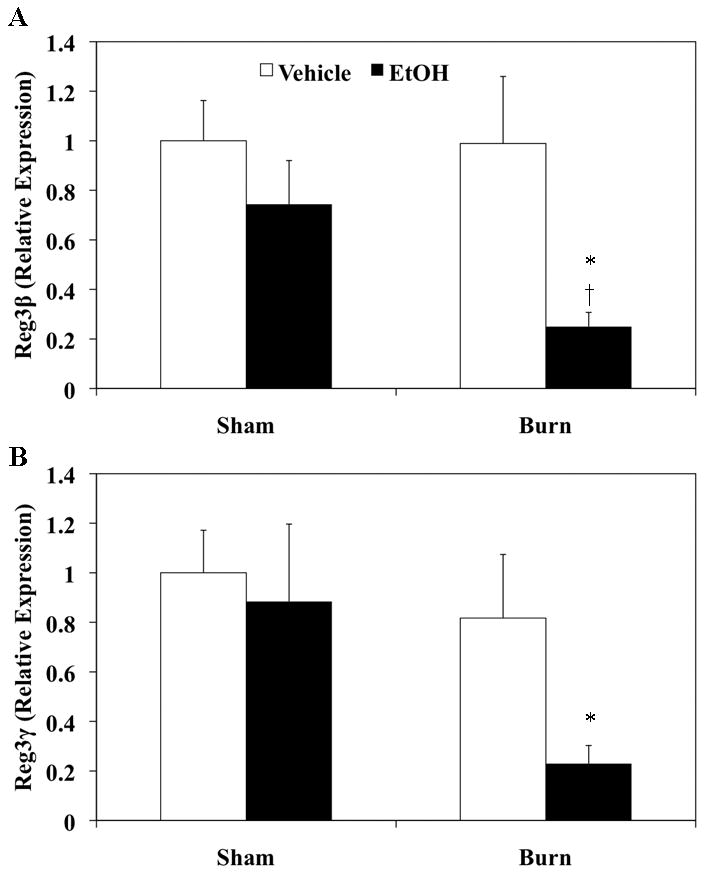
Small intestine was harvested and equal weights of tissue (25 mg) from each experimental group were used for RNA extraction using the Qiagen RNeasy Mini Kit. Reg3β, Reg3γ and GAPDH were measured by qRT-PCR using specific probes. AMP expression was normalized to GAPDH expression via the ΔΔCT method. Values shown as mean + SEM, n=3–10 animals/group from two independent experiments. *, p<0.05 as compared to sham vehicle;†, p<0.05 as compared to burn vehicle by ANOVA with Tukey’s post-hoc test.
Increased gut bacterial growth one day post EtOH exposure and burn injury
Previously, our laboratory has demonstrated increased gut bacterial translocation to regional draining mesenteric lymph nodes following EtOH and burn injury (16, 19, 25). To assess whether EtOH intoxication and burn injury facilitates gut bacterial growth, we determined total and Gram-negative bacterial load in small intestinal tissue as well as in small intestine luminal content (feces). Gram-negative bacteria are considered to be the major cause of sepsis and organ dysfunction in critically ill burn patients (26, 27). As demonstrated in Fig. 4, our results show that EtOH and burn injury alone do not cause a significant change in gut bacterial load. However, the combined insult of EtOH and burn injury resulted in a robust increase in total (data not shown) and Gram-negative bacteria in the intestinal tissue and luminal content. The raw Gram negative bacterial CFU data are included as supplement data (Supplemental Tables 1 and 2). The total Gram-negative bacterial CFU count from fecal homogenates in the sham vehicle group varies from 0–44 CFU. One sham vehicle mouse exhibited too many CFUs to count. We could not detect any bacterial CFUs in the fecal homogenates from sham EtOH animals. Similarly, except one mouse, no detectable bacterial CFUs were found in tissue or feces from the burn vehicle group. In contrast, 11 out of the 16 burn EtOH mice exhibited too many CFUs to count. The remaining 5 burn EtOH mice exhibited 1–24 CFUs in their feces one day after EtOH and burn injury. Very similar observations were made in the intestine tissue homogenates prepared from sham vehicle, sham EtOH and burn vehicle mice. However, except one, all the intestine homogenates from mice subjected to EtOH and burn injury showed too many CFUs to count. This clearly shows that EtOH and burn injury substantially increases gut bacterial load compared to EtOH or burn alone. We recognize that majority of the gut bacteria grow in anaerobic conditions and that the majority of those bacteria are not cultivable or difficult to culture. So the data presented in this manuscript may represent less than 5% of the total cultivable bacteria present in the gut and is a limitation of our study.
Figure 4. Increased Gram-negative bacterial growth one day after EtOH exposure and burn injury.
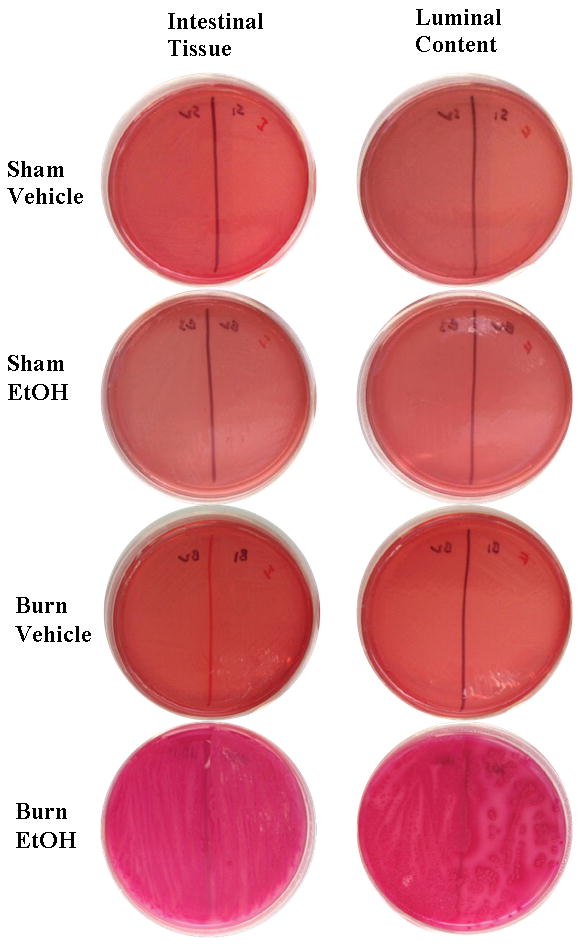
One cm-long segments of small intestine were aseptically removed, cleaned and rinsed in sterile PBS. Tissues were weighed and homogenized in sterile PBS (10 μl/mg tissue weight). Separately, fecal luminal content was harvested from terminal small intestine, weighed and resuspended in PBS (40 μl/mg fecal luminal content weight). 25 μl of the suspension containing 2.5 mg intestinal tissue or 0.625 mg fecal luminal content were plated and cultured on MacConkey agar for the analysis of Gram-negative bacteria, for 24 h at 37°C. Following incubation, plates were assessed for bacterial colony forming units (CFUs) and photographed. Pictures are representative of n=5–9 animals/group.
Treatment with IL-22
To perform these studies, sham vehicle and burn EtOH injured animals were treated with IL-22 (1 mg/kg body weight), immediately after sham or burn injury. The dose of IL-22 selected has been shown to induce beneficial physiologic effects – including hepatic regeneration and AMP expression – in a model of hepatitis (23). In our own preliminary experiments we confirmed 1 mg/kg to be optimal by measuring gut permeability using 1 mg/kg and 2 mg/kg, we found no difference between the two doses and elected to use the lower dose (data not shown). In these and subsequent experiments, we did not include all four experimental groups. Rather, we used only sham vehicle and burn EtOH, since only the burn EtOH group demonstrated decreased intestinal IL-22, increased gut permeability and decreased AMP expression.
Treatment with IL-22 prevents increased gut permeability
The effect of IL-22 treatment on gut leakiness was observed one day post injury. As demonstrated in Fig. 5, IL-22 treatment did not alter gut permeability in sham treated animal. However, IL-22 treatment prevented the increase in plasma FITC-Dextran levels following EtOH and burn injury. These data suggest that IL-22 prevents the increase in gut permeability following EtOH exposure and burn injury.
Figure 5. IL-22 treatment prevents increased gut permeability.
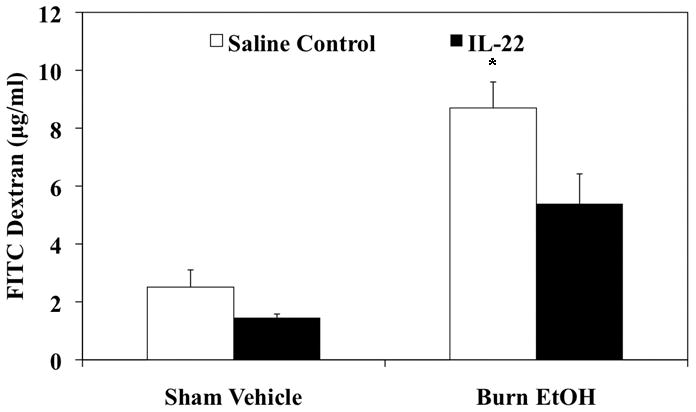
In a group of sham vehicle and burn EtOH animals, IL-22 (1 mg/kg body weight) was injected immediately after injury. One day after EtOH and burn injury, intestinal permeability was determined by monitoring the transfer of FITC-dextran from the isolated intestinal lumen to the systemic circulation, blood was drawn by cardiac puncture 90 min after FITC-dextran injection. Values shown as mean + SEM, n=4–6 animals/group. *, p<0.05 as compared to all groups by ANOVA with Tukey’s post-hoc test.
IL-22 treatment promotes intestinal AMP expression
To test whether IL-22 treatment modulates AMP expression one day following EtOH and burn injury, small intestinal tissue was collected from saline control and IL-22 treated animals. Tissues were processed for RNA extraction and the expression of Reg3β and Reg3γ analyzed by qRT-PCR. The results presented in Fig. 6 demonstrate that IL-22 treatment does not affect expression of Reg3β or Reg3γ in sham animals. However, IL-22 treatment restored Reg3β and Reg3γ expression in animals that received EtOH and burn injury.
Figure 6. Treatment with IL-22 prevents the decrease in intestinal AMP expression one day post EtOH exposure and burn injury.
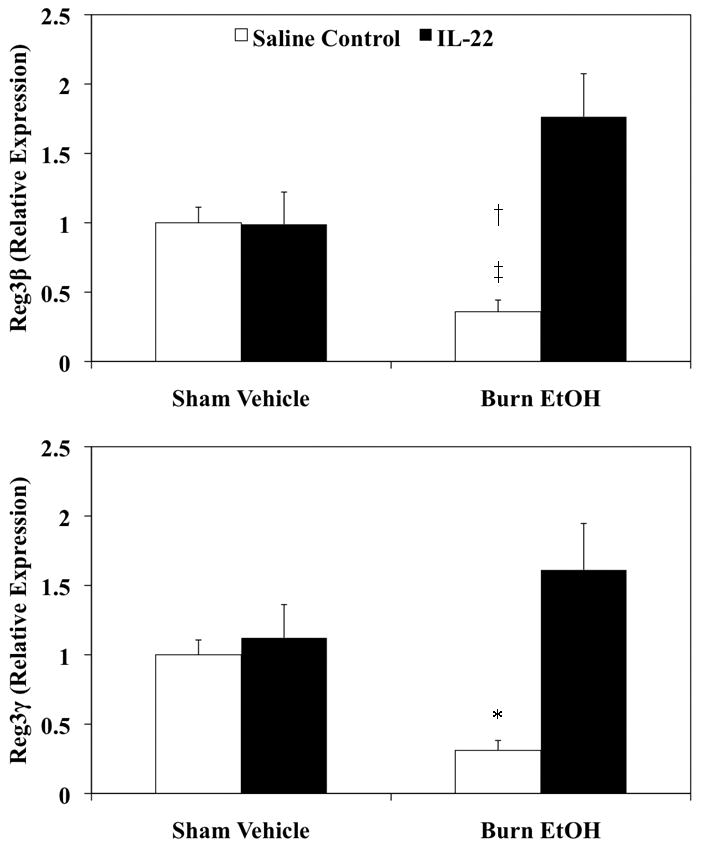
Day one after injury and IL-22 treatment, small intestine was harvested and equal weights of tissue (25 mg) from each experimental group were used for RNA extraction using the Qiagen RNeasy Mini Kit. Reg3β, Reg3γ and GAPDH were measured by qRT-PCR using specific probes. AMP expression was normalized to GAPDH expression via the ΔΔCT method. Values shown as mean + SEM, n=4–14 animals/group from three independent experiments. *, p<0.05 as compared to all groups;†, p<0.05 as compared to sham vehicle + saline control;‡, p<0.001 as compared to burn EtOH + IL-22 by ANOVA with Tukey’s post-hoc test.
Gut bacterial load and IL-22 treatment
Lastly, we examined whether IL-22 treatment has any relationship with gut bacterial load. Small intestine tissue and luminal content were harvested from saline and IL-22 treated animals, one day post sham or EtOH and burn injury. Intestinal tissue and luminal content homogenates were cultured on specific agar plates for total (TSA) and Gram-negative (MacConkey) bacteria. As shown in Fig. 7 and Supplemental Table 2, 4 out of 8 mice treated with IL-22 exhibited a substantial decrease in Gram-negative bacterial load in the intestine. Although there was some increase in bacterial CFU in sham animals treated with IL-22, the mechanism of which is not known. IL-22 did not appreciably affect total bacteria (data not shown). These findings suggest that the decrease in IL-22 may be responsible, in part, for the increase in gut bacterial load following EtOH and bun injury.
Figure 7. IL-22 treatment prevents increased Gram-negative bacterial growth in the intestine.
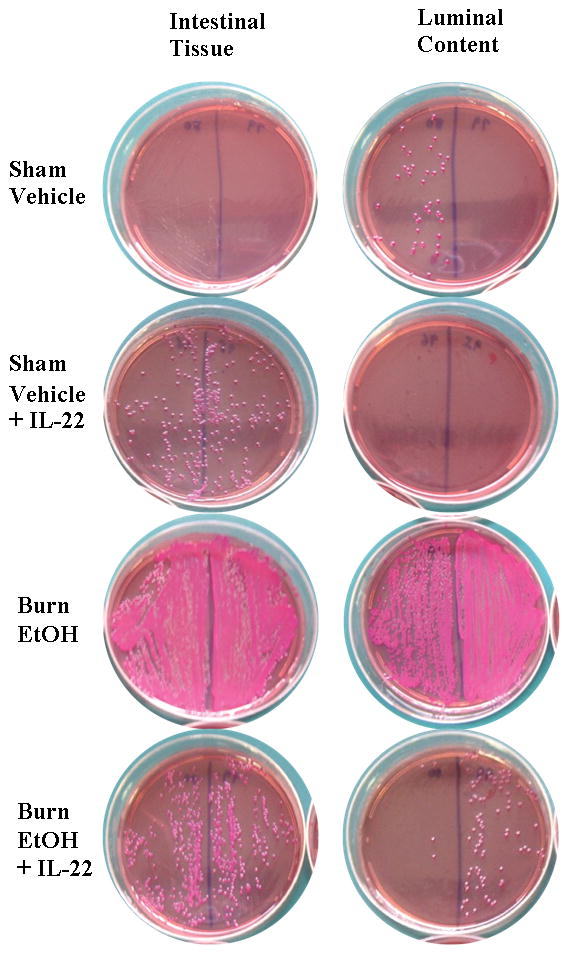
One day post injury and IL-22 treatment, a 1-cm-long segment of small intestine was aseptically removed, cleaned weighed and homogenized in sterile PBS (10 μl/mg tissue weight). Separately, fecal luminal content was harvested from terminal small intestine, weighed and resuspended in PBS (40 μl/mg fecal luminal content weight). 25 μl of the suspension containing 2.5 mg intestinal tissue or 0.625 mg fecal luminal content were plated and cultured on MacConkey agar for the analysis of Gram-negative bacteria, for 24 h at 37°C. Following incubation, plates were assessed for bacterial colony forming units (CFUs) and photographed. Pictures are representative of n=5–8 animals/group.
DISCUSSION
EtOH intoxication is a confounding factor in post-burn pathogenesis. Yet, few studies have evaluated the role of EtOH in post-burn complications. In the present study, we examined the role of EtOH intoxication on post-burn gut permeability, intestinal expression of IL-22 and AMP expression as well as total and Gram-negative gut bacterial load. Additionally, we treated animals with IL-22 to test whether it plays any role in the modulation of gut barrier, immune function and bacterial containment following EtOH and burn injury. We found that EtOH combined with burn injury resulted in a decrease in intestinal tissue levels of IL-22 as well as suppression of Reg3β and Reg3γ expression, as compared to sham injury. This was attenuated following treatment with IL-22. IL-22 treatment also prevented increased gut permeability following EtOH and burn injury. The restoration of AMP and gut barrier was accompanied with a demonstrable decrease in Gram-negative gut bacterial load in half of the animals treated with IL-22 after EtOH and bun injury. Though samples from IL-22 treated animals continued to exhibit a substantial increase in total bacteria post EtOH and burn injury (data not shown), we found decreased growth of Gram-negative bacterial colonies in intestine tissue homogenates and luminal content in half of EtOH and burn injured animals. This finding suggests that Gram-negative bacteria are modulated by IL-22 and/or AMPs. Together, our data indicate that IL-22 treatment maintains gut epithelial barrier integrity and AMP expression following EtOH and burn injury.
Unlikely most cytokines, which exert their actions on immune cells, the IL-22 receptor is only expressed on epithelial cells of the skin, intestine, liver, pancreas, lung and kidney (1). IL-22 is of particular interest to gut immunity and barrier integrity (1). The IL-22 receptor is composed of the IL-22R and IL-10R2 subunits (1). Binding of IL-22 to IL-22R1 induces its complex formation with IL-10R2 and propagation of the JAK-STAT pathway, phosphorylation/homodimerization of STAT3 and subsequent activation of gene transcription (1, 28). Among the various genes activated by IL-22 are AMPs, including Reg3β and Reg3γ, which are recognized for their role in gut immunity and barrier integrity (3, 4, 29). Moreover, IL-22 has been implicated in tissue repair and regeneration. Therapeutically, IL-22 shows improvement in models of EtOH induced liver injury, pancreatitis, hepatitis and inflammatory bowel disease(1, 3, 23, 28–30). Most recently, clinical studies demonstrate modulation of IL-22 in patients with sepsis(31).
Our current data, suggests that IL-22 treatment modulates AMP expression, bacterial load and gut permeability one day post EtOH exposure and burn. Together these data highlight the critical role that IL-22 plays in maintaining gut immune function and barrier integrity. While further experiments are needed to delineate a mechanism by which IL-22 treatment improves intestinal AMP expression, bacterial load and permeability, our data support the use of IL-22 as a therapeutic agent. Mechanistically, it is likely that IL-22 maintains gut epithelial integrity through induction of genes related to regeneration and proliferation in a STAT3 dependent manner(1, 23, 28–30). Recently, our laboratory demonstrated IL-18 dependent increased apoptosis and altered tight junction formation follow EtOH and burn injury (32). It is likely that uncontrolled IL-18 driven damage is due, in part, to decreased protective effects of IL-22. Moreover, IL-18 has been shown to modulate IL-23 induced expression of IL-22. To determine the mechanism by which exogenous IL-22 prevents increased gut permeability following EtOH and burn injury, future experiments will explore the role of IL-22 dependent proliferation and regeneration in counterbalancing the damaging effects of IL-18.
In regards to immune function, it is possible that IL-22 exerts its action through modulation of epithelial cells and induction of AMP expression. Recently, IL-22 was demonstrated to protect again K. pneumoniae infection, a Gram-negative gut pathogen (29). In vivo inoculation with K. pneumoniae induced IL-22 in the lungs of infected animals, where IL-22 blockade increased mortality as well as local and systemic bacterial dissemination. Further analysis demonstrated that IL-22 induced epithelial cell proliferation and increased transepithelial resistance to injury (29). Following EtOH and burn injury, it is possible that decreased intestinal IL-22, as observed in this study, may facilitate survival of Gram-negative bacteria. Additionally, IL-22 induces mucin production by colonic epithelial cells(30), which offers protection again bacterial invasion. As we observed reduced Gram-negative bacterial colonization following IL-22 treatment in combined injury animals, exogenous IL-22 may act to increase mucin production to prevent invasion by luminal bacteria. Conversely, the immuno-protective effects of IL-22 can result from induction of Reg3β and Reg3γ. Initially, Reg3β and Reg3γ were shown to bind peptidoglycan and kill Gram-positive bacteria (4). More recent evidence, suggest that IL-22 and Reg3 proteins play a protective role against Gram-negative bacteria(3), though the mechanism by which this occurs remains unknown. Using an in vivo model of C. rodentium infection, a Gram-negative pathogen, in IL-22 knockout mice, Zheng et al. demonstrated that IL-22 is required to clear C. rodentium (3). Specifically, IL-22 knockout mice demonstrated decreased survival following C. rodentium infection, which was improved following treatment with recombinant mouse Reg3γ-Ig fusion protein (3). Previously, Reg3β was shown to induce aggregation of E. coli. Together these data support the idea that Reg3β and Reg3γ play a role in the protection against Gram-positive as well as Gram-negative bacteria. In our model, IL-22 dependent induction of Reg3β and Reg3γ was associated with decreased Gram-negative bacterial burden in small intestinal tissue and luminal content. It is possible that Reg3β and Reg3γ do not directly kill Gram-negative bacteria, but rather prevent their invasion into intestinal crypts. Further studies to dissect the role of Reg3β and Reg3γ in the containment of gut Gram-negative bacteria following combined trauma are needed.
Maintenance of effective gut immune function and barrier are critical to containment of gut bacteria. Our data indicate that IL-22 treatment prevents increased gut permeability and increased bacterial load following EtOH exposure and burn injury. However, we recognize that gut permeability did not return completely back to that of sham vehicle animals following IL-22 treatment. Similarly, decreased bacterial load was only noted in half of animals treated with IL-22. It is possible that a higher dose or a multiple dose regimen of IL-22 maybe to necessary to fully restore gut epithelial barrier and immune function following EtOH intoxication and burn injury. Moreover, suppression of IL-22 may be one mechanism by which gut permeability is increased following EtOH and burn injury. Other factors that contribute to increased gut permeability include IL-18, neutrophil infiltration and increased apoptosis as well as suppression of T helper cells, including Th1 interferon-γ (17, 20, 21, 32). Thus more studies are needed to determine the relative contribution these factors in gut barrier disruption after ethanol and burn injury. Findings from these potential future experiments may also reveal whether IL-22 has any role in the recruitment of neutrophils to the intestine following EtOH and burn injury. The findings from these studies will help in identifying a pathway(s) that can be targeted to maintain proper gut homeostasis and reduce morbidity and mortality following combined EtOH exposure and burn injury. While it is difficult to establish whether the increase in gut permeability is a direct or indirect effect of EtOH and burn injury, our unpublished data suggest that administration of EtOH alone cause gut leakiness within 3 hours after its administration, which is then exacerbated 24 hours after burn injury. However, in the absence of subsequent burn insult, the acute effect of EtOH disappears. Based on this data, we can speculate that EtOH combined with burn injury influences the epithelial barrier within the first few hours and disrupts the intricate relationship between gut bacteria and gut barrier, which in the context of subsequent burn injury leads to increased gut bacterial translocation.
We recognize that in our study, burn injury alone, in the absence of EtOH, did not cause any demonstrable change in the gut leakiness, this is in contrast to many previous studies suggesting impaired gut integrity and increased levels of bacterial translocation after burns. While the mechanisms for these differences are not known, one potential cause for this could be due to a difference in TBSA burn used in our and other studies. The surface area of burn insult plays a significant role in the overall outcome after burn injury(33). We have used ~12.5% TBSA burn injury. In contrast, most of the previous studies have used 25–30% TBSA to produce burn injury. There is evidence that patients with major burn injury (>20% TBSA) are more likely to develop complications and are transferred to major burn centers(34). Our own findings indicate a strong correlation between burn size and intestinal tissue damage, with increasing injury size being associated with higher tissue damage(20). However, in addition to burn size, other factors such as age, gender and preclinical manifestation can also influence the outcome of burn patients, particularly in patients who sustain small burn injuries(35). Similarly, EtOH exposure at the time of burn injury is being increasingly recognized as a factor that further complicates post burn pathogenesis (5–9, 11). In this study, we have utilized a relatively smaller (~12.5%) TBSA burn injury and this by itself did not produce any adverse effects on the intestine one day after injury. However, when combined with EtOH intoxication, it caused a decrease in gut AMP levels, an increase in gut bacterial load and gut leakiness. Thus, while a small injury by itself may not have an adverse effect on gut after injury, when combined with existing conditions such as EtOH intoxication it may become detrimental.
In conclusion, our findings suggest that administration of IL-22 following EtOH and burn injury prevents increased gut leakiness, gut bacterial load and decreased AMP levels in the gut. While more studies are needed to elucidate the mechanism by which IL-22 influences these parameters, the IL-22/AMP pathway may provide a novel therapeutic target for the treatment of patients who sustain burn injury under the influence of EtOH.
Supplementary Material
Acknowledgments
This study is supported by NIH grants R01AA015731 and R01AA015731-04S1. Juan L. Rendon is supported by NIH grants F30AA020167 (JLR), T32AA013527 (EJK), the Loyola University Chicago Stritch School of Medicine Combined MD/PhD Program and the Dr. Ralph and Marian C. Falk Medical Research Trust
Footnotes
Disclosures:
The authors have no financial conflict of interest.
References
- 1.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 2.Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 4.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Burn Association. Burn incidence and treatment in the US: 2007 fact sheet. 2010. 2007 [Google Scholar]
- 6.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. J Burn Care Rehabil. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 7.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Kelley D, Lynch JB. Burns in alcohol and drug users result in longer treatment times with more complications. J Burn Care Rehabil. 1992;13:218–220. doi: 10.1097/00004630-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Albright JM, Kovacs EJ, Gamelli RL, Schermer CR. Implications of formal alcohol screening in burn patients. J Burn Care Res. 2009;30:62–69. doi: 10.1097/BCR.0b013e3181921f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Front Biosci. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–140. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Deitch EA, Rutan R, Waymack JP. Trauma, shock, and gut translocation. New Horiz. 1996;4:289–299. [PubMed] [Google Scholar]
- 15.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Rana SN, Kovacs EJ, Gamelli RL, Chaudry IH, Choudhry MA. Corticosterone suppresses mesenteric lymph node T cells by inhibiting p38/ERK pathway and promotes bacterial translocation after alcohol and burn injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R37–44. doi: 10.1152/ajpregu.00782.2004. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Chaudry IH, Choudhry MA. ERK and not p38 pathway is required for IL-12 restoration of T cell IL-2 and IFN-gamma in a rodent model of alcohol intoxication and burn injury. J Immunol. 2009;183:3955–3962. doi: 10.4049/jimmunol.0804103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhry MA, Fazal N, Namak SY, Haque F, Ravindranath T, Sayeed MM. PGE2 suppresses intestinal T cell function in thermal injury: a cause of enhanced bacterial translocation. Shock. 2001;16:183–188. doi: 10.1097/00024382-200116030-00003. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–47. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication potentiates neutrophil-mediated intestinal tissue damage after burn injury. Shock. 2008;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. J Burn Care Res. 2011;32:489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehotzky RE, Partch CL, Mukherjee S, Cash HL, Goldman WE, Gardner KH, Hooper LV. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc Natl Acad Sci U S A. 2010;107:7722–7727. doi: 10.1073/pnas.0909449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavanaugh MJ, Clark C, Goto M, Kovacs EJ, Gamelli RL, Sayeed MM, Choudhry MA. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290–296. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg Infect (Larchmt) 2009;10:389–397. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruitt BA, Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 28.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 29.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bingold TM, Ziesche E, Scheller B, Sadik CD, Franck K, Just L, Sartorius S, Wahrmann M, Wissing H, Zwissler B, et al. Interleukin-22 detected in patients with abdominal sepsis. Shock. 2010;34:337–340. doi: 10.1097/SHK.0b013e3181dc07b1. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta. 2012;1822:196–203. doi: 10.1016/j.bbadis.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 34.Mlcak RP, Dimick AR, Mlcak G. Pre-hospital management, transportation and emergency care. In: Herndon DN, editor. Total Burn Care. Philadelphia: W. B. Saunders Company Ltd; 1997. pp. 33–43. [Google Scholar]
- 35.McGwin G, Jr, George RL, Cross JM, Reiff DA, Chaudry IH, Rue LW., 3rd Gender differences in mortality following burn injury. Shock. 2002;18:311–315. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


