Abstract
Purpose
To understand cultural differences in the impact of cancer (IOC), by (1) performing an independent psychometric evaluation of the Dutch version of the Impact of Cancer Scale version 2 (IOCv2) in a non-Hodgkin lymphoma (NHL) sample and (2) examining differences between Dutch and American NHL survivors in perceived impact of cancer and identify associations with socio-demographic and clinical characteristics.
Methods
Data collected from 491 Dutch and 738 American NHL survivors were used in this study. IOCv2 responses were obtained from all survivors; the Dutch survivors also completed the EORTC QLQ-C30, which measures quality of life.
Results
Exploratory factor analysis of the Dutch version yielded a factor solution similar to the American structure but with some subscales merging into single factors. Internal consistency was good; Cronbach's alpha was 0.88 for the Positive and 0.94 for the Negative summary scales. Large differences were observed between survivors, whereby Dutch survivors reported fewer Positive (Δ−0.4,p<.001,effect size:0.27) and more Negative (Δ0.2,p≤.001,effect size:0.13) impacts of cancer independent of socio-demographic and clinical characteristics.
Conclusion
Similar impact domains of the IOCv2 were observed in the Dutch sample, providing evidence that IOCv2 scales measure common and important survivor concerns across two different Western nations. Higher positive impacts for US survivors might be explained by more personal control and availability of supportive services. Future research should focus on determinants of the impact of cancer in both Dutch and American survivors to gain better understanding of the factors that might improve it and suggest how health care may be modified toward that end.
Introduction
Advances in cancer treatment have led to an expansion in the number of cancer survivors in developed countries. Non-Hodgkin's lymphoma (NHL) is one of the diseases that has benefited from such advances. For both the Netherlands and the United States (US), the annual age-adjusted incidence of NHL is 1 in 5,000 persons, with approximately 3,000 new cases in the Netherlands1,2 and 65,000 new cases in the US3 annually. The number of NHL survivors has increased rapidly from 13,400 in 2001 to 19,600 in 2008 in the Netherlands1,2 and from approximately 347,000 in 2001 to 454,000 in 2008 in the US3. An individual has a 1 in 50 chance of being diagnosed with NHL during his or her lifetime.
As cancer survivors live longer, they develop risks such as late effects of therapy and adverse physical and psychosocial long-term effects4. These long-term effects include persistent fatigue, depression, anxiety and marital disruption that can have a negative influence on survivors' health-related quality of life (HRQOL)5–10. While cancer survivors may be expected to return to normal life soon after treatment ends, they may continue to be burdened by the physical and psychosocial effects of the cancer and related treatments.
In a recent systematic review, we found that, on average, lymphoma cancer survivors have decreased HRQOL compared to the general population even several years post-diagnosis (i.e., no resolution at more than five years post-diagnosis)11. However, most survivorship studies lack the use of an instrument that addresses the unique concerns related to the cancer experience such as those measured by the impact of cancer (IOC) scale12–14. This self-reported questionnaire was developed in the US to measure positive and negative impacts of cancer that long-term survivors attribute to their cancer experience. A translation of the IOC into Dutch has been undertaken, but its psychometric properties have not been described.
Cultural differences may affect the perception of the impact of cancer on HRQOL15,16. Moreover, attitudes towards health practice and illness may also be defined by culture17. Therefore, we undertook an examination of two samples of NHL patients in the Netherlands and the US, and compared their responses to the IOC. To better understand the commonality of psychosocial problems between cultures, it is important to examine cross-national differences18. This undertaking will provide more knowledge of culture-specific determinants of psychosocial well-being.
Therefore, the aims of the present study were to (1) perform an independent psychometric evaluation of the Dutch version of the IOCv2 in a NHL sample and (2) explore differences between Dutch and American NHL survivors regarding the impact of cancer and identify associations with socio-demographic and clinical characteristics associated with the IOC score.
Methods
Participants
Dutch sample
NHL survivors aged ≥18 were identified using the Eindhoven Cancer Registry (ECR) to select all patients who were diagnosed with NHL between January 1st, 1999 and July 1st, 2009. We included all patients with indolent (including Chronic Lymphocytic Leukemia) and aggressive B-cell NHL as defined by the International Classification of Diseases for Oncology-3 codes (ICD-O-3)19. To identify and exclude patients who were deceased, the database was linked with the database of the Central Bureau for Genealogy, which collects data on all deceased Dutch citizens through the civil municipal registries.
Data collection took place in summer 2009 and was done within PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship). PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from ECR. Details of the data collection method have been previously described20.
Of the 1026 eligible survivors who were assumed to have received an invitation, 824 (80%) returned survey materials. Non-respondents were more often diagnosed with indolent NHL (63% versus 54%, p<.05) and less often diagnosed with stage I disease (19% versus 25%, p<.05). There were no differences between respondents and non-respondents in gender or age.
American sample
NHL survivors were identified through Duke University Medical Center and University of North Carolina at Chapel (UNC) Hill Lineberger tumor registries in November 2004 as previously described21. Patients were eligible if ≥18 years old at diagnosis, and ≥2 years post-diagnosis. Prospective participants were mailed a self-administered survey. Of the 1195 eligible survivors who were assumed to have received an invitation, 886 (74%) returned survey materials. Participants, compared with non-participants, were less frequently African American (10% versus 20%, p<.001) and older at study enrollment (mean age 62.9 versus 58.8 years p<.001).
Total sample
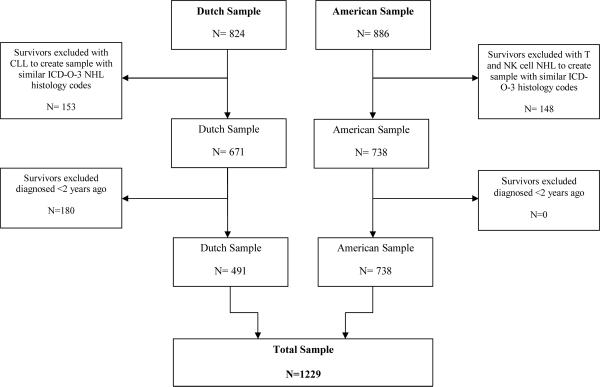
To create more comparable samples, we selected those survivors with overlapping ICDO-3 codes, i.e. excluding survivors diagnosed with Chronic Lymphocytic Leukemia in the Dutch sample and survivors with T-cell and NK-cell NHL in the American sample. We also excluded survivors diagnosed ≤2 years post-diagnosis in the Dutch sample since the IOC was developed for longer term survivors and the US sample included only this population13,14. The total sample consisted of 1229 survivors, 491 Dutch and 738 American survivors (Figure 1). Institutional Review Board approval was obtained in both countries at all institutions participating in the study and written informed consent was obtained from each participant.
Figure 1.
Flow diagram of the data sample structure.
Note. Flow diagram of the data sample structure, excluding patients with non-similar International Classification of Diseases for Oncology-3 (ICD-O-3) codes and those diagnosed less than two years post-diagnosis. CLL=Chronic Lymphocytic Leukemia, NHL=non-Hodgkin lymphoma, NK=natural killer.
Measures
The IOC presents statements regarding specific impacts of cancer to which respondents indicate their level of agreement from 1 (strongly disagree) to 5 (strongly agree). Initial psychometric scaling of a 81-item IOC questionnaire yielded the 41-item IOC version 1 (IOCv1)13,14. A more recent and comprehensive scaling of the IOC questionnaire yielded the 37-item IOC version 2 (IOCv2)12. The Dutch survivors completed the IOCv1, which is missing 7 items that are in IOCv2. A newly developed algorithm was used to impute the 7 missing IOCv2 item scores for the Dutch survivors based on their IOCv1 responses22. The American survivors completed the 81-item IOC questionnaire and had their responses scored as IOCv2 scales. Other reports from the American sample have used both the IOCv1 and IOCv2 scoring formats9,10.
The Dutch survivors also completed the Dutch validated version of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 which assesses HRQOL in cancer patients23. Response categories range from 1 to 4. After linear transformation, all scales and single item measures range in score from 0 to 100. A higher score on function scales and the global health and quality of life scale implies a better HRQOL, whereas for symptoms (scales and items) a higher score refers to more symptoms23.
For both samples, comorbidity was assessed with the Self-administered Comorbidity Questionnaire (SCQ)24. Marital status and educational level were also assessed in both samples. For the Dutch sample, clinical information was available from the ECR that routinely collects data on tumor characteristics, including date of diagnosis, tumor grade, histology, Ann Arbor stage25, primary treatment, and demographic characteristics, including gender and date of birth. Clinical data pertaining to the American sample were obtained from Duke University Medical Center and University of North Carolina at Chapel Hill Lineberger Tumor Registries and complemented with self-reported data.
Statistical analysis
All statistical analyses were performed using SAS (version 9.1 for Windows; SAS Institute Inc., Cary, NC). P values of <.05 were considered statistically significant. Differences in socio-demographic and clinical characteristics between Dutch and American NHL respondents were assessed using chi-square and t-tests.
Psychometric evaluation
An exploratory factor analysis was conducted on the 37 items of the IOCv2 of the Dutch sample. Factors were extracted using principal components; the number of factors was selected using eigenvalue >1 and scree plots and promax rotation were performed. We repeated the factor analysis three times, with six, seven and eight factors, as the scree plot showed a stabilization point after six and eight factors. Internal consistency of the IOCv2 of the Dutch sample was measured using Cronbach's alpha. The Cronbach's alpha coefficient should reach 0.7 or above to be judged as good internal consistency and reliability26. Concurrent validity was evaluated by calculating Spearman correlation coefficients between IOCv2 scales and EORTC QLQ-C30 subscales. We hypothesized that the IOCv2 positive scales would be uncorrelated with the EORTC QLQ-C30, because they measure distinct constructs. We hypothesized that the IOCv2 negative scales would be substantially correlated with the EORTC QLQ-C30, because limitations in functioning and having cancer-related symptoms could have negative impacts on one's QOL.
Comparison of Dutch and American survivors
The mean IOCv2 scores of the Dutch NHL survivors were compared with the scores of the American NHL survivors using independent sample t-tests. Multivariate linear regression analysis was performed to investigate the independent association between socio-demographic and clinical variables and IOCv2 scales for the samples (Dutch and American) separately and for the total NHL sample. Since there were no large differences between countries in associations between IOCv2 scores and socio-demographic and clinical variables, only results of the total sample are presented.
Results
Sample characteristics
Comparisons between Dutch and American NHL survivors showed significant differences on most sociodemographic and clinical characteristics (all p<.001) except for age, marital/partner status and NHL histology (Table 1). Dutch respondents were more often male, had on average a lower educational level and were less likely to be employed during study enrollment. Mean interval since diagnosis was shorter among Dutch survivors, who also had a smaller range of interval (i.e., standard deviation). Dutch survivors also reported fewer comorbid conditions. Despite statistically significant differences in disease stage and treatment, both survivor groups were most often diagnosed with stage I disease followed by stage IV disease, and chemotherapy and radiotherapy were the most common treatments received. The mean age at the time of survey for both groups was 63 years and about 80% of survivors were married or in a committed relationship.
Table 1.
Socio-demographic and clinical characteristics of Dutch and American non-Hodgkin lymphoma survivors.
| Dutch Respondents N=491 | American Respondents N=738 | ||
|---|---|---|---|
|
| |||
| N (%) | N (%) | P-value | |
| Gender | <.001 | ||
| Male | 290 (59) | 363 (49) | |
| Female | 201 (41) | 375 (51) | |
| Age at time of survey: mean (SD) | 63.0 (12.5) | 63.0 (13.3) | .98 |
| <50 years | 71 (15) | 111 (15) | |
| 50–64 years | 174 (35) | 273 (38) | |
| 65+ years | 246 (50) | 339 (47) | |
| Education $ | <.001 | ||
| Low | 111 (23) | 81 (11) | |
| Medium | 291 (61) | 353 (49) | |
| High | 74 (16) | 284 (40) | |
| Marital/partner status | .16 | ||
| Married/committed | 390 (81) | 567 (78) | |
| Not married/committed | 92 (19) | 164 (22) | |
| Employment status | <.001 | ||
| Currently employed | 116 (25) | 287 (42) | |
| Not employed or retired | 339 (75) | 400 (58) | |
| Years since diagnosis: mean (SD) | 5.3 (2.2) | 10.2 (7.3) | <.001 |
| 2–4 years | 263 (54) | 180 (24) | |
| 5–7 years | 153 (31) | 223 (26) | |
| 8–10 years | 75 (15) | 151 (17) | |
| >10 years | 0 | 293 (32) | |
| NHL histology | .89 | ||
| Indolent | 226 (46) | 314 (43) | |
| Aggressive | 265 (54) | 374 (54) | |
| Unknown | 0 | 50 (6) | |
| NHL stage at diagnosis | .001 | ||
| I | 153 (31) | 183 (28) | |
| II | 93 (19) | 149 (18) | |
| III | 75 (15) | 133 (17) | |
| IV | 146 (30) | 205 (24) | |
| Unknown# | 24 (5) | 68 (13) | |
| Primary treatment/ treatment | <.001 | ||
| Radiotherapy | 148 (30) | 363 (49) | |
| Chemotherapy | 343 (70) | 618 (84) | |
| Biologic | 0 | 224 (30) | |
| Active surveillance+ | 78 (16) | 0 | |
| Transplant* | 28 (6) | 126 (17) | |
| Surgery | 33 (7) | 237 (33) | |
| Self-reported comorbidity | <.001 | ||
| No comorbid condition | 136 (30) | 75 (10) | |
| 1 comorbid condition | 138 (31) | 135 (19) | |
| 2 comorbid conditions | 93 (21) | 141 (19) | |
| > 2 comorbid conditions | 86 (19) | 374 (52) | |
Note.
Education levels included low = no/primary school; medium = lower general secondary education/vocational training; or high = pre-university education/ high vocational training/university
Tumor stage could not be determined in some subtypes of indolent non-Hodgkin Lymphoma.
Patients are under active surveillance and receive no therapy.
Transplant= autologous stem cell or bone marrow transplantation.
Psychometric evaluation
Exploratory factor analysis
The six factor structure yielded the most interpretable solution. `Health Awareness and Worry' emerged as a single factor as did `Body Change Concerns and Life Interferences'. The additional factors represented the four other domains of the IOCv2, i.e. Meaning of Cancer, Positive Self-Evaluation, Altruism/Empathy, and Appearance Concerns (Appendix 1). Item IOC29 loaded higher on Meaning of Cancer than on Health Awareness (0.57 vs. 0.35). The emerging of Body Change Concerns and Life Interferences as a single domain was also observed in the factor analysis of the American NHL sample27. Cronbach's alpha was 0.88 for the Positive and 0.94 for the Negative Impact scales, respectively, and ranged from 0.75 to 0.93 for the subscales.
Concurrent validity
The correlations between IOCv2 Positive scales and the EORTC QLQ-C30 were all below 0.30, supporting the distinctive content of the IOCv2 Positive scales from this HRQOL measure (Appendix 2). With respect to IOCv2 Negative scales we observed an overall pattern of moderate (r≥0.30) to substantial correlation (r≥0.45) with the EORTC QLQ-C30. The strongest correlation was observed between IOCv2 Body Change Concerns and Fatigue of the EORTC QLQ-C30 (r=0.61).
Comparison of Dutch and American survivors
Significant differences were observed between Dutch and American NHL survivors on all IOCv2 scales (all p<.01) except for Meaning of Cancer and Life Interferences (Table 2). Dutch survivors scored lower on the Positive Impact subscales (i.e., Altruism/Empathy, Health Awareness and Positive Self-Evaluation) and higher on the Negative Impact subscales (i.e., Appearance Concerns, Body Change Concerns and Worry). The difference on the Positive Impact Summary scale was larger compared to the Negative Impact Summary scale (0.4 vs. 0.2 points, both p<.01).
Table 2.
Comparison of mean scores of the IOCv2 sub and total scales between Dutch and American non-Hodgkin's lymphoma survivors.
| Dutch Respondents N=491 | American Respondents N=738 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| IOCv2 Scale | Mean | SD | Range | Mean | SD | Range | Δ | p-value | Effect size r |
| Altruism/Empathy | 3.3 | 0.8 | 1–5 | 3.9 | 0.9 | 1–5 | −0.6 | <.001 | 0.33 |
| Health Awareness | 3.2 | 0.9 | 1–5 | 3.7 | 0.9 | 1–5 | −0.5 | <.001 | 0.27 |
| Meaning of Cancer | 2.7 | 0.9 | 1–5 | 2.7 | 1.1 | 1–5 | 0 | .12 | 0 |
| Positive Self-Evaluation | 3.4 | 0.8 | 1–5 | 3.9 | 1.0 | 1–5 | −0.5 | <.001 | 0.27 |
| Appearance Concerns | 1.8 | 0.9 | 1–5 | 1.7 | 0.9 | 1–5 | 0.1 | .004 | 0.06 |
| Body Change Concerns | 2.6 | 1.0 | 1–5 | 2.4 | 1.2 | 1–5 | 0.2 | .002 | 0.09 |
| Life interferences | 2.0 | 0.7 | 1–5 | 2.0 | 0.7 | 1–4.8 | 0 | .62 | 0 |
| Worry | 2.8 | 1.0 | 1–5 | 2.6 | 1.0 | 1–5 | 0.2 | <.001 | 0.10 |
| Positive Impact Scale | 3.1 | 0.6 | 1–4.9 | 3.5 | 0.8 | 1–5 | −0.4 | <.001 | 0.27 |
| Negative Impact Scale | 2.4 | 0.8 | 1–4.9 | 2.2 | 0.7 | 1–4.8 | 0.2 | .001 | 0.13 |
Note. IOCv2= Impact of Cancer Scale version 2.
Multivariate linear regression analysis also showed lower Positive IOCv2 scores and higher Negative IOCv2 scores (p<.001) for Dutch survivors (Table 3). Based on the total sample of Dutch and American NHL survivors, females scored significantly higher on several Positive Impact subscales and on Appearance Concerns. Older survivors scored significantly lower on both Positive and Negative Impact Summary scales. In addition, higher educated survivors showed less Altruism/Empathy, survivors without a partner reported more Worry, and survivors who were not employed or were retired showed less Life Interferences.
Table 3.
Multivariate linear regression analyses of IOCv2 sub and total scales of Dutch and American non-Hodgkin lymphoma survivors (N=1229).
| Positive impact scale | Positive subscales |
Negative impact scale | Negative subscales |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Altruism/ Empathy | Health Awareness | Meaning of cancer | Positive self-Evaluation | Appearance Concerns | Body Changes | Life interferences | Worry | |||
| Country | ||||||||||
| USA | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| The Netherlands | −0.25** | −0.32** | −0.25** | −0.05 | −0.23** | 0.16** | 0.18** | 0.13** | 0.08 | 0.13** |
| Gender | ||||||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Female | 0.10** | 0.09* | 0.07 | 0.06 | 0.11** | 0.03 | 0.14** | 0.00 | −0.01 | 0.02 |
| Age | −0.13** | 0.00 | −0.16** | −0.17** | −0.09 | −0.18** | −0.15** | −0.10* | −0.16** | −0.17** |
| Education | ||||||||||
| Low | 0.04 | 0.03 | 0.02 | 0.02 | 0.05 | −0.02 | 0.00 | −0.05 | −0.03 | −0.01 |
| Medium | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| High | −0.08 | −0.12** | −0.00 | −0.07 | −0.06 | 0.01 | −0.01 | −0.06 | −0.03 | 0.06 |
| Marital/partner status | ||||||||||
| Partner | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| No partner | 0.02 | 0.05 | 0.06 | −0.04 | 0.01 | 0.06 | 0.02 | 0.05 | 0.01 | 0.08* |
| Employment status | ||||||||||
| Currently employed | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Not employed/retired | 0.01 | 0.03 | −0.04 | 0.00 | 0.04 | −0.09 | −0.06 | −0.06 | −0.16** | −0.03 |
| Years since diagnosis | 0.04 | 0.01 | −0.03 | 0.03 | 0.10* | −0.15** | −0.01 | −0.19** | −0.06 | −0.16** |
| NHL histology | ||||||||||
| Indolent | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Aggressive | 0.01 | 0.00 | −0.01 | 0.02 | 0.01 | −0.05 | −0.02 | −0.02 | 0.01 | −0.09* |
| NHL stage | ||||||||||
| I | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| II | 0.09 | 0.09 | 0.07 | 0.07 | 0.05 | 0.09 | 0.05 | 0.05 | 0.06 | 0.10* |
| III | 0.08 | 0.05 | 0.08 | 0.08 | 0.05 | 0.12* | 0.08 | 0.10* | 0.10 | 0.10* |
| IV | 0.09 | 0.06 | 0.14** | 0.03 | 0.05 | 0.16** | 0.12* | 0.13* | 0.14** | 0.15** |
| Chemotherapy | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.09* | 0.08 | 0.01 | 0.05 | 0.16** | 0.05 | 0.02 | 0.13** | 0.03 | 0.01 |
| Radiotherapy | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | −0.04 | −0.02 | 0.00 | −0.09 | 0.01 | −0.01 | 0.01 | −0.00 | −0.02 | 0.01 |
| Comorbidity | ||||||||||
| None | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 1 | −0.03 | −0.03 | −0.05 | −0.01 | −0.02 | 0.09 | 0.07 | 0.10 | 0.08 | 0.07 |
| 2 | −0.01 | −0.04 | 0.03 | 0.01 | −0.01 | 0.12* | 0.11* | 0.11* | 0.09 | 0.09 |
| 3 or more | 0.01 | 0.01 | 0.05 | −0.02 | −0.03 | 0.32** | 0.23** | 0.32** | 0.31** | 0.21** |
Note.IOCv2= Impact of Cancer Scale version 2;
p<0.01;
p<.001
With respect to the clinical characteristics, survivors with a longer survival time post-diagnosis showed higher Positive Self-Evaluation scores, and less Negative Impacts on Body Change Concerns, and Worry. Survivors with an aggressive NHL histology reported less Worry. Furthermore, survivors with more advanced disease stage, especially stage IV disease, showed higher scores on Health Awareness and on all Negative Impact scales. Survivors treated with chemotherapy reported a higher Positive Self-Evaluation and Positive Impact Summary scale as well as higher scores on Body Change Concerns. Lastly, survivors with three or more comorbidities had higher Negative Impact subscale scores.
Discussion
The findings of this study show that similar impact domains were observed for Dutch and American NHL survivors, providing evidence that the IOCv2 measures common and important survivor concerns across two different Western nations. The internal reliability and consistency of the Dutch scales were good and construct validity was observed between the IOCv2 negative scales and the EORTC QLQ-C30. Unfortunately, we could not evaluate the construct validity of the IOCv2 Positive Impact subscales, since the Dutch study did not have a relevant questionnaire that measured positive growth.
We also observed significant differences between Dutch and American NHL survivors, whereby Dutch survivors reported less positive impacts and more negative impacts of cancer. These differences, combined with construct validity, suggest that the IOCv2 scales are able to distinguish between cultures of the impacts of cancer, and this questionnaire is thus culturally sensitive.
One explanation for these differences might be that living in different cultures cultivates other psychological resources which influence health. The structure of a society, such as the social safety net and health care systems, contributes to shaping population health and attitudes towards health care28. Individuals in the US are socialized to rely more on individual resources compared with collective resources in Western Europe16,29. In the US, health care programs fall under the responsibility of the individual30,31, whereas in the Netherlands they are administered by the government32,33. To be more responsible for one's own health care creates a situation wherein control must be exercised. This sense of control is reflected in the emergence of a patient autonomy movement that began in America during the 1970s. Since then, a shift was made from a more paternalistic relationship between physicians and patients to a more equal relationship34,35, whereby information provision is one of the key elements of patient autonomy36. Studies have shown that personal control is associated with better self-reported health37,38 since individuals who believe that they have some degree of control over their lives may be more likely to take action in difficult situations39. Furthermore, the sense of personal control is more prevalent in North America than in Europe15, which might result in the ability to alter perceptions of the cancer experience in a more positive way among American survivors.
Additionally, the hospitals where the American NHL survivors were treated have well-developed programs in cancer survivorship care. For example, support groups are readily available and Duke University Medical Center provides free psychosocial counseling and UNC social workers were available to assist patients free of charge. A recent study reported that social support is associated with more positive and less negative Impacts of cancer (Smith et al., under review). Therefore, the higher positive and lower negative impact scores of the American survivors might be ascribed partly to having received more social support. Other evaluation of the sample demonstrated that females scored significantly higher on the positive impacts of cancer (Smith et al., review). However, in the Dutch sample no differences in impact between men and women were observed, which may reflect other differences between the genders across the two samples.
Our results related to the impact of cancer are largely consistent with another Dutch study of 562 melanoma survivors40. In both studies, it appeared that time since diagnosis, tumor stage, and comorbidity were found to be associated with negative impacts of cancer.
The present study had some limitations. Although the response rate was high for both samples and information was available on socio-demographic and clinical characteristics of the non-respondents in both samples, it remains unknown whether non-respondents declined to participate in the study because of poor health. In addition, the seven missing items for the IOCv2 were calculated with a newly developed algorithm which has not been tested in other samples yet. Furthermore, the US data were collected from two institutions only, which limits the heterogeneity of the American sample.
In spite of these limitations, this study provides important information about the valid use of the IOCv2 in the Netherlands and with a preliminary look at the cross-national difference of the IOCv2 between Dutch and American NHL survivors. Results suggest that Dutch NHL survivors have lower positive and higher negative impacts of cancer compared with their American counterparts. Higher positive impacts for US survivors might be explained by more personal control and availability of supportive services. Future research should focus on determinants of the impact of cancer in both Dutch and American survivors to gain better understanding of the factors that might improve it.
Supplementary Material
Acknowledgments
Funding: The Dutch part of the study was financially supported by the Jonker-Driessen Foundation, ZonMW (the Netherlands organization for health research and development, #80-82500-98-01007) and the Netherlands Organization for Scientific Research (Investment Subsidy (#480-08-009). Dr. Van de Poll-Franse was supported by a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349). The American part of the study was funded by the National Cancer Institute (CA-101492), the American Cancer Society Doctoral Training Grant in Oncology Social Work (DSW-0321301-SW), and the University of North Carolina Research Council. Dr. Crespi was also supported by NIH CA16042.
References
- 1.Kankerbestrijding SKvK. Kanker in Nederland tot 2020. Trends en prognose. http://www.cijfersoverkanker.nl/selecties.
- 2.van de Schans SA, Issa DE, Visser O, et al. Diverging trends in incidence and mortality, and improved survival of non-Hodgkin's lymphoma, in the Netherlands, 1989–2007. Ann Oncol. 2011 Apr 4; doi: 10.1093/annonc/mdr055. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011, assessed on May 10, 2011. [Google Scholar]
- 4.Geffen DB, Blaustein A, Amir MC, Cohen Y. Post-traumatic stress disorder and quality of life in long-term survivors of Hodgkin's disease and non-Hodgkin's lymphoma in Israel. Leuk Lymphoma. 2003 Nov;44(11):1925–1929. doi: 10.1080/1042819031000123573. [DOI] [PubMed] [Google Scholar]
- 5.Bellizzi KM, Rowland JH, Arora NK, Hamilton AS, Miller MF, Aziz NM. Physical activity and quality of life in adult survivors of non-Hodgkin's lymphoma. J Clin Oncol. 2009 Feb 20;27(6):960–966. doi: 10.1200/JCO.2008.17.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerkeman M, Kaasa S, Hjermstad M, Kvaloy S, Cavallin-Stahl E. Health-related quality of life and its potential prognostic implications in patients with aggressive lymphoma: a Nordic Lymphoma Group Trial. Med Oncol. 2001;18(1):85–94. doi: 10.1385/MO:18:1:85. [DOI] [PubMed] [Google Scholar]
- 7.Mols F, Aaronson NK, Vingerhoets AJ, et al. Quality of life among long-term non-Hodgkin lymphoma survivors: a population-based study. Cancer. 2007 Apr 15;109(8):1659–1667. doi: 10.1002/cncr.22581. [DOI] [PubMed] [Google Scholar]
- 8.Pettengell R, Donatti C, Hoskin P, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. 2008 Mar;19(3):570–576. doi: 10.1093/annonc/mdm543. [DOI] [PubMed] [Google Scholar]
- 9.Smith SK, Crespi CM, Petersen L, Zimmerman S, Ganz PA. The impact of cancer and quality of life for post-treatment non-Hodgkin lymphoma survivors. Psychooncology. 2010 Jan 22; doi: 10.1002/pon.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009 Jul 15;115(14):3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll-Franse LV. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin's and non-Hodgkin's lymphoma survivors: a systematic review. Ann Hematol. 2011 Sep;90(9):993–1004. doi: 10.1007/s00277-011-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crespi CM, Ganz PA, Petersen L, Castillo A, Caan B. Refinement and psychometric evaluation of the impact of cancer scale. J Natl Cancer Inst. 2008 Nov 5;100(21):1530–1541. doi: 10.1093/jnci/djn340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zebrack BJ, Ganz PA, Bernaards CA, Petersen L, Abraham L. Assessing the impact of cancer: development of a new instrument for long-term survivors. Psychooncology. 2006 May;15(5):407–421. doi: 10.1002/pon.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psychooncology. 2008 Sep;17(9):891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- 15.Clarke P, Smith J. Aging in a cultural context: cross-national differences in disability and the moderating role of personal control among older adults in the United States and England. J Gerontol B Psychol Sci Soc Sci. 2011 Jul;66(4):457–467. doi: 10.1093/geronb/gbr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trill MD, Holland J. Cross-cultural differences in the care of patients with cancer. A review. Gen Hosp Psychiatry. 1993 Jan;15(1):21–30. doi: 10.1016/0163-8343(93)90087-5. [DOI] [PubMed] [Google Scholar]
- 17.Forjaz MJ, Guarnaccia CA. A comparison of Portuguese and American patients with hematological malignancies: a cross-cultural survey of health-related quality of life. Psychooncology. 2001 May-Jun;10(3):251–258. doi: 10.1002/pon.522. [DOI] [PubMed] [Google Scholar]
- 18.Collings JA. International differences in psychosocial well-being: a comparative study of adults with epilepsy in three countries. Seizure. 1994 Sep;3(3):183–190. doi: 10.1016/s1059-1311(05)80187-6. [DOI] [PubMed] [Google Scholar]
- 19.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. ed 3rd World Health Organisation; Geneva: 2000. [Google Scholar]
- 20.van de Poll-Franse LV, Horevoorts N, Eenbergen MV, et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: Scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011 May 26; doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Smith SK, Zimmerman S, Williams CS, Preisser JS, Clipp EC. Post-traumatic stress outcomes in non-Hodgkin's lymphoma survivors. J Clin Oncol. 2008 Feb 20;26(6):934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespi CM, Ganz PA, Petersen L, Smith SK. A procedure for obtaining Impact of Cancer version 2 scores using version 1 responses. Qual Life Res. 2012 Feb 3; doi: 10.1007/s11136-012-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar 3;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 24.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003 Apr 15;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 25.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- 26.Cronbach L. Coefficient alpha and the internal structure of test. Psychometrica. 1951;16:297–334. [Google Scholar]
- 27.Crespi CM, Smith SK, Petersen L, Zimmerman S, Ganz PA. Measuring the impact of cancer: a comparison of non-Hodgkin lymphoma and breast cancer survivors. J Cancer Surviv. Mar;4(1):45–58. doi: 10.1007/s11764-009-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonough P, Worts D, Sacker A. Socioeconomic inequalities in health dynamics: a comparison of Britain and the United States. Soc Sci Med. 2010 Jan;70(2):251–260. doi: 10.1016/j.socscimed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Mayer KU. Structural constraints on the life course. Human Development. 1986;29:163–170. [Google Scholar]
- 30.Robinson JC. Health savings accounts--the ownership society in health care. N Engl J Med. 2005 Sep 22;353(12):1199–1202. doi: 10.1056/NEJMp058097. [DOI] [PubMed] [Google Scholar]
- 31.Steinbrook R. Imposing personal responsibility for health. N Engl J Med. 2006 Aug 24;355(8):753–756. doi: 10.1056/NEJMp068141. [DOI] [PubMed] [Google Scholar]
- 32.Bal R, Zuiderent-Jerak T. The practice of markets in Dutch health care: are we drinking from the same glass? Health Econ Policy Law. 2011 Jan;6(1):139–145. doi: 10.1017/S1744133110000368. [DOI] [PubMed] [Google Scholar]
- 33.Schafer W, Kroneman M, Boerma W, et al. The Netherlands: health system review. Health Syst Transit. 2010;12(1):v–xxvii. 1–228. [PubMed] [Google Scholar]
- 34.Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: Part II: The autonomy model. Chest. Jun;139(6):1491–1497. doi: 10.1378/chest.11-0516. [DOI] [PubMed] [Google Scholar]
- 35.Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: Part I: The beneficence model. Chest. Mar;139(3):669–673. doi: 10.1378/chest.10-2532. [DOI] [PubMed] [Google Scholar]
- 36.Hoving C, Visser A, Mullen PD, van den Borne B. A history of patient education by health professionals in Europe and North America: from authority to shared decision making education. Patient Educ Couns. 2010 Mar;78(3):275–281. doi: 10.1016/j.pec.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Krause N. Age and decline in role-specific feelings of control. J Gerontol B Psychol Sci Soc Sci. 2007 Jan;62(1):S28–35. doi: 10.1093/geronb/62.1.s28. [DOI] [PubMed] [Google Scholar]
- 38.Wickrama KA, Surjadi FF, Lorenz FO, Elder GH., Jr. The influence of work control trajectories on men's mental and physical health during the middle years: mediational role of personal control. J Gerontol B Psychol Sci Soc Sci. 2008 May;63(3):S135–145. doi: 10.1093/geronb/63.3.s135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. J Pers Soc Psychol. 1998 Mar;74(3):763–773. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- 40.Holterhues C, Cornish D, van de Poll-Franse LV, et al. Impact of melanoma on patients' lives among 562 survivors: a Dutch population-based study. Arch Dermatol. 2011 Feb;147(2):177–185. doi: 10.1001/archdermatol.2010.433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.