The midbrain dopamine system is implicated in mediating the reactivity of the organism to salient environmental stimuli,1,2 including emotional events,3 at different timescales2. Preclinical and human neuroimaging studies implicate the dopamine system in coding stimulus salience4 through its innervations of nigrostriatal and mesocorticolimbic pathways,1,2 and dysfunction of this system plays an important role in neuropsychiatric disorders such as schizophrenia and depression that are accompanied by impaired social and emotional cognition. However, the role of dopamine in modulating transient (in milliseconds) distributed neural representation of human emotional cognition remains undefined. We examined the relationship of midbrain presynaptic dopamine (directly measured with positron emission tomography [PET]) to “sustained” and “transient” neural responses to salient emotional facial expressions (measured with fMRI and magnetoencephalography [MEG], respectively).
Twenty-one healthy participants (mean age=31, six females) consented according to NIH-IRB and Radiation Safety Committee guidelines before undergoing 90-minute PET scans following IV administration of 16-mCi[18F]fluoroDOPA (FDOPA). A cerebellar reference region was used to determine FDOPA-Ki, a reproducible measure of presynaptic DA stores and synthesis.5 After coregistration of FDOPA scans onto native space MRIs, a manually-defined midbrain volume of interest within each individual's MRI was used for extraction of mean Ki values (Supplementary Information).
All 21 FDOPA-PET participants underwent event-related fMRI while viewing videos of 10 different actors portraying dynamic fearful, happy and neutral expressions. Sixteen of the 21 participants repeated the same behavioral paradigm during MEG acquired using a CTF-275 system. Global facial movement parameters for each video were measured to determine average timecourses of the emotional expressions. fMRI data (dynamic emotional>dynamic neutral expressions) were analyzed using SPM5; MEG data (assessing both fear>neutral, and happy>neutral dynamic expressions) were analyzed with 200ms sliding windows with synthetic aperture magnetometry, a beamformer method for voxelwise source power analysis, in AFNI.
Midbrain FDOPA-Ki values were then used as regressors of BOLD response to dynamic facial expression and of valence-specific transient gamma band activity (GBA) as follows: we first mapped correlations between the “sustained” BOLD response and midbrain FDOPA-Ki (p<0.001), and then used implicated brain regions to guide the search for more time-resolved correlations between FDOPA-Ki and MEG oscillatory power during fearful and happy relative to neutral dynamic expressions (p<0.001). We focused on the low-[30–50Hz] and high-[65–140Hz] GBA because of their role in emotional processing6 and in routing attention and cortical information flow,7 and because dopamine-mediated midbrain-cortex gamma-synchronization has been demonstrated in rats.8
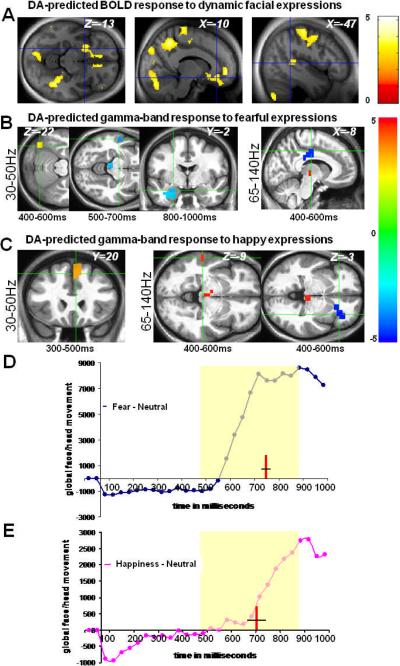
FDOPA-BOLD correlations were observed in occipital, superior temporal sulcus (STS), supplementary motor areas (SMA), and amygdala, hippocampus, striatum, insula, medial-frontal/orbitofrontal and cingulate cortices (Figure 1A). Searching in similar brain areas, we found FDOPA-GBA correlation patterns that were regionally, temporally, and directionally valence-specific (Figure 1B–C; Supplementary Information): during observation of fearful expressions, midbrain FDOPA-Ki predicted 30–50Hz GBA at 400–600 ms in the occipital face area and at 500–700ms in STS, consistent with previous data regarding human face processing9,10, and 65–140Hz GBA in posterior cingulate at 400–600ms, followed at 500–700ms and 800–1000ms by correlations between FDOPA-Ki and 30–50Hz GBA in the amygdala/hippocampus; during observation of happy expressions, FDOPA-Ki predicted 30–50Hz GBA at 300–500ms in the SMA/anterior cingulate, followed by correlations between FDOPA-Ki and 65–140Hz GBA at 400–600ms in STS and anterior insula. The latter corresponds to the same anatomical insula region found to be responsive to social signaling in a major meta-analysis of functional neuroimaging studies.11
Figure 1.
Neural correlates of emotional salience as predicted by midbrain presynaptic dopamine synthesis and tone. A, correlations in sensorimotor and frontolimbic regions between midbrain FDOPA Ki and BOLD response to videos of dynamic facial expressions independent of valence (p<.001) B, for fearful expressions: correlation between FDOPA Ki and low-[30–50Hz] and high-[65–140Hz] GBA and the corresponding time windows within which these responses survived p<0.001 statistical thresholds. C, for happy expressions: correlation between FDOPA Ki and low and high GBA. X,Y,Z values represents MNI coordinates; t-values for the resulting maps in A, B & C are shown on the color bars. Blue clusters represent regions showing negative correlations between GBA and FDOPA measures, whereas red clusters represents positive correlations between GBA and FDOPA measures. D, E timecourses of behaviorally validated facial expressions of fear and happiness15, relative to neutral assessed with the PerceptualDiff software; red lines and black crossing lines within the yellow shaded areas in D & E indicate the points in time (mean ±SEM, in milliseconds) of subjective recognition of fear (757.42ms ±14.27) and happiness (709.93ms ±45.14), respectively (see supporting online information).
The relations between midbrain dopamine tone and transient GBA response showed valence-specificity. Correlations between FDOPA and fear-evoked GBA were predominantly negative (at 500–700ms in STS and midbrain, 800–1000ms in the amygdala, and 400–600ms in the cingulate), in line with earlier findings showing inhibitory dopamine influence on neural response to aversive experiences,2 in marked contrast to the predominantly positive correlations with happiness-evoked GBA (in SMA/cingulate at 300–500ms, and in STS and midbrain at 400–600ms), in line with an excitatory DA influence on neural response to rewarding experiences.2 Since FDOPA measures dopamine synthesis and tone (rather than release) and this measure spans a very different time scale than the MEG data, our findings likely reflect a modulatory rather than causative role for the dopaminergic system as it affects neural coding of emotion processing.
These findings, together, suggest dopaminergic modulation of transient sensorimotor and mesocorticolimbic representations of facial emotional salience. Consistent with this proposal, the observed correlations between midbrain FDOPA-Ki and GBA evoked by fearful and happy expressions predominantly occurred during the emergence and peaking of this salience, as demonstrated both by measures of facial movements, which developed between 500–900ms, and by the average time at which facial emotion was recognized (757ms for fear, and 710ms for happiness; Figure 1D–E).
Here, we demonstrate that midbrain dopamine tone predicts sustained BOLD and transient GBA responses to environmentally valid, dynamic emotional cues in regions known to code perceptual, mnemonic and experiential aspects of emotional signals,12 thereby providing novel evidence that midbrain dopaminergic tone relates not only to the anatomically-distributed neural response to emotional dynamics, but also to the finely-tuned, transient nature with which this neural response evolves over time. The temporal coincidence of the emergence of emotional salience and the time windows within which midbrain dopamine tone predicted transient distributed neural response to these cues supports a dopaminergic modulation of spatiotemporal gamma-band coding of emotional salience.13 Understanding how this system is perturbed in neuropsychiatric disorders with prevalent impairments in emotion cognition4 may offer therapeutic insight.
Supplementary Material
Acknowledgements
We thank our colleagues at the NMR centre, MEG core facility and PET department for technical support. We also thank Drs. Christian van der Gaag and Christian Keysers for access to the video stimuli, and Drs. Caroline F. Zink and Alex Martin for helpful comments. This work is supported by the intramural research program of the national institute of mental health.
Footnotes
Supplementary information is available at Molecular Psychiatry's website
Author Contributions: Conceived and designed the experiments and wrote the manuscript, M.J. Performed the experiments and analyzed the data, T.N., M.J., P.K., A.I., D.R., J.S.K., T.H., F.C., J.M., and S.R. Contributed analytical tools and performed video timecourse analysis, P.K. R.C., and K.F.B., supervised all aspects of this project. All authors contributed to the manuscript.
Authors have no conflict of interest to declare
REFERENCES
- 1.Grace AA. Neuroscience. 1991;41:1. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 2.Schultz W. Annu Rev Neurosci. 2007;30:259. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 3.Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Clin Neuropharmacol. 2005;28:228. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]
- 4.Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Neuron. 2004;42:509. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- 5.Vingerhoets FJ, Snow BJ, Schulzer M, Morrison S, Ruth TJ, Holden JE, et al. J Nucl Med. 1994;35:18. [PubMed] [Google Scholar]
- 6.Luo Q, Mitchell D, Cheng X, Mondillo K, Mccaffrey D, Holroyd T, Carver F, et al. Cereb Cortex. 2009;19:1896. doi: 10.1093/cercor/bhn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries P. Annu Rev Neurosci. 2009;32:209. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 8.Lee KM, Ahn TB, Jeon BS, Kim DG. Neurosci Res. 2004;49:179–84. doi: 10.1016/j.neures.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Tsao DY, Moeller S, Freiwald WA. Proc Natl Acad Sci U S A. 2008;105:19514–9. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haxby JV, Hoffman EA, Gobbini MI. Trends Cogn Sci. 2000;4:223. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 11.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. Brain Struct Funct. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolan RJ. Science. 2002;298:1191. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- 13.Uhlhaas PJ, Singer W. Nat Rev Neurosci. 2010;11:100. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.