Abstract
Numerous studies utilizing drug self-administration have shown the importance of conditioned cues in maintaining and reinstating addictive behaviors. However, most used simple cues that fail to replicate the complexity of cues present in human craving and addiction. We have recently shown that music can induce behavioral and neurochemical changes in rats following classical conditioning with psychostimulants. However, such effects have yet to be characterized utilizing operant self-administration procedures, particularly with regard to craving and relapse. The goal of the present study was to validate the effectiveness of music as a contextual conditioned stimulus using cocaine in an operant reinstatement model of relapse. Rats were trained to lever press for cocaine with a musical cue, and were subsequently tested during reinstatement sessions to determine how musical conditioning affected drug seeking behavior. Additionally, in vivo microdialysis was used to determine basolateral amygdala involvement during reinstatement. Lastly, tests were conducted to determine whether the putative anti-addictive agent 18-methoxycoronaridine (18-MC) could attenuate cue-induced drug seeking behavior. Our results show that music-conditioned animals exhibited increased drug seeking behaviors when compared to controls during reinstatement test sessions. Furthermore, music-conditioned subjects exhibited increased extracellular dopamine in the basolateral amygdala during reinstatement sessions. Perhaps most importantly, 18-MC blocked musical cue-induced reinstatement. Thus, music can be a powerful contextual conditioned cue in rats, capable of inducing changes in both brain neurochemistry and drug seeking behavior during abstinence. The fact that 18-MC blocked cue-induced reinstatement suggests that α3β4 nicotinic receptors may be involved in the mechanism of craving, and that 18-MC may help prevent relapse to drug addiction in humans.
Keywords: Conditioning, Music, Cues, Cocaine, Reward, 18-MC
1. Introduction
Relapse to drug usage following abstinence is a significant obstacle in the treatment of drug abuse and addiction (Koob, 2000; See, 2002). A distinctive characteristic of craving and drug seeking is that it can be induced and maintained by conditioned stimuli (CS), even following extended periods of abstinence. Exposure to CS previously paired with drug reward has revealed that these cues are able to elicit craving and cause drug seeking behavior in both human and animal models of relapse (Childress et al., 1999; Di Ciano and Everitt, 2003; Fuchs et al., 2008; O’Brien et al., 1998). These drug-paired cues acquire increased salience through repeated association with the rewarding effects of a drug, producing conditioned reinforcement that is not easily diminished (Lee et al., 2006; Weiss et al., 2001). Therefore, it is clearly important to elucidate the behavioral and biochemical mechanisms through which drug-associated stimuli exert their effects.
The animal reinstatement model of relapse has become a popular assay to investigate the impact that cues have on drug seeking behavior (Shaham et al., 2003). It is a powerful model to study drug craving, and the results of these studies conclusively show that discriminative, discrete, and contextual cues all have the ability to reinstate drug-seeking behavior (Atkins et al., 2008; Bossert et al., 2005; Crombag et al., 2008; Crombag and Shaham, 2002; Fuchs et al., 2007; Gabriele and See, 2010). However, the vast majority of these studies have used either simple discriminative or discrete cues (e.g., tone or light), or contextual cues (e.g., color, floor texture, bedding) that fail to replicate the complexity of environmental triggers that are likely to be present during human drug experiences.
Recent investigations show that music can serve as an effective contextual CS in rats. For instance, music has been shown to enhance MDMA-conditioned reward in rats (Feduccia and Duvauchelle, 2008); this study revealed increases in both locomotor activity and extracellular dopamine in the nucleus accumbens (NAc) after music was paired with MDMA during operant self-administration. Another recent investigation established that rats have the ability to differentiate music composed by Bach versus Stravinsky, and even transfer this ability to novel musical selections by the same composers (Otsuka et al., 2009). Furthermore, recent clinical studies have indicated that music can be used as an effective treatment for a variety of disorders. Music therapy has shown promise as an efficacious treatment for sleep disorders, anxiety, chronic stress, pain, psychosis, autism, depression, post-traumatic stress disorder, respiratory disease, and importantly, as an adjunct therapy for addiction (Bauldoff, 2009; Bradt and Dileo, 2009; de Niet et al., 2009; Gold et al., 2009; Jung and Newton, 2009; Nilsson, 2008; Rossignol, 2009). Considering the enormous potential that music therapy offers, there is an increasing need to develop preclinical models that utilize music as a significant variable. With this aim in mind, our laboratory has recently shown that after repeated pairings between music and methamphetamine, music alone can produce significant increases in locomotor activity and extracellular dopamine release in both the basolateral amygdala (BLA) and NAc in rats (Polston et al., 2011). In a subsequent study we showed that rats demonstrate preferences between musical selections by Miles Davis and Beethoven, and that these preferences can be altered after cocaine-paired conditioning (Polston and Glick, 2011). Taken together, these reports indicate that music can serve as an effective contextual CS in rats. However, at this time, music has yet to be used as a contextual CS in an animal reinstatement model of relapse.
One neural site shown to be crucial for cue-induced drug seeking is the BLA. The BLA is a key limbic-related region within the brain that projects heavily to the NAc, another region consistently implicated in addiction. Inactivation of the BLA through lesion or drug blockade results in attenuation of cue-induced drug seeking behaviors (Feltenstein and See, 2007; Fuchs and See, 2002). Additionally, significant increases in dopaminergic neurotransmission have been detected in the BLA after cue-induced classical conditioning procedures (Hori et al., 1993; Polston et al., 2011). Cocaine-associated cues have also been shown to elicit drug seeking behavior and activate the BLA after four months of abstinence, illustrating the efficacy of drug-conditioned cues even after extended periods of drug deprivation (Ciccocioppo et al., 2001). Adaptations of the cortico-limbicstriatal circuitry that take place during subjective human drug experiences may influence associative learning mediated by the BLA, the brain area thought to be ultimately responsible for cue-induced reinstatement of drug-seeking behavior (McLaughlin and Floresco, 2007).
18-Methoxycoronaridine (18MC) is an α3β4 nicotinic antagonist that has been proposed as a treatment for addiction to a number of substances. It has been shown to reduce nicotine, cocaine, morphine, methamphetamine, and ethanol self-administration (Glick et al., 1996; Glick et al., 2000a; Maisonneuve and Glick, 1999; Rezvani et al., 1997) in rats. It has also been shown to block acquisition of a cocaine conditioned place preference (McCallum and Glick, 2009). 18-MC’s primary mechanism of action appears to be through selective blockade of α3β4 nicotinic receptors (Glick et al., 2002; Pace et al., 2004). The mechanism of action of nearly every abused drug appears to involve the dopaminergic mesolimbic system; although 18-MC affects the mesolimbic dopamine (DA) system, it does so in an indirect way via other pathways (Maisonneuve and Glick, 2003). In the brain, α3β4 nicotinic receptors are preferentially localized in the medial habenula and interpeduncular nucleus, while lower densities of these receptors reside in the ventral tegmental area (Klink et al., 2001; Quick et al.,1999) and other brain regions such as the dorsolateral tegmentum and basolateral amygdala (Perry et al., 2002; Zhu et al., 2005). Our working hypothesis is that 18-MC decreases drug self-administration by indirectly modulating the dopaminergic mesolimbic pathway via blockade of α3β4 nicotinic receptors in the habenulo-interpeduncular pathway and the basolateral amygdala.
The present study had three objectives: (1) validate the effectiveness of music as a contextual conditioned stimulus in an operant reinstatement model of relapse; (2) determine, using in vivo microdialysis, if dopaminergic changes occurred during music-induced reinstatement of drug seeking; and (3) assess the efficacy of 18-MC to abate cue-induced drug seeking behaviors. All studies were conducted using a model of self-administration, extinction and reinstatement in which rats made lever presses for cocaine in the presence or absence of a musical cue (Table 1). The results of the present study provide novel insight into the mechanisms underlying contextual cues and associated drug-seeking behavior, and also demonstrate the effectiveness of 18-MC as a potential treatment for relapse, even in the presence of complex contextual cues.
Table 1.
Musical conditioning assignments during cocaine training, self-administration, extinction, and reinstatement test sessions.
| Experiment | Group | Training | Self-Administration | Extinction | Reinstatement |
|---|---|---|---|---|---|
| One | Music | Music | Music | No Music | Music |
| NMCond | No Music | No Music | No Music | Music | |
| NMTest | Music | Music | No Music | No Music | |
| Two | Microdialysis | Music | Music | No Music | Music |
| Three | 18MC | Music | Music | No Music | Music |
| Saline | Music | Music | No Music | Music |
2. Materials and Methods
2.1 Animals
Naïve female Sprague-Dawley rats (Taconic Germantown, NY), weighing approximately 250g at the start of the experiments, were housed individually in a temperature and humidity controlled colony room under a standard 12:12 light/dark cycle. Food and water were provided ad libitum. Protocols were designed and implemented in accordance with the “Guide for the Care and Use of Laboratory Animals” (1996) and were approved by the Institutional Animal Care and Use Committee of Albany Medical College. Rats were given one week of acclimation prior to experimental procedures.
2.2 Drugs
Cocaine hydrochloride (~0.4 mg/kg/infusion, Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% sodium chloride with a 2 mg/ml drug to saline ratio, and then brought to a neutral physiological pH before use in intravenous (i.v.) self-administration sessions. 18-MC (40 mg/kg, Obiter Research LLC. Champaign, IL) and saline were both administered intraperitoneally (i.p.). Animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) for both intrajugular and microdialysis cannulation surgeries. Sodium methohexital (10 mg/kg) was used to verify catheter patency. All other reagents used in conjunction with microdialysis experiments were obtained from local suppliers and were of analytical grade.
2.3 Music
Miles Davis’ “Four” (Prestige Blue Haze, 1954) was the musical track used as a contextual cue in these experiments. The Miles Davis selection was chosen because it had been used successfully in past conditioning paradigms in our laboratory (Polston et al., 2011). This musical selection was originally chosen because it has a repetitive beat and melody, helping to make it easily recognizable and identifiable. During drug training, self-administration, and applicable test sessions, “Four” was played on a continuous loop, at a volume staying between 65 and 75 decibels. This decibel range was chosen because it had been used successfully in past investigations involving rats and music (Feduccia and Duvauchelle, 2008; Otsuka et al., 2009; Polston et al., 2011).
2.4 Apparatus
Experiments were conducted in rat operant conditioning chambers (ENV-009, Med Associates, St. Albans, VT) located within sound attenuated boxes outfitted with acoustical foam. The operant boxes were continuously ventilated with a house fan, and equipped with two retractable levers spaced approximately 20 cm apart on the front wall, with a house light mounted on the back wall of the test chamber. Infusion pumps (PHM-100VS, Med Associates, St. Albans, VT) located beneath the operant test chamber were used in combination with polyethylene tubing and Instech (375/22PS) swivels for i.v. drug delivery. Stereo speakers (Orb Audio, New York, NY) were mounted from the ceiling and suspended above the middle of the operant boxes. These speakers were interfaced with a stereo receiver (Sony Inc., Tokyo, Japan) that controlled the musical acoustics in the operant test chambers. Additionally, infrared digital video cameras (Clover Inc., Cerritos, CA) were mounted from the ceiling of the operant boxes, allowing an unobstructed view of the test chamber floor. These cameras were used in conjunction with Any-Maze™ video tracking software (Stoelting Inc., Wood Dale, IL) to analyze locomotor activity and the time spent in predefined spatial areas within the apparatus. By operationally defining the floor (30.5cm × 31.8cm) of the test chamber, and dividing it into three spatial zones, the program automatically generated detailed readings of the time spent in each zone in seconds and the distance that the animal traveled in meters. We defined the “active zone” (15.25cm × 15.9cm) of the apparatus as the area containing the active drug-paired lever and the surrounding spatial area. The “inactive zone” (15.25cm × 15.9cm) contained the inactive lever and surrounding spatial area, and the “back zone” (15.25cm × 31.8cm) consisted of the back half of the test chamber. By operationally dividing the test chamber in this way, our system provided an automated way to determine spatial preferences within the apparatus. Videos were also periodically recorded and analyzed to ensure that Anymaze was functioning correctly.
2.5 Self-Administration Procedure
During initial shaping of the lever press response, a modular pellet dispenser (ENV-203M, Med Associates, St. Albans, VT) and receptacle were added to the operant test chamber, allowing delivery of a 45 mg sucrose chocolate flavored pellet (Bio-Serv, Frenchtown, NJ). Food-deprived rats were trained to lever press for sucrose pellets during an overnight 16h session under a fixed-ratio 1 (FR1) schedule of reinforcement. Both retractable levers were present during training, but only one (active lever) was associated with reward delivery. Responses on the other lever (inactive lever) were recorded but did not have any programmed consequences. Active lever responses resulted in immediate delivery of a food pellet, followed by retraction of both levers for a 20s timeout period. Following the timeout, the house light would flash for 0.5s, and the levers would re-emerge from the front wall of the apparatus. Rats were considered “trained” if they successfully completed 200 active lever presses during the 16hr session.
2.5.1 Experiment 1
Once the rats had successfully learned to lever press for food, they were randomly assigned to one of three treatment groups: Music, NMCond, or NMTest (refer to table 1 for a detailed account of all musical treatments). Rats were subsequently anesthetized with sodium pentobarbital (50mg/kg) and catheters were implanted in the external jugular vein according to procedures described by Weeks (1972). Rats were given a minimum of three days recovery time before drug self-administration sessions commenced. Self-administration testing began with a 16h nocturnal session. Each rat’s catheter was flushed with 0.05 ml of saline and immediately placed in the operant box, where the animal was tethered to the drug infusion tubing. If applicable (Table 1), the music was then started along with the behavioral tracking system, and the levers in the operant box were deployed, initiating the beginning of the cocaine self-administration session. An active lever-response (FR1) produced a 50 μl infusion of cocaine over the course of one second, followed by retraction of both levers for a 20s timeout period. Following the timeout, the house light would flash for 0.5s, and the levers would re-emerge from the front wall of the apparatus. Since all rats generally weighed 250±20g, each response delivered approximately 0.4 mg/kg of cocaine during the infusion. Responses on the inactive lever resulted in no programmed consequences but were recorded. Assignment of the active lever within the operant chamber was counterbalanced among subjects. At the end of the session, rats were removed from the operant box, their catheters were flushed with heparinized saline, and they were returned to the colony room. Animals had to make a minimum of 100 active cocaine responses during the overnight training session in order to move into daily self-administration sessions.
Daily self-administration sessions followed the same protocol outlined above for the 16h nocturnal sessions; that is, rats were transported to the operant boxes and allowed to self-administer cocaine, either in the presence or absence of the contextual music cue. The Music treatment group was exposed to the musical cue during cocaine conditioning sessions, and reintroduced to the music during the reinstatement test. The NMCond control group was not exposed to the music during conditioning, but did receive the musical cue during the reinstatement test. The NMTest control group received music during daily cocaine sessions, but did not receive music during the reinstatement test. The duration of each of the 15 daily sessions was 90 min. A FR1 schedule of reinforcement was used on days 1–12, at which time rats were subsequently moved to a FR3 schedule of reinforcement for the final three cocaine-self administration sessions and all subsequent extinction and reinstatement sessions. Following the final self-administration session, catheter patency was checked by infusing a small dose (10 mg/kg) of sodium methohexital, which would immediately render the rat ataxic if the cannula was functioning properly. Only rats whose catheters were patent on day 15 were allowed to continue to the extinction and reinstatement parts of the experiment.
Following self-administration training, rats began daily 90 min extinction sessions for five consecutive days (days 16–20). During these sessions, no music was present for any of the three treatment groups, and responses on either the previously drug paired lever or the inactive lever resulted in no drug infusions. Additionally, animals underwent 24 days of abstinence, with housing in the colony room, prior to reinstatement testing. Following this period of extinction and abstinence, both treatment (Music) and control animals (NMCond, NMTest) were tested (day 45) to determine what effect the music-drug conditioning would have on drug seeking behaviors. During extinction and reinstatement test sessions, the house lights, levers, and 20s timeout period remained consistent with the conditions during daily self-administration sessions. This model of self-administration, extinction, abstinence, and reinstatement testing followed a previously established rat protocol of reinstatement (Kelamangalath and Wagner, 2009).
2.5.2 Experiment 2
Animals in this experiment underwent the exact same treatment conditions as the animals in the Music group in Experiment 1; that is, their cocaine training, extinction/abstinence, and reinstatement music conditions were identical (Table 1). However, at the time of the intrajugular catheterization surgery, these animals underwent an additional stereotaxic surgery for implantation of microdialysis guide cannulae. This surgery was conducted in accordance with a previously established protocol (Maisonneuve and Glick, 1999). Each rat had two microdialysis guide cannulae (CMA/Microdialysis AB, Stockholm, Sweden) implanted into the basolateral amygdala (BLA). Coordinates were determined according to Paxinos and Watson (Paxinos and Watson, 1986) such that, when inserted, the dialysis probe was located in the BLA (in mm, AP = −2.2; ML = ±4.6; DV = −5.0, 0° lateral angle insertion). On the afternoon prior to assessment (day 44), microdialysis probes were calibrated for DA, DOPAC, and HVA to ensure recovery higher than 15% (Glick et al., 1994). Probes were discarded if they did not meet the 15% criteria. The subjects were transiently anesthetized with 25 mg/kg of Pentothal (Hospira, INC., Lake Forest, IL), and then placed into our operant chambers, where microdialysis probes were inserted and connected via a custom harness and tubing to both the self-administration tether and microdialysis tubing. The subjects were monitored until the effects of anesthesia had subsided, and were provided with ad libitum food and water throughout the night.
On the day of microdialysis reinstatement testing (day 45), samples were collected in tubes containing 2 μl of 1.1 N perchloric acid solution (containing 50 mg/l Na2EDTA and 50 mg/l sodium metabisulfite). The probe was continuously perfused at a flow rate of 1 μl/min with artificial cerebrospinal fluid (146 mM NaCl, 2.7 mM KCL, 1.2 mM CaCl2, 1.0 mM MgCl2). A test sample was collected for 20 min from each probe for each experimental subject. Six, 20 min baseline samples were obtained during the first 2h of sample collection. Immediately following baseline sample collection, the reinstatement test session commenced, and the conditioned music cue was presented; four 20 min samples were collected during behavioral testing. The cue was removed (music turned off) at the end of the 90 min session, and an additional five 20 min samples were collected. The dialysate samples were transferred from collection to analysis vials for DA, DOPAC, and HVA analysis by high performance liquid chromatography with electrochemical detection (HPLC-EC). Immediately following the microdialysis reinstatement experiment, subjects were sacrificed; their brains were removed and preserved for histological confirmation of guide cannulae placements. The BLA was chosen for study because it had been previously shown to respond to musical cues after drug conditioning, and had previously never shown a response after music/saline conditioning (Polston et al., 2011).
2.5.3 Experiment 3
Animals in this experiment underwent the exact same treatment conditions as the animals in the music group in Experiment 1; that is, their cocaine training, extinction/abstinence, and reinstatement music conditions were identical (Table 1). However, these animals received i.p. injections of either 18-MC (40 mg/kg) or saline 20 min prior to the reinstatement test session.
2.6 Histology
Brains were frozen at −80°C until histology was performed utilizing a cryostat (Microm HM500M, Walldorf, Germany). Probe placements were mapped directly from the cryostat sections, and data were excluded from analysis if the probe was not located within region-specific boundaries for the BLA (refer to 3b).
2.7 HPLC
The dialysate samples were analyzed utilizing a high performance liquid chromatography system with electrochemical detection (HPLC-EC). The system consisted of an ESA 540 autosampler (ESA, North Chelmsford, MA), an ESA solvent delivery unit, an ESA column (MD-150/RP-C18; diameter =3.0 μm), and an ESA Coulochem II electrochemical detector with an ESA 5020 guard cell and an ESA 5014B analytical cell. The potential of the glass carbon working electrode was set at 300 mV with respect to the reference electrode. The MD-TM mobile phase (ESA, North Chelmsford, MA), composed of 75 mM sodium dihydrogen phosphate monohydrate, 1.7 mM 1-octanesulfonic acid sodium salt, 100 μl/l triethylamine, 25 μM EDTA in 10% acetonitrile (pH=3.0), was pumped at a flow rate of 0.530 ml/min. The electrochemical data were processed with Agilent Technologies Chem Station Plus software (Agilent Technologies, Wilmington, DE). The software produced chromatographs, visual depictions of DA, DOPAC, and HVA concentrations (in pmol) plotted on the y-axis against the temporal representation (in min) for ion affinity plotted along the x-axis.
2.8 Data Analysis
All data are presented as mean ± SEM. For Experiment 1, active and inactive lever presses were analyzed using a factorial Analysis of Variance (ANOVA) with Condition (Music, NMCond, or NMTest) and Trial as the independent variables. Locomotor activity data and the amount of time spent in the active, inactive, and back zones were analyzed at three time points (final day of self-administration, final day of extinction, and reinstatement test day) using one-way ANOVAs with Condition as the independent variable. All significant results were further examined by Newman-Keuls post-hoc tests.
For analysis of the microdialysis data in Experiment 2, basal levels of DA and its metabolites were expressed as pm/10 μl and were analyzed using a repeated measures ANOVA with Time as the repeated measures variable. As no significant differences were observed in the basal levels, DA and its metabolites were expressed as a percentage of the corresponding baseline means, and the percent baseline values were then used in subsequent analyses. A repeated measures ANOVA was used to evaluate differences between basal and treatment samples with Time (20 min samples, 15 total) as the repeated measure. Significant results were further examined by Newman-Keuls post-hoc testing. To determine if animals receiving microdialysis differed in behavior prior to the reinstatement test, a factorial ANOVA was conducted on active and inactive lever presses comparing the microdialysis animals with all other groups that received the same conditioning during training, self-administration, and extinction.
In Experiment 3, active and inactive lever presses were analyzed using a factorial ANOVA with Treatment (18-MC or NaCl) and Trial as the independent variables. Locomotor activity data and the amount of time spent in the active, inactive and back zones were analyzed at three time points (final day of self-administration, final day of extinction, and reinstatement test day) using a one-way ANOVA with Treatment as the independent variable. All significant results were further examined by Newman-Keuls post-hoc tests.
3. Results
3.1 Experiment 1 – Music-induced reinstatement
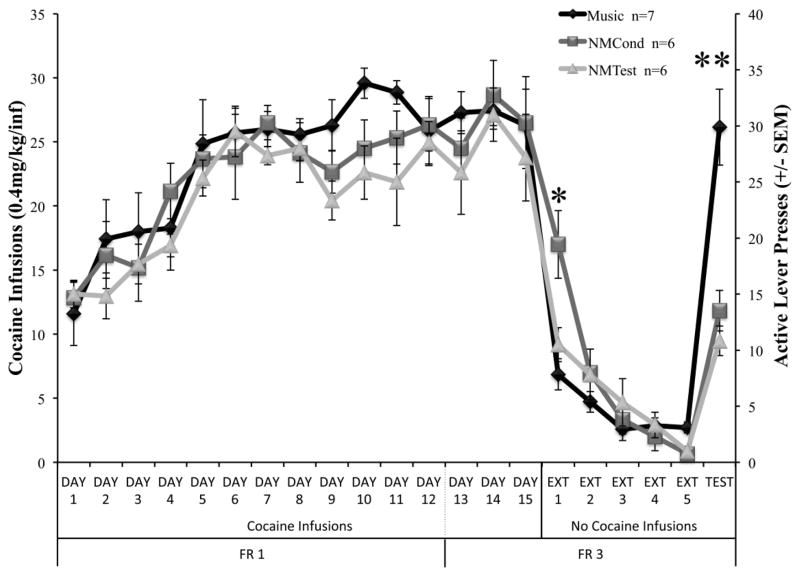
Figure 1 depicts the average responses made for cocaine reinforcement during self-administration trials, extinction sessions, and the reinstatement test day. The factorial ANOVA revealed a significant effect of Trial (F(20,336)=55.052, p<0.001) and a significant Condition × Trial interaction (F(40,336)=1.643, p<0.01) but not an effect of Condition alone (F(2,336)=1.369, p=0.256). Post-hoc analysis revealed that a significant difference was observed on the first day of extinction (Ext 1), where animals that had not been conditioned with music during self-administration (NMCond) made significantly more responses than animals that had been trained with music (p<0.05). Furthermore, animals in the Music condition made significantly more responses on the active lever on the reinstatement test day (Test) compared to animals in the NMCond (p<0.001) and NMTest (p<0.001) groups. There were, however, no significant differences between NMCond and NMTest groups (p=0.98) during the reinstatement test.
Figure 1.
Effects of music conditioning on active lever responding during daily cocaine self-administration sessions, extinction, and the reinstatement test session. Data depicted as mean cocaine infusions (±SEM) during self-administration trials, and theoretical infusions earned during extinction sessions. Active lever presses (±SEM) during the reinstatement test session are plotted accordingly with the right x-axis. Animals were on an FR1 schedule of reinforcement on days 1–12, and changed to an FR3 schedule for the remainder of the experiment. Animals in the Music condition made significantly more responses on the drug-paired lever on the reinstatement test day (Test) than animals in the NMCond and NMTest groups. Additionally, significant differences were observed on the first day of extinction (Ext 1), where animals that had not been conditioned with music during self-administration (NMCond) made significantly more responses than animals that had been trained with music. * p < 0.05, *** p < 0.001
Locomotor activity data are shown in Figure 2a. The ANOVA showed that there was no effect of Condition at any of the days tested (final day of self-administration: F(2,16)=0.027, p=0.974; final day of extinction: F(2,16)=0.786, p=0.472; reinstatement test day: F(2,16)=0.800, p=0.466). The amount of time spent in the active zone (the area corresponding to the active lever), in the inactive zone (corresponding to the inactive lever), and in the back zone (corresponding to the remainder of the chamber) is shown in Figure 2b. ANOVA revealed a significant effect on the reinstatement test day for the active (F(2,16)=12.039, p<0.001) but not inactive (F(2,16)=0.258, p=0.775) or back zones (F(2,16)=1.270, p=0.308). Post-hoc analysis revealed that rats conditioned with music spent an increased amount of time in the active zone on the reinstatement day compared to NMCond (p<0.01) and NMTest (p<0.001) groups. The one-way ANOVA did not reveal any significant effects of Condition for the final day of self-administration (Active: F(2,16)=0.218, p=0.807; Inactive: F(2,16)=0.049, p=0.952; Back: F(2,16)=0.133, p=0.876) or final day of extinction (Active: F(2,16)=0.916, p=0.420; Inactive: F(2,16)=0.166, p=0.849; Back: F(2,16)=0.120, p=0.888).
Figure 2.
Effects of music conditioning on locomotor activity and spatial preferences within the apparatus. a) Locomotor activity depicted as distance traveled in meters (±SEM) during the final day of self-administration, the final day of extinction, and the reinstatement test session. No significant differences were observed in locomotor activity at any of these time points. b) Time spent in the active zone in seconds (±SEM) during the final day of self-administration, the final day of extinction, and the reinstatement test session. Animals in the Music condition spent significantly more time in the spatial area surrounding the active drug-paired lever (active zone) during the reinstatement test session than animals in the NMCond and NMTest groups. *** p < 0.001
3.2 Experiment 2 – Music-induced dopamine release in the BLA
Table 2 shows the average concentration of basal dopamine, DOPAC and HVA levels. There were no significant differences in the basal levels of dopamine (F(5,20)=0.6371, p=0.674) or its metabolites (DOPAC: F(5,20)=2.123, p=0.105; DOPAC: F(5,20)=1.637, p=0.196). Therefore, data in Figure 3a depicts the dopaminergic responses during the microdialysis trials as a percent of baseline. As can be seen from the graph, there was a significant efflux of dopamine (F(14,56)=5.204, p<0.001) following onset of the music cue (120 min) compared to baseline (140 min: p<0.01; 160 min: p<0.01). No significant changes were observed in the levels of DOPAC (F(14,56)=1.734, p=0.105) or HVA (F(14,56)=1.259, p=0.262). The behavioral comparison between microdialysis animals and the other groups that received the same musical conditioning during training, self-administration and extinction sessions showed no differences in active or inactive lever presses between the groups (F(6,878)=0.810, p=0.563) and no group × trial interaction (F(38,878)=0.615, p=0.999). Mean (± SEM) active lever presses during the reinstatement test session were 14.6 (±1.86) and inactive lever presses during the reinstatement test session were 2.40 (±1.03).
Table 2.
Average basal levels of extracellular DA, DOPAC, and HVA in rats during the reinstatement test session. Mean + SEM expressed as pm/10 μl.
| Region | Neurotransmitter | Treatment | N= | Mean ± SEM |
|---|---|---|---|---|
| BLA | Dopamine | Music | 5 | 0.023 ± 0.00112 |
| DOPAC | Music | 5 | 4.962 ± 0.12372 | |
| HVA | Music | 5 | 4.773 ± 0.08988 |
Figure 3.
Effects of the conditioned musical cue on dopamine release within the basolateral amygdala. a) Time course of extracellular dopamine (mean ±SEM) during microdialysis testing on the reinstatement test day as a percentage of baseline. Musical cue presentation was initiated at the 120min time point and terminated at the 210min time point (indicated by arrows). There was a significant efflux of dopamine following onset of the musical cue at both the 140 and 160min time points compared to baseline samples. Graph in the upper right corner depicts the active lever responses by this microdialysis group during the reinstatement test session b) Representative probe placements for the basolateral amygdala. Shown at AP: −2.2 in Paxinos and Watson (1986). ** p < 0.01
3.3 Experiment 3 – 18-MC effect on cue-induced reinstatement
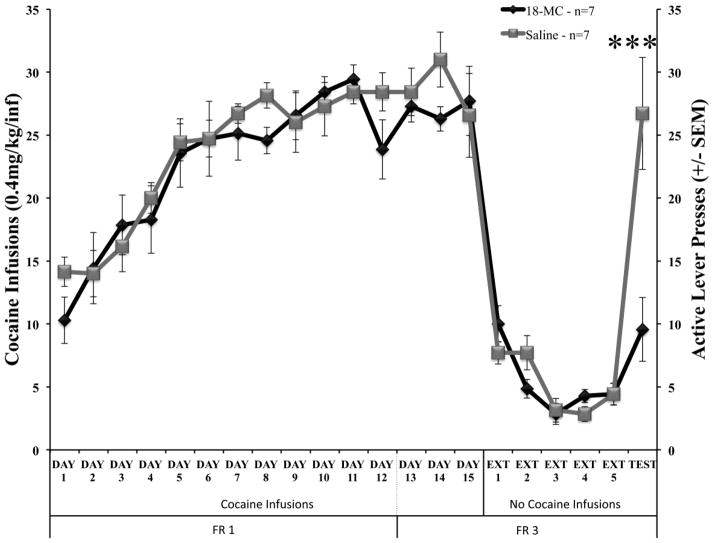
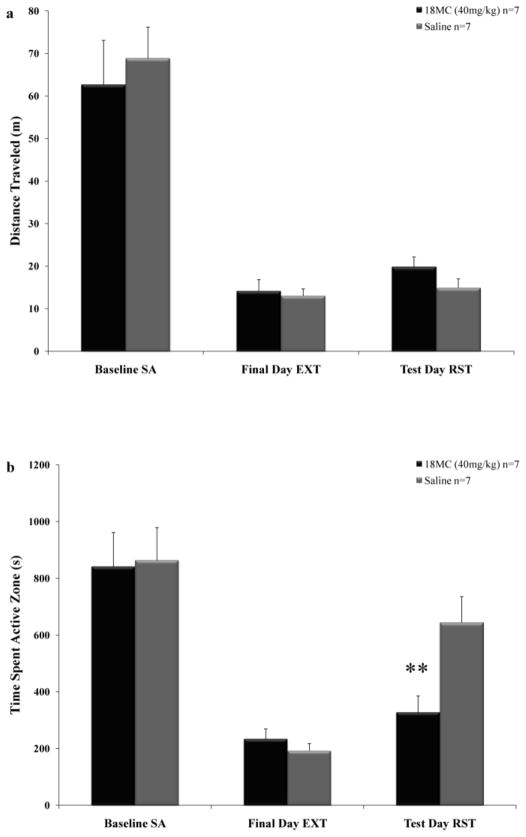
Figure 4 depicts the average responses made for cocaine reinforcement during self-administration trials, extinction sessions, and the reinstatement test day. The factorial ANOVA revealed a significant effect of Treatment (F(2,251)=3.606, p<0.05) and Trial (F(40,502)=25.172, p<0.001) and a significant Treatment × Trial interaction (F(40,502)=1.726, p<0.01). As can be seen from the graph, post-hoc testing showed there were no significant differences between 18-MC and saline treated groups during self-administration or extinction sessions. However, animals treated with 18-MC made significantly fewer active lever responses on the reinstatement test day (p<0.001). Figure 5a depicts the locomotor activity of the Treatment groups, with no significant differences between groups on the final day of self-administration (F(1,12)=0.235, p=0.637), the final day of extinction (F(1,12)=0.141, p=0.714) or the reinstatement test day (F(1 12)=2.454, p=0.143). Figure 5b shows the average amount of time spent in the active zone during the final day of self-administration, the final day of extinction, and the reinstatement test session. ANOVA revealed no significant difference between groups in time spent in the active zone on the final day of self-administration (F(1,12)=0.018, p=0.896) or the final day of extinction (F(1,12)=0.900, p=0.362). There was, however, a significant decrease in the amount of time spent in the active zone for rats treated with 18-MC prior to the reinstatement test session (F(1 12)=8.523, p<0.01). There was no effect of Treatment on time spent in the inactive or back zones of the operant chambers on the final day of self-administration (inactive: F(1 12)=0.021, p=0.888; back: F(1 12)=0.001, p=0.981), the final day of extinction (inactive: F(1 12)=0.007, p=0.933; back: F(1 12)=0.282, p=0.605) or the reinstatement test session (inactive: F(1 12)=0.511, p=0.488; back: F(1 12)=3.606, p=0.082).
Figure 4.
Effects of 18-MC on musical cue-induced reinstatement. Data depicted as mean cocaine infusions (±SEM) during self-administration trials, and theoretical infusions earned during extinction sessions. Active lever presses (±SEM) during the reinstatement test session are plotted accordingly with the right x-axis. Animals were on an FR1 schedule of reinforcement on days 1–12, and changed to an FR3 schedule for the remainder of the experiment. Administration of 18-MC (40mg/kg) prior to the reinstatement test session (Test) significantly attenuated active lever responses for the musical cue. *** p < 0.001
Figure 5.
Effects of 18-MC on locomotor activity and spatial preferences within the apparatus. a) Locomotor activity depicted as distance traveled in meters (±SEM) during the final day of self-administration, the final day of extinction, and the reinstatement test session. Administration of 18-MC (40mg/kg) prior to the reinstatement test session had no significant impact on locomotor activity. b) Time spent in the active zone in seconds (±SEM) during the final day of self-administration, the final day of extinction, and the reinstatement test session. Administration of 18-MC (40mg/kg) prior to the reinstatement test session significantly attenuated the amount of time that the animals spent in the spatial area surrounding the drug-paired lever (active zone) ** p < 0.01
4. Discussion
While the influence of conditioned cues has been extensively investigated with regard to goal directed behavior, the impact of complex environmental cues has not been comparably explored. Using the animal reinstatement model of relapse, we show for the first time that musical drug-paired CS have the ability to profoundly influence drug-seeking behavior following repeated pairings during cocaine self-administration. To mimic the intricate psychological processes that occur during human drug experiences, we utilized a complex contextual cue to assess associative learning processes that occur during craving. Complementing previous work (Polston and Glick, 2011; Polston et al., 2011), the present findings further support the notion that rats have the capacity to distinguish complex musical passages, and suggest that rats could be used in other preclinical models involving musical interventions.
The results of Experiment 1 demonstrate that animals conditioned with a musical cue (Music) show increased drug-seeking behaviors when compared to the NMCond and NMTest control groups. Music-conditioned rats made significantly more active lever responses during the reinstatement test session, indicative of increased drug craving in the presence of the musical cue (Fig 1). These results are consistent with other cue-induced reinstatement paradigms, in which drug-paired CS have been consistently found to increase drug-seeking behavior (Crombag and Shaham, 2002; Fuchs et al., 2008; Gabriele and See, 2010). It could be argued that the increased lever responding we observed was due to chronic cocaine alone. However, both the NMCond and NMTest groups received the same cocaine reinforcement during the acquisition and maintenance phases of the experiment, and neither was significantly different from the Music group during daily self-administration sessions. It could also be argued that the increased responding we observed was in part due to spontaneous recovery, particularly since our rats were exposed to 24 days of home cage abstinence between the final day of extinction and the reinstatement test day. Previous work has shown that rats are prone to spontaneous recovery, which is resumption of the extinguished conditioned response that occurs after time has passed following extinction (Shaham et al., 1997). The latter study showed rats would spontaneously recover operant responding for nicotine after 21 days of abstinence. However, our NMCond and NMTest control animals were exposed to the same 24 days of home cage abstinence as our music test group, and neither control group exhibited spontaneous recovery when comparing the final day of extinction to the reinstatement test day. Therefore, differences observed between the music-conditioned animals and the control animals during the reinstatement session were most likely an effect of condition, as the music acquired increased salience during acquisition and daily cocaine sessions.
When compared to the subjects that had received the musical cue during training and daily self-administration sessions, the subjects that did not receive music conditioning (NMCond) showed significantly increased active lever responding on the first day of extinction. This result is consistent with other studies showing that rats experiencing cues during self-administration extinguish more readily when those cues are removed and more readily than rats that have not had the opportunity to develop CS-drug associations (Arroyo et al., 1998; Panlilio et al., 2000). Indeed, drug-related cues produce an enduring resistance to extinction due to the associative learning that takes place during conditioned reinforcement (Weiss et al., 2001). Thus the observed differences in extinction were likely attributable to the absence of music for subjects accustomed to it during previous reinforcement sessions. It could be argued that we should have employed a CS- paired with a different musical selection in order to test the specificity of our music-induced reinstatement. However, in previous investigations we have paired both the Miles Davis song used here, and Beethoven’s “Fur Elise” with saline, and have never seen a behavioral or neurochemical effect (Polston and Glick, 2011; Polston et al., 2011). It is also possible that our results could have been influenced by the fact that we used female rats for this investigation. While there is ample literature demonstrating differential sensitivity and drug effects between male and female rats, there is also literature showing that there is little effect of estrous, particularly when looking at fixed-ratio schedules of reinforcement with cocaine (Anker and Carroll, 2011; Lynch, 2008). Although previous studies show that female rats could be more sensitive to reinstatement procedures, it is highly unlikely that this played a role in our findings. Our control groups were all comprised of female rats, and they did not display increased sensitivity, as there were no statistically significant differences for either of the control groups between the last day of extinction and the reinstatement test day. Therefore, the most parsimonious explanation of our differential extinction and reinstatement results is that the music-conditioned animals were profoundly impacted by the contextual conditioning they received, indicative of conditioned learning, and the differences observed on the first extinction day and the reinstatement test day were a function of this conditioned response.
One finding that was somewhat surprising is that we failed to find a locomotor effect in music-conditioned animals during reinstatement test sessions (Fig 2a). Other investigations using similar reinstatement procedures have found cue-induced locomotor activation during final testing (Feduccia and Duvauchelle, 2008). Moreover, it is quite common to find locomotor activation to cues that were previously associated with drug reward (Bevins et al., 2001; Rodríguez-Borrero et al., 2006). However, other studies have found no differences in locomotor activation to CS after drug-paired conditioning, and reviews show that contextual cues in particular yield mixed locomotor results (Martin-Iverson and Reimer, 1996; Tzschentke, 1998). Interestingly, closer examination of our groups’ behaviors revealed that, although they did not show differences in locomotor activity, animals conditioned with the musical cue spent significantly more time in the spatial area surrounding the active lever during the reinstatement test session (Fig 2b). This indicates that animals developed an effect analogous to a conditioned place preference (CPP) within the apparatus in the presence of the cue previously associated with cocaine reinforcement. In a typical CPP paradigm, a primary reinforcer is paired with contextual stimuli, which acquire secondary reinforcing properties. These secondary reinforcing properties, established due to classical conditioning, are capable of inducing an operant approach response or place preference. Indeed, CPP results consistently show that drug-paired environmental stimuli are capable of producing drug-seeking behavior during abstinence, indicative of drug craving (McCallum and Glick, 2009; Tzschentke, 2007). The fact that our animals essentially “camped out” by the previously active drug-paired lever is indicative of goal-directed behavior, and it certainly helps explain the lack of locomotor activation. An analogy to human behavior would be that an addict, after experiencing a drug-paired contextual CS, decided to “hang out” by the door of his drug distributor, rather than running aimlessly all over town.
During reinstatement sessions in Experiment 2, using in vivo microdialysis, we examined the dopaminergic response to the cue previously associated with cocaine self-administration. We found that the presence of the musical cue elicited a substantial increase in extracellular dopamine within the BLA (Fig 3a). Immediately following presentation of the musical cue, extracellular dopamine increases of approximately 100% were observed for the 40 mins following cue initiation. This finding is consistent with previous work in our laboratory showing that, following repeated classical conditioning sessions with methamphetamine, music alone can increase extracellular DA in the BLA (Polston et al., 2011). Moreover, the present microdialysis results are further corroborated by studies that have shown cue-induced increases in BLA DA in other conditioning paradigms (Suzuki et al., 2002; Yokoyama et al., 2005). These results are also consistent with the literature showing that inactivation of the BLA through lesion or drug blockade results in attenuation of cue-induced drug seeking behaviors (Feltenstein and See, 2007; Fuchs and See, 2002). Behaviorally, the animals undergoing microdialysis showed no significant differences when compared to other animals that received the same musical conditioning during training, daily self-administration sessions, and extinction. Although these rats did not make as many active lever presses during the reinstatement session, this is readily explained by the differences in protocol required for microdialysis sample collection as well as possibly by the custom harness designed for these experiments. While these harnesses were designed with intent to minimize any possible discomfort, the additional tubing and probes required for microdialysis procedures did slightly inhibit overall behavioral responding. Regardless, the animals made sufficient responses to exhibit reinstatement-like behavior, and this provided an important neurochemical measure regarding the impact of the musical cue. It might be argued that our microdialysis results are incomplete, or should be discounted due to the lack of a specific control group to rule out the nonspecific effect of music on DA release. While we agree that a control group that did not receive the music during cocaine conditioning would have been ideal, we have already ruled out the nonspecific effect of music on DA release in a similar previously published investigation (Polston et al., 2011). In this previous study we tested the same Miles Davis selection used in this experiment, and did not observe nonspecific effects of this musical selection in the brain area tested here (BLA), or in other addiction related brain areas, such as the NAc or the prefrontal cortex. Therefore, in light of the inherent difficulty of this experimental paradigm, and the fact that we have never seen nonspecific effects of music in the BLA in our laboratory, we thought a NMCond control group was unwarranted, and that the inherent within-subject baseline control that is utilized in microdialysis procedures would be sufficient.
While we were able to validate the effectiveness of the musical conditioned cue in Experiment 1, perhaps the most significant and important finding of this investigation is that 18-MC was able to block the cue-induced reinstatement produced by the musical CS. As can be seen in Fig 4, 18-MC significantly attenuated responses on the previously active drug-paired lever during the reinstatement test session. While 18-MC has been shown to attenuate self-administration for multiple drugs of abuse, it has not been studied as extensively in animal models of craving (Glick et al., 1996; Glick et al., 2000a; Maisonneuve and Glick, 1999; Rezvani et al., 1997). One model of craving that 18-MC has been applied to is CPP, and it was shown that 18-MC was able to block the acquisition of a cocaine CPP (McCallum and Glick, 2009). Therefore, the fact that 18-MC was able to block cue-induced reinstatement in the form of active lever pressing was a notable finding, considering the potential it has shown for treating active drug abuse. Also of interest was the finding that 18-MC produced no changes in locomotor activity when administered prior to the reinstatement test session (Fig 5a). These results are consistent with other findings in our laboratory showing that 18-MC produces no locomotor effects alone when compared to saline treated rats (Glick et al., 2000b). However, 18-MC was able to attenuate the music-induced CPP effect that we had previously seen in Experiment 1. As can be seen from Fig 5b, administration of 18-MC (40 mg/kg) prior to the reinstatement test session significantly decreased the time spent in the active zone (i.e., corresponding to the previously drug-paired lever). Thus 18-MC was able to block musical-cue induced drug seeking behaviors, both by decreasing active lever pressing and by abolishing a CPP-like effect. These effects could not be attributed to locomotor differences since 18-MC had no effect on locomotor activity. Rather, the results suggest that 18-MC’s ability to attenuate drug seeking behaviors in this paradigm is due to a specific behavioral effect where subjects showed decreased interest in reinstated lever responding and decreased interest in the spatial area associated with previous drug experiences.
Although we cannot be certain as to why 18-MC was so effective at attenuating drug-craving behaviors, we can hypothesize on some of the potential mechanisms involved in this phenomenon. 18-MC appears to act in three circuits: the medial habenula-interpeduncular nucleus, basolateral amygdala-nucleus accumbens, and the dorsolateral tegmentum-ventral tegmental area. All three of these circuits appear to potentially modulate the mesolimbic dopaminergic pathway, which is the primary circuitry consistently implicated in drug addiction (Maisonneuve and Glick, 2003). However, the relative importance of these various pathways for the actions of 18-MC appear to vary with the particular reward (e.g., methamphetamine vs. sucrose; cf. Glick et al., 2008). Interestingly, the BLA, which has been shown to be critical for cue-induced reinstatement, is apparently much less important for opioid reward than for stimulant reward (Alderson et al., 2000; Olmstead and Franklin, 1997). Perhaps this helps explain why we have consistently found extracellular dopamine increases in the BLA in response to music-induced cues paired with stimulants in both a previous noncontingent drug-CS (methamphetamine) investigation (Polston et al., 2011) and in the current investigation where drug (cocaine) was contingently administered in a reinstatement paradigm. The common factor in both of these paradigms was that the musical cue was paired with a stimulant (methamphetamine or cocaine); it would be interesting to determine if these musical cues would be as effective both behaviorally and neurochemically (i.e., within the BLA) with an opioid.
Alpha3beta4 nicotinic receptors are preferentially localized in the medial habenula and interpeduncular nucleus, with lower densities in the basolateral amygdala (Perry et al., 2002; Zhu et al., 2005). Our working hypothesis is that 18-MC decreases drug self-administration by indirectly modulating the dopaminergic mesolimbic pathway via blockade of α3β4 nicotinic receptors in the habenulo-interpeduncular pathway and the basolateral amygdala. Perhaps a similar mechanism helps explain why 18-MC was so effective at blocking drug craving in our current model, as disruption of the BLA circuitry appears to be necessary to prevent cue-induced reinstatement (McLaughlin and Floresco, 2007). 18-MC has been proposed as a treatment for addiction to multiple drugs, as well as showing promise as a treatment for obesity (Maisonneuve and Glick, 2003; Taraschenko et al., 2008). Antagonism of α3β4 nicotinic receptors represents a relatively novel approach to treating multiple addictive disorders, dampening the impact of the mesolimbic pathway through indirect modulation via the habenulo-interpeduncular pathway. Pleasurable music induces neurological reactions in humans that are comparable to the effects induced by drugs of abuse. For example, highly enjoyable music has been shown to activate brain regions such as the nucleus accumbens, ventral tegmental area, amygdala, and prefrontal cortex. Enhanced functional connectivity between brain regions that mediate reward may help explain why listening to music is regarded as a highly pleasurable human experience (Blood and Zatorre, 2001; Menon and Levitin, 2005). It has also been demonstrated that music increases dopaminergic neurotransmission in the brain (Sutoo and Akiyama, 2004). The fact that human beings find music rewarding may help explain why music therapy has shown such promising results across a vast spectrum of disorders. However, our goal was not to see if rats had an appreciation for Miles Davis, but rather to determine whether a complex musical passage could effectively be used as a contextual CS in an animal reinstatement paradigm. Studies demonstrating that music can serve as an effective contextual CS in rats are an important first step in creating preclinical models that involve music.
While the influence of simple CS on goal-directed behavior has been explored thoroughly, more complex contextual CS have not been adequately investigated. Utilization of a complex contextual musical cue allowed for examination of associative learning that may be comparable to the psychological processes that occur during subjective human drug experiences. The present study is the first to give instrumental control of cocaine to a lower order species in the presence of a complex musical cue. The results clearly showed that music will indeed serve as an effective contextual CS in rats. Most importantly, our findings demonstrated that 18-MC has the ability to block musical cue-induced reinstatement, consistent with its potential use to treat drug seeking and taking in humans.
Highlights.
Novel reinstatement paradigm that utilized music as a contextual CS in rats.
Drug-paired music reinstated drug seeking and produced spatial conditioning.
Drug-paired music significantly increased extracellular DA in the BLA of rats.
18-MC pretreatment blocks context-induced drug seeking and spatial conditioning.
Acknowledgments
This work was supported by National Institute on Drug Abuse training grant 5T32DA007307-10 and National Institute on Drug Abuse grant 5R01DA016283-05.
Footnotes
COI Statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderson HL, Robbins TW, Everitt BJ. Heroin self-administration under a second-order schedule of reinforcement: acquisition and maintenance of heroin-seeking behaviour in rats. Psychopharmacology (Berl) 2000;153:120–133. doi: 10.1007/s002130000429. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauldoff GS. Music: More than just a melody. Chron Respir Dis. 2009;6:195–197. doi: 10.1177/1479972309346752. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacology Biochemistry and Behavior. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. European Journal of Pharmacology. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bradt J, Dileo C. Music for stress and anxiety reduction in coronary heart disease patients. Cochrane Database Syst Rev. 2009:CD006577. doi: 10.1002/14651858.CD006577.pub2. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- de Niet G, Tiemens B, Lendemeijer B, Hutschemaekers G. Music-assisted relaxation to improve sleep quality: meta-analysis. J Adv Nurs. 2009;65:1356–1364. doi: 10.1111/j.1365-2648.2009.04982.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behavioral Neuroscience. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Duvauchelle CL. Auditory stimuli enhance MDMA-conditioned reward and MDMA-induced nucleus accumbens dopamine, serotonin and locomotor responses. Brain Research Bulletin. 2008;77:189–196. doi: 10.1016/j.brainresbull.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiology of Learning and Memory. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Gabriele A, See RE. Reversible inactivation of the basolateral amygdala, but not the dorsolateral caudate putamen, attenuates consolidation of cocaine-cue associative learning in a reinstatement model of drug-seeking. Eur J Neurosci. 2010;32:1024–1029. doi: 10.1111/j.1460-9568.2010.07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Dong N, Keller RW, Jr, Carlson JN. Estimating extracellular concentrations of dopamine and 3,4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: Relationships between in vitro and in vivo recoveries. Journal of Neurochemistry. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18- Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000a;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann N Y Acad Sci. 2000b;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- Gold C, Solli HP, Kruger V, Lie SA. Dose-response relationship in music therapy for people with serious mental disorders: systematic review and meta-analysis. Clin Psychol Rev. 2009;29:193–207. doi: 10.1016/j.cpr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Hori K, Tanaka J, Nomura M. Effects of discrimination learning on the rat amygdala dopamine release: a microdialysis study. Brain Res. 1993;621:296–300. doi: 10.1016/0006-8993(93)90119-8. [DOI] [PubMed] [Google Scholar]
- Jung XT, Newton R. Cochrane Reviews of non-medication-based psychotherapeutic and other interventions for schizophrenia, psychosis, and bipolar disorder: A systematic literature review. Int J Ment Health Nurs. 2009;18:239–249. doi: 10.1111/j.1447-0349.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Wagner JJ. Effects of abstinence or extinction on cocaine seeking as a function of withdrawal duration. Behav Pharmacol. 2009;20:195–203. doi: 10.1097/FBP.0b013e32832a8f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. Journal of Neuroscience. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18- methoxycoronaridine. European Journal of Pharmacology. 1999;383:15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Reimer AR. Classically conditioned motor effects do not occur with cocaine in an unbiased conditioned place preferences procedure. Behav Pharmacol. 1996;7:303–314. doi: 10.1097/00008877-199608000-00001. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Glick SD. 18-Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett. 2009;458:57–59. doi: 10.1016/j.neulet.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146:1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Nilsson U. The anxiety- and pain-reducing effects of music interventions: a systematic review. Aorn J. 2008;87:780–807. doi: 10.1016/j.aorn.2007.09.013. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can t hey explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of lesions of various CNS sites. Behav Neurosci. 1997;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Yanagi J, Watanabe S. Discriminative and reinforcing stimulus properties of music for rats. Behavioural Processes. 2009;80:121–127. doi: 10.1016/j.beproc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Effects of compounding drug-related stimuli: escalation of heroin self-administration. J Exp Anal Behav. 2000;73:211–224. doi: 10.1901/jeab.2000.73-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, Ca: Academic Press Inc; 1986. [Google Scholar]
- Polston JE, Glick SD. Music-induced context preference following cocaine conditioning in rats. Behav Neurosci. 2011;125:674–680. doi: 10.1037/a0024341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE, Rubbinaccio HY, Morra JT, Sell EM, Glick SD. Music and methamphetamine: Conditioned cue-induced increases in locomotor activity and dopamine release in rats. Pharmacol Biochem Behav. 2011;98:54–61. doi: 10.1016/j.pbb.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Borrero E, Bernardo Colón A, Burgos-Mártir MA, Álvarez Carillo JE, Estrada del Campo Y, Abella-Ramírez C, Maldonado-Vlaar CS. NMDA antagonist AP-5 increase environmentally induced cocaine-conditioned locomotion within the nucleus accumbens. Pharmacology Biochemistry and Behavior. 2006;85:178–184. doi: 10.1016/j.pbb.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry. 2009;21:213–236. [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sutoo D, Akiyama K. Music improves dopaminergic neurotransmission: demonstration based on the effect of music on blood pressure regulation. Brain Res. 2004;1016:255–262. doi: 10.1016/j.brainres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ishigooka J, Watanabe S, Miyaoka H. Enhancement of delayed release of dopamine in the amygdala induced by conditioned fear stress in methamphetamine-sensitized rats. Eur J Pharmacol. 2002;435:59–65. doi: 10.1016/s0014-2999(01)01563-1. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Rubbinaccio HY, Maisonneuve IM, Glick SD. 18-methoxycoronaridine: a potential new treatment for obesity in rats? Psychopharmacology (Berl) 2008;201:339–350. doi: 10.1007/s00213-008-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett. 2005;379:37–41. doi: 10.1016/j.neulet.2004.12.047. [DOI] [PubMed] [Google Scholar]