Abstract
Adenosine 3′, 5′-cyclic adenosine monophosphate (cAMP) activates intracellular signaling by regulating Protein Kinase A (PKA), calcium influx, and cAMP-binging guanine nucleotide exchange factors (Epac or cAMP-GEF). cAMP inhibits cytokine-induced expression of nitric oxide synthase (iNOS) in hepatocytes by a PKA-independent mechanism. We hypothesized that Epac mediates this effect. A cyclic AMP analogue that specifically activates Epac, 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (OPTmecAMP) and overexpression of liver specific Epac2 both inhibited IL-1β/IFNγ–induced iNOS expression and nitrite production. OPTmecAMP inactivated Raf1/MEK/ERK signaling but ERK had no effect on iNOS expression. OPTmecAMP induced a persistent Akt phosphorylation in hepatocytes that lasted up to 8 hours. Overexpression of a dominant negative Akt blocked the inhibitory effect of OPTmecAMP on iNOS production. A specific PI3K inhibitor, LY294002, attenuated the inhibition of nitrite production and iNOS expression produced by overexpressing a liver specific Epac2 (LEpac2). OPTmecAMP also induced c-Jun N-terminal kinase (JNK) phosphorylation in hepatocytes. Overexpression of dominant negative JNK enhanced cytokine- induced iNOS expression and nitrite production and reversed the inhibitory effects of LEpac2 on nitrite production and iNOS expression. We conclude that Epac regulates hepatocyte iNOS expression through an Akt- and JNK- mediated signaling mechanism.
Keywords: Epac2, Nitric Oxide, Akt, JNK, hepatocyte
INTRODUCTION
Cyclic adenosine monophosphate (cAMP) is a second messenger that activates several intracellular signaling pathways to regulate cell function and cellular gene expression. The mechanisms involved in the cAMP-induced activation of Protein Kinase A (PKA) and its regulation of regulating hepatocyte metabolism have been extensively studied[1, 2]. Cyclic AMP also mediates PKA-independent signaling by binding to guanine nucleotide exchange factors (cAMP-GEFs, also known as Exchange Protein directly activated by cAMP, Epac) and activating GTPases [3–5]. Activation of GTPases such as Rap1 and Raf1 regulates downstream signaling pathways such as ERK, p38, and PI3K in what may be a cell- and tissue-specific manner [3–8]. GEFs also activate calcium-mediated pathways and can regulate downstream signaling through mechanisms other than binding to GTPase that are incompletely understood [9–11].
Two isoforms of Epac, Epac1 and Epac2, are found in mammalian cells [3, 12] and both are expressed in relatively low levels in liver tissue compared to other organs [12]. Instead, a specific short form of Epac2 mRNA and protein that uses a translation start site located in exon 10 of the “regular Epac2” is enriched in liver tissue [13]. The liver specific Epac2 lacks the first cAMP binding domain and the DEP (Dishevelled, Egl, Pleckstrin) domain [13], giving it a structure even shorter than Epac1[14]. Although this liver specific isoform showed GEF activity toward Rap1, the role of liver specific Epac in hepatocyte physiology is incompletely understood. Cyclic AMP suppresses hepatocyte apoptosis using both PKA-dependent and PKA-independent mechanisms and Epac-mediated signaling regulates the PKA-independent effect on apoptosis [15]. The role of Epac in regulating the effects of cAMP in other areas of hepatic function is unknown.
We have demonstrated that Glucagon and cAMP decrease hepatocyte iNOS expression and activity [16, 17] and does so through a mechanism that involves JNK in a manner that is primarily PKA-independent [18]. We therefore tested the hypothesis that Epac regulates the PKA-independent effect of cAMP on iNOS. Our data demonstrate that activation of Epac signaling suppresses hepatocyte iNOS expression and activity and does so through effects on both Akt and JNK.
MATERIALS AND METHODS
Reagents
Williams Medium E, penicillin, streptomycin, L-glutamine, LipofectAMINE, Human recombinant interleukin 1β (IL-1β), murine recombinant interferon γ (IFNγ) and HEPES were all from Invitrogen Life Science Inc. (Carlsbad, CA). Insulin was from Lilly (Indianapolis, IN). 8- (4- Chlorophenylthio)adenosine- 3′, 5′-cyclic monophosphate (CPTcAMP), 8- Bromoadenosine- 3′, 5′-cyclic monophosphate ( 8-Br-cAMP), 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic Monophosphate ( OPTmecAMP), and N6- Phenyladenosine- 3′, 5′-cyclic monophosphate (PhecAMP ) were purchased from Biolog Life Science Institute (Germany)[6]. Polyclonal antibodies to iNOS were purchased from BD Bioscience (Billerica, MA). Antibiodies to Raf1 (phosphorylated at serine 259), Akt, ERK, and actin were purchased from Cell Signaling Technology (Danvers, MA). LY294002 and FTI were purchased from Calbiochem (San Diego, CA). The plasmid expressing dominant negative Akt was provided by Drs. Burgering and Triest from Utrecht University, Belguim. H-Ras cDNA (dominant negative) in pUSEamp vector was purchased from Upstate (Charlottesville, VA). Recombinant adenovirus expressing dominant negative JNK (DNJNK) was provided by Dr. Hideaki Kaneto from Osaka City University Medical School, Japan. This DNJNK is a kinase-dead mutant (the ATP-binding site is mutated) and can be phosphorylated itself but cannot phosphorylate c-Jun [12]. All other reagents were from Sigma (St. Louis, MO).
Hepatocyte isolation and culture
Primary hepatocytes were isolated from male Sprague Dawley rats (200–250g) using the modified collagenase perfusion technique as previously described [1]. All animal care was in accordance with the University of Louisville’s Animal Care and Use Committee and followed guidelines proscribed by the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Purified hepatocytes (>98% pure with > 95% viability by trypan blue exclusion) were cultured onto collagen-coated 100mm dishes in Williams Medium E with L-arginine (0.5 mM), L- glutamine (2 mM), HEPES (15 mM) penicillin, streptomycin and 10% low endotoxin calf serum (HyClone Laboratories, Logan, UT). Once cells attached, the media was changed to insulin-free media with 5% calf serum. The cells were further incubated for 16 hours and the experimental conditions were established. Conditions were performed in duplicate or triplicate and experiments were repeated three times to ensure reproducibility.
Adenovirus mediated expression of constitutively active liver specific Epac2
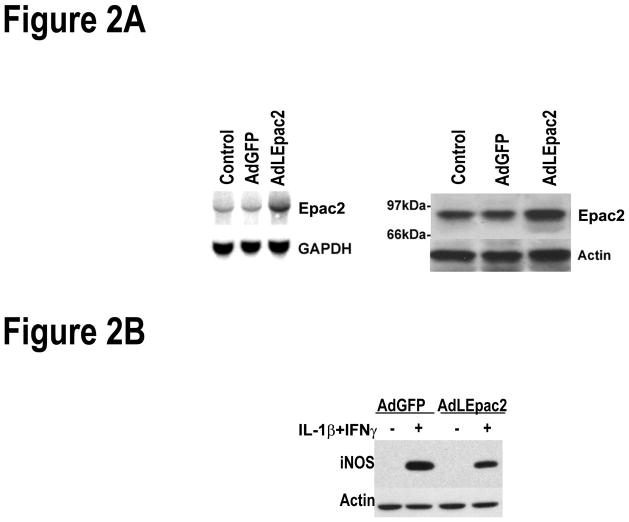
A unique Xho1 site was introduced at −12bp upstream of the ATG start codon in pSD5 -mouse liver cAMP-GEFII (a plasmid containing exon 10-3′UTR of Epac2 provided by Dr. Susumu Seino from Kobe University Graduate School of Medicine, Japan)[13] without interrupting the Kozak cassette. The VLVLE motif in the LID region was then mutated to AAAAA creating LEpac2 (Ala)5, a constitutively activated cDNA sequence [19]. After DNA sequencing, the Xho1-EcoR1 fragment was then sub cloned into the Xho1-EcoR1 site of a shuttle vector (pAdTrackCMV) from pAdEasy system (Clontech Laboratories. Mountain View, CA). The linearized shuttle plasmid containing constitutively activated LEpac2 was homogenously recombined with an adenoviral backbone plasmid, pAdEasy-1, in BJ5183 E. coli cells. Successful recombinants were digested with PacI and packaged in the HEK cell line. Viral titers were determined under a florescent microscope as described by Kaneto et al [12]. Briefly, confluent 293 cells were infected with a 1:10,000 dilution of the final lysate containing AdLEpac2. After 18 hours of incubation, the effective titer was determined by the following formula: 107 × the average number of GFP-positive cells per field (×100 magnification), which was considered equivalent to plaque-forming units (pfu)/ml. This number was considered to be proportional to the number of infective particles in the original lysate. LEpac2 mRNA and protein expressed by the created adenovirus (AdLEpac2) in hepatocytes were confirmed by Northern blot and Western blot analysis (Figure 2),
Figure 2. Over expression of liver specific Epac2 decreases IL-1β + IFN.
γ induced iNOS expression.
A. Hepatocytes were infected with AdGFP or AdLEpac2 by MOI (multiplicity of infection) 1:5. Total RNA was isolated and analyzed by Northern blot. 32P labeled BamH1 and Xba1 fragment (1.16 kb) from mouse Epac2 cDNA was used detect Epac2 mRNA and GAPDH was probed as loading control. B. Hepatocytes were infected with AdGFP or AdLEpac2. After recovery, the cells were treated with IL-1β + IFNγ for 24 hours and proteins were prepared for Western blot analysis using antibodies against iNOS and actin. The blots shown are representative of three independent experiments.
Western Blot
Hepatocytes were washed with ice-cold PBS and then scraped from the plate in 500 μl of lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2-EDTA, 1mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1 μg/ml leupeptin, and 1mM PMSF). After 30 min at 4° C, the lysates were centrifuged (15,000 × g for 15 minutes) and stored at −80° C until use. Proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding was blocked with TBS-T (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% non-fat milk for 1 hour. Primary antibodies were diluted and incubated with membranes for 1–2 hours at room temperature or overnight at 4 °C with agitation. After washing three times with TBS-T, secondary antibodies were incubated at 1:10,000 dilution for 1 hour. After 5 additional washes with TBS-T, the bands were visualized with chemiluminescence according to the manufacturer’s instructions. Membranes were then stripped and reprobed with antibodies for unphosphorylated proteins or actin as described.
Plasmid Transfection
Hepatocytes were plated in 6-well plates (106cells/well) and transfected with plasmids as previously described [18]. Briefly, hepatocytes were transfected using LipofectAMINE for 6 hours, allowed to recover overnight, and the experimental conditions established after washing away unincorporated particles.
Nitrite analysis
Supernatent NO2− was measured as an index of NO production by the Griess reaction as described [20].
Statistical analysis
Data are shown as the mean ± S.D. The Student t test or analysis of variance (ANOVA) using Sigmastat (Systat Software Inc, San Jose, CA) was used to determine statistical significance. A value of p < 0.05 was considered significant.
RESULTS
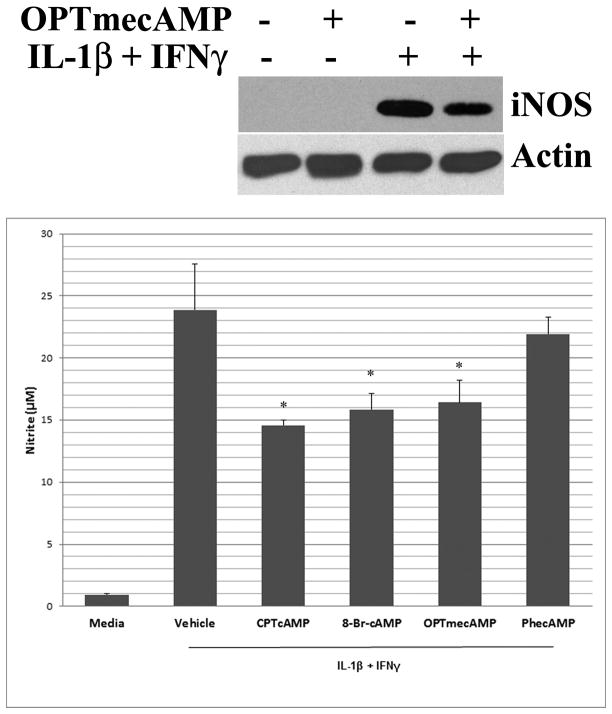
Cyclic AMP analogues modified in the 2′-ribose position activate Epac signaling to a greater extent than they activate PKA [21]. We therefore evaluated the ability of OPTmecAMP to inhibit cytokine-stimulated iNOS activation compared to CPTcAMP and 8-Br-cAMP, cAMP analogues that activates both PKA and Epac. The analogue PhecAMP which does not activate Epac was used to selectively activate cAMP-dependent protein kinase A (PKA type 1 and type 2) (Figure 1). When cultured hepatocytes were exposed to IL-1β + IFNγ to induce iNOS, OPTmecAMP inhibited nitrite production to a degree similar to that of CPTcAMP and 8-Br-cAMP while the inhibitory effect of phecAMP was much less and not different to vehicle control. OPTmecAMP also effectively suppressed iNOS protein expression as measured by Western Blot (Figure 1).
Figure 1. Epac activation inhibits cytokine-induced iNOS expression.
Hepatocytes were stimulated with IL-1β (200 U/ml) + IFNγ (100 U/ml) with 100 μM of CPTcAMP, 8-Br-cAMP, OPTmecAMP, or PhecAMP for 24 hours. The supernatant was analyzed for nitrite by Griess assay (lower) and the proteins were analyzed by Western blot with an antibody against iNOS (upper). Data represent the mean ± S.D. from three independent experiments. *p<0.05 versus IL-1β + IFNγ alone (vehicle) by t-test, n=6.
To confirm the effect of Epac on cytokine-induced iNOS, we developed a recombinant adenovirus that expressed constitutively active liver specific Epac2 (AdLEpac2) in cultured hepatocytes. After 24 hours of culture, hepatocytes infected with AdLEpac2 had increased expression of Epac2 mRNA and 97 KDa Epac2 protein compared to non-infected hepatocytes or hepatocytes infected with a control virus (AdGFP) (Figure 2A). Notably, basal Epac2 mRNA and protein expression were detected in control hepatocytes and were unchanged by the control virus (Figure 2A). When hepatocytes infected with either AdLEpac2 or AdGFP were stimulated to express iNOS by IL-1β + IFNγ, decreased iNOS protein expression was seen in LEpac2-overexpressing cells (Figure 2B).
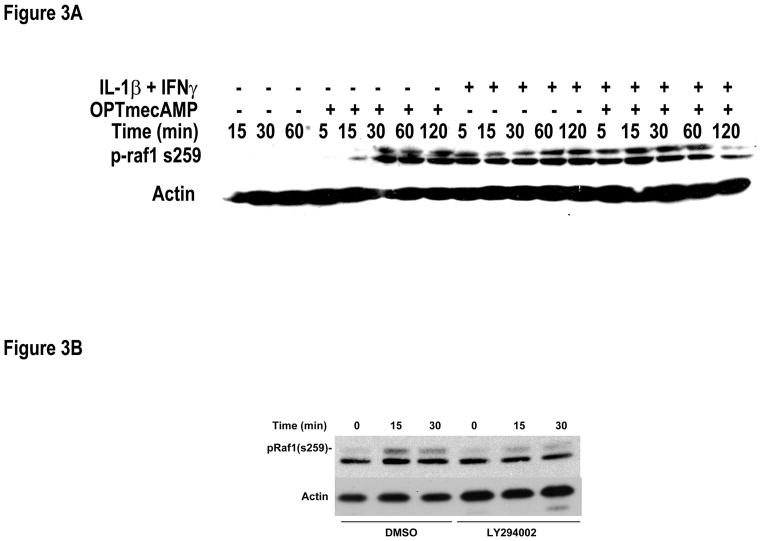
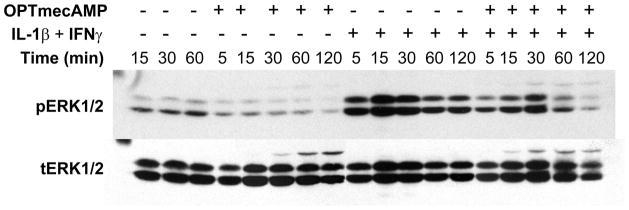
The signaling pathways responsible for Epac’s effect on hepatocyte iNOS are not known. Epac regulates MAPK through Rap1 and Ras/Raf1 signaling [3–7, 22]. We therefore assessed the ability of OPTmecAMP to regulate Raf1 in cultured hepatocytes. OPTmecAMP induced Raf1 phosphorylation at serine 259 (Figure 3A). Raf-1 phosphorylation at ser259 serves as a binding point for the regulatory adapter protein 14-3-3 and stabilizes the basal inactive Raf-1 conformation, serving as a negative regulatory site [23]. IL-1β + IFNγ phosphorylated Raf1 s259 to a lesser degree then OPTmecAMP. The combination of IL-1β + IFNγ and OPTmecAMP produced an earlier phosphorylation of Raf1-s259 then either stimulus alone. This result suggests that Epac stabilizes Raf1 and inhibits MEK/ERK signaling in hepatocytes. The phosphorylation of Raf1-s259 induced by OPTmecAMP was mediated in part by PI3K since Raf1 phosphorylation at ser259 was suppressed by the PI3K inhibitor, LY294002 (Figure 3B). Phosphorylation of Raf1 at serine 259 decreases downstream MEK/ERK activation and OPTmecAMP produced a corresponding decrease in IL-1β + IFNγ induced ERK1/2 phosphorylation in hepatocytes (Figure 4). However, the ERK inhibitor, PD98059, had no effect on IL-1β + IFNγ–induced nitrite production and overexpressing a constitutively active ERK2 failed to restore the OPTmecAMP-induced suppression of iNOS expression (data not shown). In addition, OPTmecAMP did not change p38 phosphorylation in hepatocytes stimulated with IL-1β + IFNγ and overexpression of a dominant negative p38 had no effect on the Epac-induced suppression of iNOS (data not shown). These data suggest that while the OPTmecAMP-decreased MAPK activation in hepatocytes, this effect was not responsible for the suppression of iNOS expression produced by Epac.
Figure 3. Epac activation regulates Raf-1 in hepatocytes.
A. Hepatocytes were treated with IL-1β (200 U/ml) + IFNγ (100 U/ml) with or without OPTmecAMP (0.1 mM). Proteins were collected at the indicated time point for Western blot analysis using antibodies against raf-1 phosphorylated at serine 259 and Actin. B. Hepatocytes were pre-incubated with 10 μM LY294002 for 30 minutes. The cells were then treated with 0.1 mM OPTmecAMP total protein collected at 0, 15 and 30 minutes. Western blot analysis was performed with antibodies against p-raf1 (s259) and actin. The blots shown are representative of three independent experiments.
Figure 4. Epac activation ERK phosphorylation in hepatocytes.
Hepatocytes were treated with IL-1β (200 U/ml) + IFNγ (100 U/ml) for the indicated time. Total cell lysates were harvested for Western blot analysis. The membranes were probed with antibodies specific for phosphorylated and total ERK p44/42. The blots shown are representative of three independent experiments.
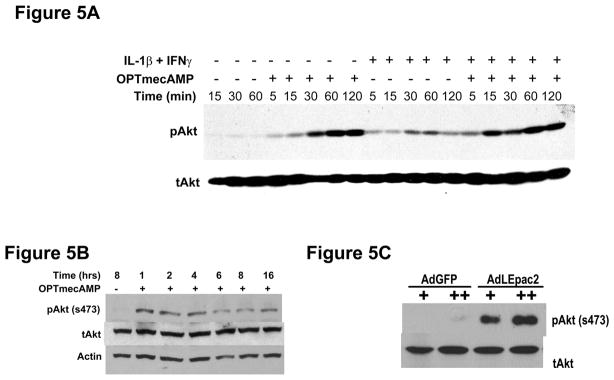
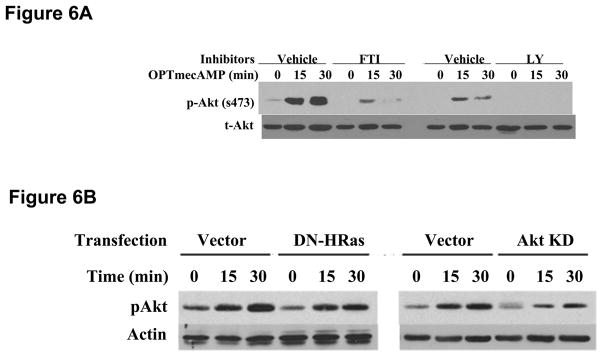
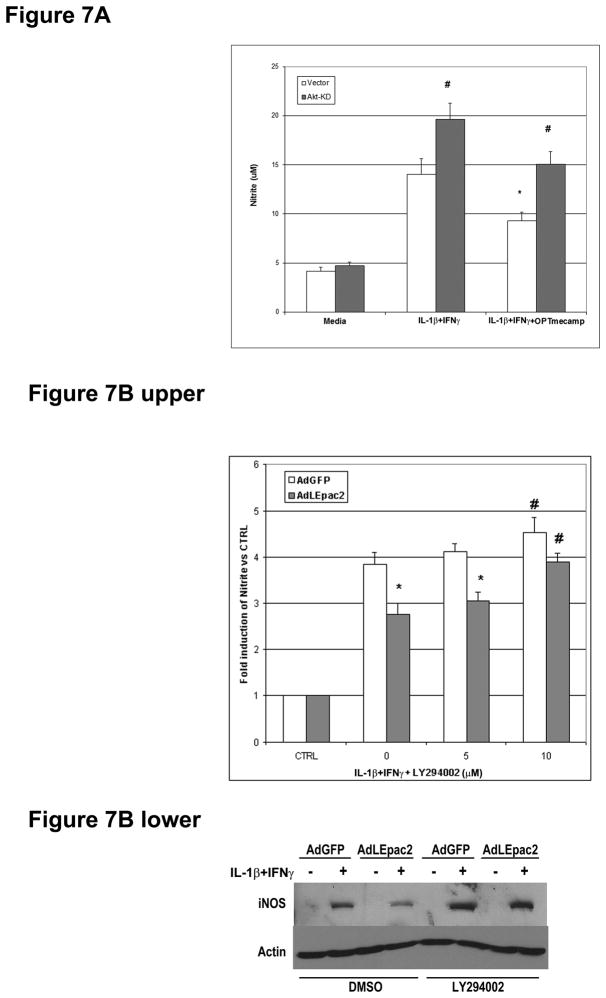
Cyclic AMP can activate [24] or inhibit [25] PI3K/Akt signaling depending on the cell being studied. In hepatocytes, Epac phosphorylates Akt and prevents apoptosis in a PI3K-dependent manner [15]. We therefore evaluated whether Epac activates PI3K/Akt to mediate its effect on iNOS. OPTmecAMP alone induced Akt phosphorylation up to 16 hours of culture (Figure 5A and 5B). Overexpression of LEpac2 also increased Akt activation in a dose-dependent manner compared to GFP control (Figure 5C). Ras Farnesyltransferase Inhibitor (FTI) and overexpression of dominant negative HRas decreased OPTmecAMP-induced Akt phosphorylation to an extent similar to that of the specific PI3K inhibitor LY294002 (Figure 6A) or overexpression of dominant negative Akt (Figure 6B). These results suggest that Epac-induced Akt activation is mediated by the Ras/PI3K pathway. We then explored the role of Epac-induced Akt activation on hepatocyte iNOS expression (Figure 7). Overexpression of dominant negative Akt increased nitrite production in IL-1β + IFNγ-stimulated hepatocytes, suggesting that Akt is involved in the endogenous regulation of cytokine-stimulated NO production. Dominant negative Akt blocked the inhibitory effect of OPTmecAMP on IL-1β + IFNγ-nitrite production (Figure 7A). Furthermore, the PI3K inhibitor LY294002 reduced the inhibition of nitrite production and iNOS expression produced by overexpressing LEpac2 in cytokine-stimulated hepatocytes (Figure 7B). These data support the hypothesis that Epac mediates its effects on iNOS expression through PI3K and Akt.
Figure 5. Epac induces Akt phosphorylation.
A and B. Hepatocytes were treated with IL-1β (200 U/ml) + IFNγ (100 U/ml) for the indicated time with or without OPTmecAMP (0.1 mM) (A) or OPTmecAMP alone (B). In C, hepatocytes were infected with AdLEpac2 or AdGFP and the cells were harvested 6 hours after recovery. Total cellular proteins were collected for Western blot analysis using antibodies against Akt phosphorylated at s473, total Akt, and Actin. The blots shown are representative of three independent experiments.
Figure 6. Ras mediates Epac-induced Akt phosphorylation.
A. Hepatocytes were pre-incubated with 10 μM farrnesyltransferase inhibitor (FTI) or 10 μM P13K inhibitor (LY294002) for 30 minutes, and then stimulated with OPTmecAMP (0.1 mM) for 0, 15 or 30 minutes. Cells were lysed for Western Blot analysis and probed with antibodies against phosphorylated Akt (s473). The blot shown is representative of three independent experiments. B: Hepatocytes were transfected with a dominant negative human Ras expression plasmid (DN-HRas) or dominant negative Akt expression plasmid (AktKD) for 6 hours. After recovery overnight, the cells were stimulated with OPTmecAMP (0.1 mM) for 0, 15 or 30 minutes. The cell lysates were subjected to Western blot using antibodies against phosphorylated Akt (s473), total Akt, and actin. The blots shown are representative of three independent experiments.
Figure 7. Dominant negative Akt and PI3K inhibitors attenuate the effects of Epac on iNOS.
A. Hepatocytes were transfected with dominant negative Akt plasmid (Akt KD). After recovery, the cells were treated with IL-1β + IFNγ with or without 0.2mM OPTmecAMP for 24 hours. n=9, * p<0.05 vs IL-1β + IFNγ with vector control transfection; # p<0.05 vs control by ANOVA. B. Hepatocytes were infected with AdGFP or AdLEpac2. After recovery, the cells were treated with IL-1β + IFNγ for 20 hours with either DMSO or 10 μM LY294002. The supernatants were collected for Griess assay (Upper) and the cell lysates were collected for Western blot analysis (Lower). Data shows the fold induction of Nitrite vs CTRL, n=9, * p<0.05 vs AdGFP infection; # p<0.05 vs IL-1β + IFNγ alone by ANOVA. The blot shown is representative of three independent experiments.
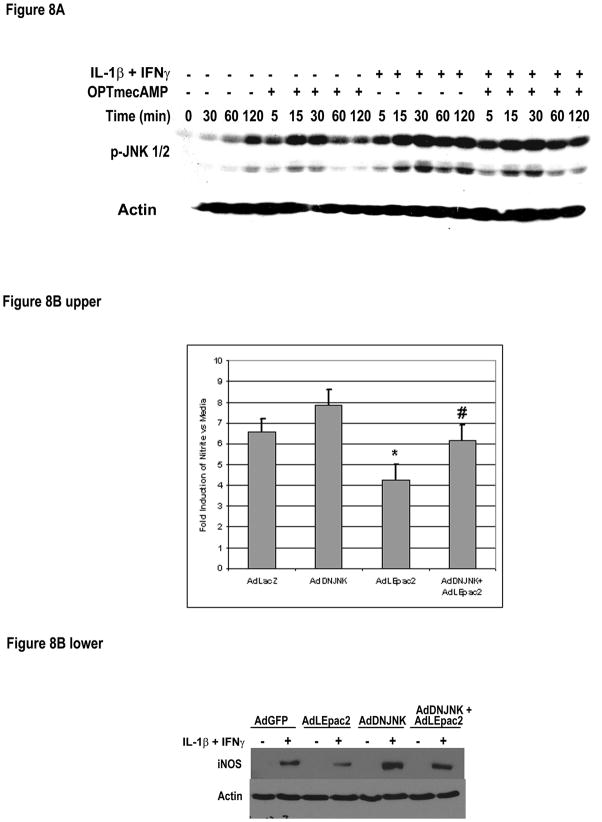
Cyclic AMP can activate JNK through GEFs [11] and JNK activation mediates the inhibition of IL-1β + IFNγ–induced iNOS expression by cAMP [18]. We therefore evaluated whether JNK was activated by Epac and regulated iNOS production in hepatocytes. OPTmecAMP (0.2mM) alone induced JNK1/2 phosphorylation which was detectable early (5 minutes) and peaked at 30 minutes (Figure 8A). We then expressed LEpac2 with or without dominant negative JNK in hepatocytes and stimulated them with IL-1β + IFNγ to induce iNOS. Overexpression of LEpac2 decreased IL-1β + IFNγ –induced nitrite production as previously shown (Figure 2) while dominant negative JNK enhanced cytokine-stimulated iNOS expression (Figure 8B). Coexpression of DN-JNK reversed the inhibitory effects of LEpac2 on both nitrite production and iNOS expression (Figure 8B).
Figure 8. Epac activates JNK and regulates iNOS expression. A.
Hepatocytes were treated with IL-1β (200 U/ml) + IFNγ (100 U/ml) with or without OPTmecAMP (0.1 mM). The cells were harvested at the indicated time point for Western blot analysis using antibodies against phosphorylated JNK1/2 and actin. The blot shown is representative of three independent experiments. B. Hepatocytes were infected with AdDNJNK, AdLEpac2 or both, using AdLacZ as control to ensure the same MOIs in each group. After recovery, the cells were treated with IL-1β + IFNγ for 20 hours in 5% FBS. The supernatants were subjected to Griess assay (Upper) and the cell lysates were subject to Wester blot analysis (Lower). Data shows the fold induction of Nitrite vs control (AdLacZ) hepatocytes, n=9, * p<0.05 vs AdLacZ, # p<0.05 vs AdLEpac2 by t-test. The blot shown is representative of three independent experiments.
DISCUSSION
Hepatocyte NO synthesis is an important part of the liver’s response to shock and sepsis and excessive NO production by iNOS induces tissue injury and organ dysfunction [16, 26]. Glucagon and other cAMP-elevating compounds inhibit hepatic NO synthesis in vitro and in vivo [16] but this regulation is complex. Despite PKA signaling being the best characterized pathway mediating cAMP-induced changes in cellular gene expression, we have found that the inhibitory effect of cAMP on cytokine-induced hepatocyte NO synthesis and iNOS expression is predominantly PKA-independent[27]. While cAMP-mediated changes in NF-κB and JNK contribute to this finding, they do not account for all of the PKA-independent effects of cAMP on NO [27]. Epac is a guanine nucleotide exchange factor that can activate several distinct intracellular signaling pathways in a PKA-independent manner. Studies have implicated Epac in the regulation of several inflammatory responses including the regulation of endothelial cell–cell junction stability [28], the suppression of cytokine-induced pro-inflammatory signaling [29] and development of hepatocyte apoptosis [15]. We therefore explored the role of Epac in mediating the PKA-independent inhibition of hepatocyte iNOS by cAMP. Our data demonstrate that activation of Epac signaling with OPTmecAMP or overexpression of LEpac2 decreased cytokine-induced iNOS expression.
Epac regulates Ras/Raf/MEK/ERK signaling Epac can inhibit or upregulate MAPK depending on the cell type being investigated [25, 30, 31]. We found that Epac stabilized Raf1 in hepatocytes through phosphorylation of serine 259 associated with the suppression of ERK activation but had no effect on p38 MAPK. However, we could find no role for either p38 MAPK or p44/42 MAPK in regulating hepatocyte iNOS expression. Our data do not exclude the possibility that Epac induced changes in p38 or p44/42 MAPK can regulate the expression of other hepatocyte genes.
Although cAMP inhibits Akt activation in a number of cell types [25, 32], it activates PI3K/Akt signaling in a PKA-independent manner in hepatocytes [15, 33]. We found that pharmacologic activation of Epac signaling with OPTmecAMP or overexpression of liver specific Epac2 increased Akt phosphorylation. The activation of Akt by OPTmecAMP was inhibited by FTI, an inhibitor of Ras, and LY294002, an inhibitor of PI3K. These data demonstrate that Epac mediated a Ras- and PI3K-dependent Akt activation in cultured rat hepatocytes. Both LY294002 and dominant negative Akt blocked the inhibitory effect of Epac signaling on iNOS expression in cytokine-stimulated hepatocytes, demonstrating that the effect of Epac on iNOS is mediated through PI3K/Akt.
JNK inhibits iNOS activation and contributes to the PKA-independent effect of cAMP on iNOS [18]. We therefore examined the role of JNK in mediating the effect of Epac activation on iNOS. In hepatocytes, OPTmecAMP increased JNK phosphorylation alone and in combination with cytokines. Expression of a dominant negative JNK reversed the inhibitory effect of Epac2 on iNOS suggesting that Epac mediates some of its effects through regulation of JNK. Other studies have shown that Epac activates JNK through Rap1-independent signaling [11] in the HEK cell line but Rap2 mediates JNK activation in excitatory synapses [34]. In hepatocytes, the mechanisms involved in the activation of JNK by Epac are unclear. It is possible that Epac-induced Akt signaling may lead to downstream JNK activation that can subsequently alter c-Jun or other transcription factors important in iNOS expression. These mechanisms will require further investigation to define.
We cannot exclude a role for other PKA-independent signaling pathways in mediating the effects of Epac on hepatocyte iNOS expression seen in these studies. Epac regulates Ca2+ mobilization in pancreatic β cells [5] and Ca2+ regulates the activity of calmodulin-dependent kinases that can alter transcription factor activation and subsequent gene expression[35]. Whether Epac-induced changes in Ca2+ orCa2+-dependent transcription factor activation contribute to the PKA-independent effects of Epac on hepatocyte iNOS will require further study.
In conclusion, we demonstrate that Epac inhibits cytokine-stimulated hepatocyte iNOS expression and does so through effects on PI3K/Akt and JNK. These signaling pathways may represent important regulators of in vivo nitric oxide synthesis during inflammation and sepsis. Additional work will be required to elucidate the specific Akt-and JNK- dependent processes regulating the iNOS gene during shock and sepsis.
Acknowledgments
We appreciate Drs. Burgering and Triest for providing the plasmids that express dominant negative Akt and Dr. Kaneto for providing the recombinant adenovirus that express dominant negative JNK.
Grant sponsor: NIH; Grant number: NIDDK 055664 to Brian G Harbrecht
References
- 1.Zhang B, Liu S, Perpetua MD, Walker WH, Harbrecht BG. Cytokines increase CRE binding but decrease CRE-mediated reporter activity in rat hepatocytes by increasing c-Jun. Hepatology. 2004;39:1343–52. doi: 10.1002/hep.20200. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Li S, Harbrecht BG. Akt-mediated signaling is induced by cytokines and cyclic adenosine monophosphate and suppresses hepatocyte inducible nitric oxide synthase expression independent of MAPK P44/42. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamcr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 4.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–8. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 5.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–6. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 7.Richards JS. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–18. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 8.Barnier JV, Papin C, Eychene A, Lecoq O, Calothy G. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J Biol Chem. 1995;270:23381–9. doi: 10.1074/jbc.270.40.23381. [DOI] [PubMed] [Google Scholar]
- 9.Keiper M, Stope MB, Szatkowski D, Bohm A, Tysack K, Vom Dorp F, Saur O, Oude Weernink PA, Evellin S, Jakobs KH, Schmidt M. Epac- and Ca2+-controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem. 2004;279:46497–508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–4. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 11.Hochbaum D, Tanos T, Ribeiro-Neto F, Altschuler D, Coso OA. Activation of JNK by Epac is independent of its activity as a Rap guanine nucleotide exchanger. J Biol Chem. 2003;278:33738–46. doi: 10.1074/jbc.M305208200. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–9. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Shibasaki T, Iwanaga T, Takahashi K, Yokoyama Y, Liu LM, Yokoi N, Ozaki N, Matsukura S, Yano H, Seino S. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics. 2001;78:91–8. doi: 10.1006/geno.2001.6641. [DOI] [PubMed] [Google Scholar]
- 14.Rehmann H, Prakash B, Wolf E, Rueppel A, De Rooij J, Bos JL, Wittinghofer A. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol. 2003;10:26–32. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- 15.Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G334–43. doi: 10.1152/ajpgi.00517.2003. [DOI] [PubMed] [Google Scholar]
- 16.Harbrecht BG, Perpetua M, Fulmer M, Zhang B. Glucagon regulates hepatic inducible nitric oxide synthesis in vivo. Shock. 2004;22:157–62. doi: 10.1097/01.shk.0000131579.22409.33. [DOI] [PubMed] [Google Scholar]
- 17.Harbrecht BG, Taylor BS, Xu Z, Ramalakshmi S, Ganster RW, Geller DA. cAMP inhibits inducible nitric oxide synthase expression and NF-kappaB-binding activity in cultured rat hepatocytes. J Surg Res. 2001;99:258–64. doi: 10.1006/jsre.2001.6200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Perpetua M, Fulmer M, Harbrecht BG. JNK signaling involved in the effects of cyclic AMP on IL-1beta plus IFNgamma-induced inducible nitric oxide synthase expression in hepatocytes. Cell Signal. 2004;16:837–46. doi: 10.1016/j.cellsig.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Rehmann H, Rueppel A, Bos JL, Wittinghofer A. Communication between the regulatory and the catalytic region of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003;278:23508–14. doi: 10.1074/jbc.M301680200. [DOI] [PubMed] [Google Scholar]
- 20.Harbrecht BG, Nweze I, Smith JW, Zhang B. Insulin inhibits hepatocyte iNOS expression induced by cytokines by an Akt-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G116–22. doi: 10.1152/ajpgi.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 22.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–6. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Avruch J, Khokhlatchev A, Kyriakis JM, Luo Z, Tzivion G, Vavvas D, Zhang XF. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog Horm Res. 2001;56:127–55. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 24.Machida K, Inoue H, Matsumoto K, Tsuda M, Fukuyama S, Koto H, Aizawa H, Kureishi Y, Hara N, Nakanishi Y. Activation of PI3K-Akt pathway mediates antiapoptotic effects of beta-adrenergic agonist in airway eosinophils. Am J Physiol Lung Cell Mol Physiol. 2005;288:L860–7. doi: 10.1152/ajplung.00131.2004. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Liu F, Adamo ML. Cyclic AMP inhibits extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways by inhibiting Rap1. J Biol Chem. 2001;276:37242–9. doi: 10.1074/jbc.M105089200. [DOI] [PubMed] [Google Scholar]
- 26.Tsao CM, Huang HC, Chen ZF, Liaw WJ, Lue WM, Chen A, Chen SJ, Wu CC. Beneficial effects of hyperoncotic albumin on liver injury and survival in peritonitis-induced sepsis rats. Shock. 2011;35:210–6. doi: 10.1097/SHK.0b013e3181f229f8. [DOI] [PubMed] [Google Scholar]
- 27.Hong G, Zhang B, Harbrecht BG. Cyclic AMP inhibits IL-1beta plus IFNgamma-induced NF-kappaB translocation in hepatocytes by a PKA independent mechanism. J Surg Res. 2010;159:565–71. doi: 10.1016/j.jss.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Sand C, Jakobs KH, Michel MC, Weernink PA. Epac and the cardiovascular system. Curr Opin Pharmacol. 2007;7:193–200. doi: 10.1016/j.coph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–46. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balan V, Leicht DT, Zhu J, Balan K, Kaplun A, Singh-Gupta V, Qin J, Ruan H, Comb MJ, Tzivion G. Identification of novel in vivo Raf-1 phosphorylation sites mediating positive feedback Raf-1 regulation by extracellular signal-regulated kinase. Mol Biol Cell. 2006;17:1141–53. doi: 10.1091/mbc.E04-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewi DA, Abayasekara DR, Wheeler-Jones CP. Requirement for ERK1/2 activation in the regulation of progesterone production in human granulosa-lutein cells is stimulus specific. Endocrinology. 2002;143:877–88. doi: 10.1210/endo.143.3.8677. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Jee K, Kim D, Koh H, Chung J. Cyclic AMP inhibits Akt activity by blocking the membrane localization of PDK1. J Biol Chem. 2001;276:12864–70. doi: 10.1074/jbc.M001492200. [DOI] [PubMed] [Google Scholar]
- 33.Gates A, Hohenester S, Anwer MS, Webster CR. cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 beta/alpha in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2009;296:G764–74. doi: 10.1152/ajpgi.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, Sheng M, Zhu JJ. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–16. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y, Moon JS, Lee KS, Park SY, Cheong J, Kang HS, Lee HY, Kim HD. Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates the expression of iNOS through IKK and NF-kappaB activity in LPS-stimulated mouse peritoneal macrophages and RAW 264.7 cells. Biochem Biophys Res Commun. 2004;314:695–703. doi: 10.1016/j.bbrc.2003.12.153. [DOI] [PubMed] [Google Scholar]