Abstract
The lipoxygenases (LOs) are principal enzymes involved in the oxidative metabolism of polyunsaturated fatty acids, including arachidonic acid. 12- and 15-LO and their lipid metabolites have been implicated in the development of insulin resistance and diabetes. Adipose tissue, and in particular visceral adipose tissue, plays a primary role in the development of the inflammation seen in these conditions. 12- and 15-LO and their lipid metabolites act as upstream regulators of many of the cytokines involved in the inflammatory response in adipose tissue. While the role that 12- and 15-LO play in chronically inflamed adipose tissue is becoming clearer, there are still many questions that remain unanswered regarding their activation, signaling pathways, and roles in healthy fat. 12- and 15-LO also generate products with anti-inflammatory properties that are under investigation. Therefore, 12- and 15-LO have the potential to be very important targets for therapeutics aimed at reducing insulin resistance and the comorbid conditions associated with obesity.
Keywords: 12/15-lipoxygenase, adipose tissue, inflammation, insulin resistance, diabetes, adipogenesis, obesity
Introduction
Polyunsaturated fatty acids (PUFAs) are essential fatty acids necessary in our diet on which various enzymes act to generate a variety of lipid metabolites that serve as signaling molecules required for normal cellular function [1]. The eicosanoids are metabolites generated from the oxidation of twenty-carbon omega-6 (ω-6) or omega-3 (ω-3) PUFAs that under certain conditions are pro-inflammatory [2,3]. One essential ω-6 PUFA is arachidonic acid (AA). AA is normally esterified in the cell membrane phospholipids and released by phospholipase A2 in response to various peptides, such as growth factors and cytokines, induced by cellular stress [4]. AA then can be oxidized by three classes of enzymes to generate lipid products: the lipoxygenases (LOs) to generate leukotrienes, lipoxins, hepoxilins, hydroperoxyeicosatetraenoic acids (HpETEs), and hydroxyeicosatetraenoic acids (HETES); the cyclooxygenases (COX-1 and COX-2) to generate prostaglandins and thromboxanes; and the cytochrome p-450 epoxygenases to generate epoxides [5]. In addition, LOs can act upon ω-3 docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) to generate lipid metabolites, such as the resolvins, maresins, and protectins, that directly act as anti-inflammatory metabolites or drive resolution of an acute inflammatory response [6].
Animal LO enzymes include 5-LO, 12-LO (epidermal-, platelet-, and leukocyte-type), 15-LO, and eLOX-3 (epidermis-type LO-3) (Table 1) named according to the carbon position at which they oxygenate their PUFA substrate [7]. The LOs are found in plants and animals, including humans, and are expressed from the LOX and ALOX genes, respectively (Table 1). However, comparison of the homologous isoforms across species is not always straightforward due to species-specific differences in affinity for different substrates and the products generated by the LO isoforms and thus care must be taken in interpreting data across species. Finally, the LOs are expressed in a variety of tissues including the vasculature, kidney, nervous system, liver, pancreatic islet, and adipose tissue [2, 8–10].
Table 1.
Nomenclature for human and mouse lipoxygenase enzymes.
| Lipoxygenase | Human Gene (Trivial Name) | Mouse Gene (Trivial Name) |
|---|---|---|
| 5-LO | ALOX5 | ALOX5 |
| Leukocyte-type 12-LO | ALOX15 (15-LO-1) | ALOX15 (12/15-LO) |
| 15-LO | ALOX15B (15-LO-2) | ALOX15B (15-LO) |
| Platelet-type 12-LO | ALOX12 (12(S)-LO or 12-LO) | ALOX12 |
| 12(R)-LO | ALOX12B | ALOX12B |
| Epidermal-type 12-LO | not expressed | ALOX12e |
| Epidermis-type LO-3 | ALOXE3 (eLOX-3) | ALOXE3 (eLOX-3) |
For the purpose of this review, we will focus on the updated role of the 12- and 15-LOs in adipose tissue in the inflammatory obese condition. For a more comprehensive review covering the role of 12- and 15-LOs in tissues in both the normal and disease state, please refer to [2].
12- and 15-Lipoxygenases in the Adipose Tissue
Fat – The Newly Appreciated Endocrine Organ
Adipose tissue (AT), or fat, is found as either brown or white with very distinct physiological characteristics [11,12]. In particular, brown adipose tissue (BAT) is found primarily in neonates (and in certain conditions in adults) in the paravertebral, periadrenal, and supraclavicular regions and developed as a means to provide thermogenesis in periods of cold weather. As such, BAT is heavily laden with mitochondria with multilocular lipid droplets. On the other hand, white adipose tissue (WAT) functions largely as a lipid storage unit characteristic of large unilocular lipid droplets for the purpose of providing a readily available fuel source for cells during times of fasting. However, our dependence on these fat organs has diminished given our modern housing and abundance of food supplies. In particular, WAT has now become a sinkhole for the storage of excess nutrients, leading to the generation of new metabolic problems.
WAT is not simply a repository for excess lipids in the diet, but also functions as an endocrine organ [13]. AT is comprised of adipocytes in addition to cells comprising the stromal vascular fraction (SVF), including preadipocytes, leukocytes, macrophages, and endothelial cells. AT is a complex milieu of cells and is actively involved in responses to various cellular stimuli and inflammatory responses. It produces and secretes inflammatory cytokines and adipocyte-specific hormone-like proteins, called adipokines, that affect local AT and systemic bodily functions. These hormones and cytokines are actively involved in regulating lipid metabolism, insulin sensitivity, and satiety. Emerging evidence points towards a significant role for 12- and 15-LO function in WAT adipogenesis and adipocyte health, and disruption of normal 12- and 15-LO function by the inflammatory obese condition promotes adipocyte dysfunction and overall metabolic disease including insulin resistance and diabetes.
12- and 15-LO Function in Adipogenesis
Adipogenesis involves the generation of adipocytes from the preadipocytes, or mesenchymal stem cells, within the SVF of AT. Peroxisome proliferator-activated receptor gamma (PPARγ) is a necessary and sufficient requirement for adipogenesis and no other factor is able to perform this role [12]. In addition, PPARγ activation is dependent upon an exogenous supply of free fatty acids and many of the lipoxygenase-derived metabolites activate PPARγ [14,15]. AA is necessary for proper glucose uptake in white adipocytes and has been shown to be dependent on LO activity [16]. The in vitro utilization of 3T3-L1 fibroblasts has allowed for detailed examination of adipogenesis given that these fibroblasts can be induced to fully differentiate into a pure population of white adipocytes simply by the addition of a differentiation cocktail [17,18]. Analysis of the expression profile of the various lipoxygenases in 3T3-L1 cells reveals that eLOX-3 is expressed in pre-adipocytes and early differentiated adipocytes while the leukocyte- and platelet-type 12-LO are not, and that adipogenesis is only sensitive to LO inhibitors during the early adipogenic stages [19]. Overexpression of eLOX-3 or addition of its hepoxilin eicosanoid products to 3T3-L1 preadipocytes promotes adipocyte differentiation while knockdown of eLOX-3 prevents adipogenesis [14]. However, expression of 5-LO and leukocyte-type 12-LO significantly increases at the time of terminal differentiation while epidermal- and platelet-type 12-LO and eLOX-3 are absent at this stage, suggesting that 5-LO and leukocyte-type 12-LO may have a role in the terminal differentiation of adipogenesis [20,21].
Pro-Inflammatory Roles of 12- and 15-LO in Adipocyte Dysfunction
As mentioned earlier, AT functions as an active endocrine organ regulating a variety of cellular processes including lipid synthesis and metabolism, insulin sensitivity, and hormonal regulation of bodily functions such as satiety. However, when the intake of nutrients excessively surpasses the caloric needs of the body, the extra nutrient source is stored as triaglycerols in the adipocyte [22]. Eventually, the overwhelming demand for intracellular lipid storage within the adipocyte will lead to overexpansion of the WAT with ensuing shear stress of the cell membrane marked by a chronic low-grade inflammation in this tissue, which can lead to systemic insulin resistance, type 2 diabetes, and cardiovascular complications [22]. Leukocyte-type 12-LO, or 12/15-LO (rodent leukocyte-type 12-LO is often labeled as 12/15-LO as it can oxygenate substrate at both the 12- and 15-carbon position), appears to be a key player in the progression of adipocyte dysfunction and resultant systemic decline. Firstly, 12/15-LO is upregulated in WAT in the obese state. C57BL/6J mice that have been on a high-fat diet for 8 weeks exhibit increased expression of 12/15-LO in isolated white adipocytes [20]. Zucker obese rats, a genetically-induced rodent model of obesity and insulin resistance, also exhibit increased expression of 12/15-LO in isolated white adipocytes compared to lean controls [21].
To examine the significance of the increased 12/15-LO expression in WAT, the differential responses of wild-type C57BL/6J and 12/15-LO-deficient mice on either a normal chow or a 42% high-fat “Western” type diet for 8–24 weeks were examined [23]. 12/15-LO deficiency preserved normal glucose metabolism (as measured by glucose and insulin tolerance tests) and fasting insulin and glucose levels when fed a high-fat diet compared to wild-type mice on a high-fat diet, suggesting improvements in insulin sensitivity in these mice. Furthermore, while circulating cytokine levels of TNF-α and IL-6 increased and the adipokine, adiponectin, decreased when wild-type mice were fed the high-fat diet, 12/15-LO-deficient mice fed a high-fat diet retained normal levels of these factors. Consistent with the observed improvements in insulin sensitivity, TNF-α and IL-6 are both implicated in the development of insulin resistance in part through promoting adipocyte lipolysis while adiponectin is a key adipokine that promotes insulin sensitivity [24–26]. Additionally, while body weight and epididymal WAT weight did not differ between the groups on a high-fat diet, significant reductions in macrophage infiltration and MCP-1 staining in the epididymal WAT was observed in the high-fat diet-fed 12/15-LO-deficient mice compared to wild-type mice. In agreement with these studies, another study examined wild-type C57BL/6J and 12/15-LO-deficient mice placed on a similar 41% high-fat diet for as little as 2–4 weeks and revealed that WAT inflammation (including TNF-α and IL-6 protein expression), macrophage infiltration, and whole body insulin resistance (as measured by euglycemic hyperinsulinemic clamp studies) was prevented in the 12/15-LO-deficient mice on the high-fat diet [27]. Thus these results indicate that 12/15-LO is required for mediating the early stages of WAT inflammation and whole body insulin resistance induced by a high-fat diet. While little study has been devoted to the role of platelet-type 12-LO in mediating diet-induced obesity, platelet-type 12-LO is also upregulated in isolated epididymal white adipocytes from C57BL/6J mice fed a high-fat diet [28]. Human relevance for 12/15-LO and platelet-type 12-LO is provided by data revealing that the respective human equivalents, 15-LO-1 and 12-LO, are upregulated in WAT of obese patients [29]. It is also worth mentioning that expression of 5-LO and its metabolites are upregulated in white adipocytes and adipose tissue of Zucker obese rats and diet-induced obese mice, concomitant with the increased 12/15-LO and cytokine (TNF-α, IL-6, and MCP-1) expression [21, 30]. However, the role of 5-LO in human WAT from obese individuals remains unclear. Therefore multiple LO pathways may play a role in mediating obesity-associated chronic-low grade inflammation.
To confirm a direct role of 12- and 15-LO activity in mediating inflammation and insulin resistance in adipocytes, examination of the addition of 12/15-LO products to 3T3-L1 adipocytes was performed by Chakrabarti et al. [20]. Addition of the major products of 12/15-LO, 12(S)-HETE and its precursor 12(S)-HpETE, directly to fully differentiated 3T3-L1 adipocytes significantly induced pro-inflammatory gene expression and secretion of many pro-inflammatory cytokines, including TNF-α, MCP-1, IL-6, and IL-12p40. In addition, the anti-inflammatory adiponectin was significantly decreased under these conditions. Consistent with the idea that increased inflammation leads to increased phosphorylated c-Jun NH2-terminal kinase (JNK)-1 and thereby reduced insulin signaling, we observed that phosphorylated JNK-1 was indeed increased and insulin-mediated activation of key insulin signaling proteins such as Akt and IRS (insulin receptor substrate)-1 were decreased after addition of 12(S)-HETE to 3T3-L1 adipocytes. Finally, to give relevance for these in vitro findings in the context of diet-induced obesity, palmitic acid, a free fatty acid that is a major component of the high-fat diet, was added to 3T3-L1 adipocytes [20]. Palmitic acid induced 12/15-LO expression with concomitant increased cytokine expression. Collectively the data reveals that diet-induced obesity is marked by a chronic, low-grade inflammation that promotes 12/15-LO activation and further amplification of the inflammatory cascade with ensuing insulin resistance and metabolic decline (Figure 1).
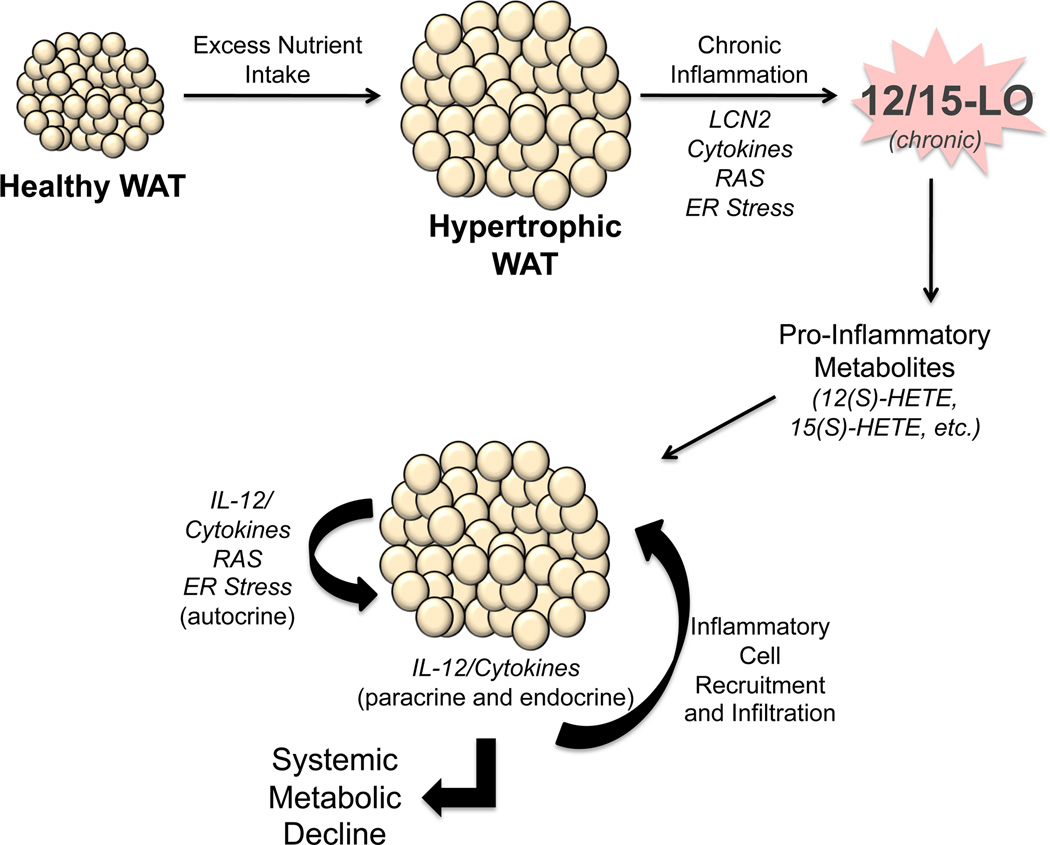
Figure 1.
The role of 12- and 15-LO in adipose tissue in the obese state. Excess consumption of energy demands an increased nutrient storage capacity of adipocytes in white adipose tissue (WAT). As a result, adipocytes become hypertrophic and stressed, leading to dysfunction marked by ensuing inflammation. Expression of pro-inflammatory cytokines, lipocalin-2 (LCN2), renin angiotensin system (RAS) markers, and ER stress markers by these stressed adipocytes and stromal vascular fraction (SVF) of the WAT leads to chronic leukocyte-type 12-LO (12/15-LO) activation in the adipocyte and SVF and subsequent generation of pro-inflammatory lipid metabolites, such as 12(S)-HETE and 15(S)-HETE. 12/15-LO activity promotes further amplification of pro-inflammatory pathways, in particular the interleukin-12 (IL-12) pathway. This inflamed fat promotes the recruitment of macrophages and other inflammatory cells into the fat bed, further propagating the inflammatory autocrine cascade. In addition, WAT exerts paracrine and endocrine pro-inflammatory effects through secreted cytokines on various organ systems, including the pancreas, liver, and vasculature, leading to metabolic decline.
Adipocyte dysfunction is not only marked by chronic inflammation and insulin resistance of WAT, but also by a phenomenon called endoplasmic reticulum (ER) stress [31,32]. The ER is a highly specialized organelle that in the adipocyte is responsible for protein and lipid biosynthesis. However, in the chronically-inflamed obese state, the ER is overwhelmed due to the excess of nutrients and thus the accumulation of misfolded proteins occurs, leading to the activation of the unfolded protein response (UPR). The UPR functions to essentially increase the ER machinery necessary to meet the demands of protein and lipid biosynthesis. The UPR functions through 3 major arms: the PKR-like ER-regulated kinase (PERK), inositol requiring enzyme 1α (IRE1α), and the activating transcription factor 6 (ATF6). While all arms will increase ER-biogenesis related genes, activated PERK leads to attenuation of protein synthesis while activated IRE1α and ATF6 upregulate protein chaperones necessary for protein folding. Obesity-associated ER stress has been shown to increase inflammation and insulin resistance [31,32]. In support of this, insulin-resistant obese patients or mice exhibiting increased ER stress in WAT reveal that caloric restriction can decrease inflammation and restore insulin sensitivity [33–38]. Interestingly, recent evidence from our lab demonstrates that 12/15-LO is a novel inflammatory pathway that mediates ER stress in the adipocyte [39] (Figure 1).
We treated 3T3-L1 adipocytes with 12(S)-HETE and 12(S)-HpETE and observed that ER stress markers associated with each UPR arm were activated [39]. Furthermore, if cells were pretreated with the 12/15-LO inhibitor, CDC (cinnamyl1–3, 4-dihdroxy-α-cyanocinnamate), induction of the ER stress response by the chemical inducer tunicamycin (an N-glycosylation inhibitor of de novo protein synthesis) was significantly ameliorated [39]. Additionally, isolated epididymal white adipocytes from C57BL/6J or 12/15-LO-deficient mice treated with tunicamycin revealed that ER stress induction was significantly impaired in the absence of 12/15-LO [39].
Peripheral Pro-Inflammatory Effects of Adipose Tissue-Specific 12- and 15-LO Activity
Current published studies reveal a significant role for 12/15-LO function in the white adipocyte. However, little is known about the systemic impact of 12/15-LO activity in WAT. Our lab has generated a mouse model whereby 12/15-LO is conditionally removed from the WAT by the Cre transgene driven by the adipocyte-lipid binding protein (aP2) promoter. Control and 12/15-LO fat-specific-deficient mice were placed on either a chow or 60% high-fat diet for 16 weeks. As expected, preliminary unpublished observations reveal that 12/15-LO deletion from WAT was able to reduce inflammation in and macrophage infiltration into the epididymal WAT. Of considerable note was the observed reduction in fasting blood glucose levels and non-fasting serum insulin levels in the 12/15-LO fat-specific-deficient mice compared to control mice on a high-fat diet. In addition these same mice exhibited improvements in insulin sensitivity and secretion as measured by glucose and insulin tolerance tests. Inflammation in the pancreatic islet was also reduced in the high-fat diet-fed 12/15-LO fat-specific-deficient mice compared to controls. These observations are consistent with improvements in pancreatic β-cell function and systemic insulin sensitivity seen in the global 12/15-LO-deficient mice on high-fat diets. These data suggest an interesting crosstalk between 12/15-LO expression in WAT and inflammation in pancreatic tissue, revealing a considerable systemic impact of chronic 12/15-LO activity in fat in diet-induced obesity.
Obesity is also associated with the hepatic manifestation of nonalcoholic fatty liver disease (NAFLD) whereby the hepatocytes are characterized by lipid accumulation with subsequent inflammation and cell injury [40]. Mice deficient for 12/15-LO on the hyperlipidemia- and atherosclerotic-prone apolipoprotein E−/− (ApoE−/−) background were protected from the development of NAFLD [41]. In addition, plasma lipidomic analysis revealed an increase in lipoxygenase metabolites from patients with NAFLD compared to healthy patients [42]. Finally, specific markers of ER stress were reduced in liver from 12/15-LO-deficient mice fed a high-fat diet compared to wild-type controls [39]. Targeting 12/15-LO function in WAT may be a novel therapeutic target in treating local WAT and systemic organ complications associated with obesity [23,27,39] (Figure 1).
As mentioned earlier, AT is a complex milieu of cell types and therefore analysis of 12/15-LO function not only in the adipocyte will be necessary to decipher the exact role of chronic 12/15-LO activity in inflamed fat. In particular, 12/15-LO is highly expressed in the macrophage and vascular endothelial cells, components of the SVF [2,5,43]. 12/15-LO is required for interleukin-12 (IL-12) expression in the macrophage and is activated in vascular endothelial cells [5,43]. Therefore, it is clear that extensive crosstalk between 12/15-LO activity in the various cell-types will have a significant impact on regulating inflammation and ensuing adipocyte dysfunction.
12- and 15-LO Pathways in Human Adipose Tissue and Effects of Obesity
An important barrier in LO research is the limited translational potential of data due to substantial species differences in substrate preference, lipid mediators, cellular expression, and functional roles of the different LO isoforms [2]. In addition, the role of different isoforms co-expressed in various tissues was very difficult to establish until recently when isoform-specific selective inhibitors became available [44–46]. In humans, six LO isoforms have been identified: 5-LO, 12(S)-LO (platelet-type 12-LO or 12-LO), 12(R)-LO, 15-LO-1 (leukocyte-type 12-LO), 15-LO-2, and eLOX-3 (epidermis-type LO-3) (Table 1). The human 12-LO was reportedly expressed in the platelets, endothelial and smooth muscle cells of large arteries, as well as in monocytes [47,48]. In a recent publication, we reported 12-LO mRNA and protein expression in human WAT with exclusive localization in the SVF both in the subcutaneous (SC) and in the omental (OM), or visceral, fat [29]. This result does not recapitulate the localization in rodents, where adipocytes are an abundant source of 12/15-LO [20,21]. In humans, the two 15-LO isoforms have different substrate specificity and generate different lipid products. 15-LO-2 can metabolize both AA and linoleic acid (LA) with some evidence for a higher preference for AA, while 15-LO-1 has comparable affinity for AA, LA, and DHA as substrates [49]. When utilizing AA as substrate, 15-LO-1 produces 90% 15-HpETE and 10% 12-HpETE, while 15-LO-2 produces exclusively 15-HpETE [50,51]. We have shown selective expression of the 15-LO isoforms in human visceral WAT. Expression of 15-LO-2 was found in both SC and OM visceral human WAT and was exclusively localized in the SVF [29]. Intriguingly, the 15-LO-1 mRNA and protein expression were undetectable in the SC AT, but showed robust expression in OM WAT, in cells of the SVF only [29]. We also showed that all of the isoforms are expressed both in the CD34+ fraction of the SVF and in the CD34− fraction containing monocytes and various lymphocytes [29]. Furthermore, by immunohistochemistry we showed robust expression of the 15-LO-1 in the WAT vasculature. There are no reports indicating the role of the 12- and 15-LO pathways in human WAT. Increased expression of all of the 12- and 15-LO enzyme isoforms in OM vs. SC WAT suggests that the pathways may contribute to the pro-inflammatory milieu prominently associated with visceral fat in obesity [29].
Recent evidence suggests a pro-inflammatory role of 12- and 15-LO pathways in humans. 15-LO gene variants in humans are associated with induced expression of IL-6, TNF-α, and IL-1β, indicating a broad role for the enzyme in systemic inflammation [52]. Also, a recent paper demonstrated through gene array analysis that AA metabolism is the second most significantly upregulated pathway in human OM WAT compared to SC WAT in human obese subjects with a 7.6-fold higher expression of 15-LO-1 in OM fat [53]. Importantly we recently found using a lipidomic approach that both the 12- and 15-HETEs are significantly higher in OM compared to SC fat and that 12-HETE is significantly increased in the WAT of subjects with morbid obesity and type 2 diabetes compared to non-diabetic obese subjects (unpublished observations). The very limited information on 12- and 15-LO functional roles and changes with different pathological conditions in human WAT warrants future studies to identify the roles of different isoforms and the lipid mediators that are key for regulation of inflammation in human obesity and type 2 diabetes. The availability of selective inhibitors of the 12- and 15-LO enzymes will also facilitate our understanding of the pathologic roles of these enzymes in the metabolic and vascular disturbances associated with obesity.
12- and 15-LO in Adipose Tissue: Activation and Signaling Pathways
A number of stimuli for the activation and upregulation of 12- and 15-LO in rodent and human tissues have been described. Brinckmann et al. demonstrated that the presence of calcium led to the binding of 15-LO to the inner plasma membrane in human eosinophils with subsequent evidence of enzymatic activity [54]. Upregulation of 15-LO has also been seen in human blood monocytes after exposure to various cytokines, including IL-4 and IL-13 [55,56]. In vitro studies using human and mouse islet cell lines demonstrated that a combination of cytokines with pro-inflammatory activity (IL-1β, IFN-γ, and TNF-α) led to the activation of leukocyte-type 12-LO, as evidenced by the production of 12(S)-HETE [57]. In porcine aortic vascular smooth muscle cells, hyperglycemic conditions, as well as the addition of the potent vasoconstricting and pro-inflammatory hormone angiotensin II (Ang II) led to increases in leukocyte-type 12-LO mRNA and protein levels, as well as increased enzyme activity, suggesting important roles for both hyperglycemia and Ang II in the increased lipoxygenase activity seen in individuals with diabetes [58]. Ang II increases the expression of leukocyte-type12-LO in vitro in human aortic smooth muscle cells, and may have its effect via the AT1 receptor [28,47]. Hyperglycemia has also been shown to increase the expression of 12/15-LO in cultured rat renal mesangial cells [59]. Interestingly, hypoxia has been associated with upregulation of 15-LO in vivo, including in human macrophages, where 15-LO-2 is expressed [60,61]. The hormone aldosterone can also upregulate 12- and 15-LO-2 expression and activity in human vascular smooth muscle cells [62]. Models of cerebral ischemia have demonstrated that ischemic insults stimulate local 12/15-LO expression in both neurons and vascular endothelial cells [63]. Iron deficiency has also been shown to induce the expression of intestinal and hepatic 12/15-LO in iron-deficient animals [64].
The activation and signaling pathways involving 12- and 15-LO that are specific to WAT are becoming clearer. As described earlier 12/15-LO expression in white epididymal adipocytes is upregulated after high-fat feeding in mice, and the addition of palmitic acid, a saturated fatty acid found in high-fat diets, increases 12/15-LO expression in vitro in 3T3-L1 adipocytes [20]. The adipokine lipocalin-2 (LCN2), which has been associated with obesity, type 2 diabetes, and inflammation, has been demonstrated to activate 12-LO activity [65–68]. Law et al. set out to determine what role LCN2 deficiency would have on systemic insulin sensitivity, and discovered that LCN2 knockout (LCN2-KO) mice had significantly reduced 12-LO activity in epididymal WAT, as assessed by reduced concentrations of the metabolite 12(S)-HETE [66]. There were no differences in 12(S)-HETE concentrations in liver or muscle, suggesting that LCN2 plays a role in 12-LO activation that is specific to WAT. Further, overexpression of LCN2 in LCN2-KO mice (through the use of a recombinant adenovirus) was associated with significant increases in the expression of 12-LO and in 12(S)-HETE production, specifically in WAT. The authors measured the concentration of the inflammatory cytokine TNF-α, that is typically produced downstream of 12-LO activity, and showed that the WAT of LCN2-KO animals expressed less TNF-α, and that overexpression of LCN2 was associated with an increase in TNF-α production. This increase in TNF-α was blocked by the addition of the previously described 12-LO inhibitor, CDC [66]. Of note, the authors found that LCN2 deficiency was associated with protection from the insulin resistance seen in aging and obesity and concluded that these changes were in part mediated by changes in 12-LO activity.
As previously discussed there is evidence that different components of the renin-angiotensin system (RAS), and in particular Ang II, may play a role in the regulation of 12/15-LO activity [47,58] (Figure 1). 12(S)-HETE has been shown to increase AT1 receptor expression in the glomeruli of type 2 diabetic rats [69]. Abdel-Rahman et al. demonstrated that valsartan blocked 12(S)-HETE production in the renal interstitium of streptozotocin-induced diabetic rats [70]. Blockade of the angiotensin type 2 receptor (AT2) with the inhibitor PD123319 had no effect on 12(S)-HETE production, indicating a primary role for the AT1 receptor in the mechanism linking Ang II with 12-LO activity. Interestingly the combination of AT1 and AT2 receptor blockade led to high levels of 12(S)-HETE, suggesting that the AT2 receptor may also have a role in 12-LO activity regulation. Studies in the obese Zucker rat have shown increased expression of 12/15-LO in the renal cortex, and that this expression can be blocked with the AT1 receptor blocker losartan [71]. In regard to WAT, as previously described, our laboratory has provided evidence that C57BL6/J mice fed a high-fat Western diet have an increase in the expression of the platelet-type 12-LO in adipocytes, that is abolished with the addition of the AT1 receptor blocker valsartan [28]. Further studies regarding the role that the RAS plays in the activation of 12- and 15-LO in WAT are needed.
The cytokine interleukin-12 (IL-12) is one primary cytokine through which the products of 12/15-LO lead to increased inflammation and WAT dysfunction (Figure 1). IL-12 is necessary for the development of pro-inflammatory T helper cells (Th1 cells) [72]. IL-12 signaling activates the signal transducer and activator of transcription 4 (STAT4), a transcription factor that has been associated with autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, and Sjögren’s syndrome [73]. IL-12 and STAT4 have also been implicated in the inflammatory actions of 12/15-LO in AT. As described previously, Chakrabarti et al. demonstrated that 12/15-LO mRNA was increased 8-fold in visceral adipocytes from obese Zucker rats compared with lean controls [21]. 12-HETE also was increased 2-fold and was associated with increased adipocyte-derived expression of the inflammatory cytokines IL-6, MCP-1, TNF-α, IL-1α, and IL-1β. Treating obese Zucker rats with the anti-inflammatory small molecule lisofylline (LSF), 1-(5-R-hydroxyhexyl)-3,7-dimethylaxanthine, which reduces the phosphorylation and activity of STAT4, and thereby downregulates the inflammatory effects of the IL-12 pathway, was shown to reduce the levels of 12/15-LO, as well as IL-6, MCP-1, and IL-1α in both adipocytes and AT. Immunohistochemical analysis of WAT from obese Zucker rats treated with LSF had reduced staining for 12/15-LO compared to untreated obese Zucker rats and lean controls. Furthermore, immunohistochemical staining for MCP-1 and western blot analysis for IL-6 were reduced in the WAT from the LSF-treated obese Zucker rats. Immunohistochemical studies also showed that phosphorylated-STAT4 (p-STAT4) was upregulated in WAT from obese Zucker rats when compared with control lean animals, and treatment with LSF reduced p-STAT4 expression as seen by immunohistochemistry of WAT and western blot analysis of adipocytes from obese Zucker rats treated with LSF. In 3T3-L1 adipocytes LSF was shown to reduce the expression of the inflammatory cytokines IL-6, MCP-1, and IL-12p40. Interestingly treatment with LSF was associated with increased WAT mass, and increased feeding efficiency in obese rats, without having an effect on total body weight. The authors hypothesized that LSF can improve the storage of fatty acids by adipocytes, and may perhaps reduce the deposition of ectopic fat that has been associated with reductions in insulin sensitivity. Of note, LSF reduces the insulin resistance seen in animals fed a high-fat diet [74].
Identification of receptors for lipid metabolites generated by 12- and 15-LO are also underway. An exciting study published recently by Guo et al. described a plasma membrane orphan G protein-coupled receptor (GPCR31) that displayed high affinity for 12(S)-HETE [75]. The authors defined this as the 12(S)-HETE receptor (12-HETER). This orphan receptor was cloned from a human prostate cancer cell line. These authors had previously demonstrated that 12(S)-HETE signaling involved PKC and the tyrosine kinase Src, as well as extracellular signal-related kinase 1/2 (ERK 1/2) and phosphatidylinositol 3-kinase [76–78]. In the latest study the authors showed that 12(S)-HETE binding to the 12-HETER led to NFκB activation, as well as activation of the protein kinases ERK 1/2 and MEK. The role that this receptor may play in the adipocyte and in WAT is unknown at this time. Interestingly the authors reported performing an analysis of array data in the Gene Expression Omnibus (GEO) that suggested a possible role for the 12-HETER in multiple diseases, including Alzheimer’s disease and diabetic nephropathy. It will be important to see if there is any role for this receptor in the metabolic dysfunction that occurs in obesity and diabetes.
Many other pathways may be involved in 12- and 15-LO signaling in WAT. The incretin pathway may play a role in the regulation of 12- and 15-LO and its metabolites in WAT. The dipeptidyl peptidase IV inhibitor sitagliptin was shown to reduce adipocyte and WAT mRNA expression of 12/15-LO in C57Bl/6J mice [79]. In the insulin-producing α-cell of the pancreas, 12(S)-HETE may have its detrimental effects by stimulating the stress-activated p38-mitogen-activated protein kinase pathway [80]. Whether or not this pathway is involved in 12- and 15-LO-regulated WAT inflammation is not known at this time and warrants further study. 12- and 15-LO products have been shown to stimulate protein kinase C (PKC) activity in rat adrenal glomerulosa cells [81], and 12-HETE also leads to increased c-Jun amino-terminal kinase (JNK) activity, as well as increased p21-activated kinase activity, in a Chinese hamster ovary fibroblast cell line overexpressing the rat vascular type-1a Ang II receptor [82,83]. As described previously studies in 3T3-L1 adipocytes have demonstrated that 12/15-LO products (12(S)-HETE and 12(S)-HpETE) increase JNK-1 phosphorylation, and that this was associated with impaired insulin signaling [20], and again, in a recent publication our laboratory reported that the addition of 12(S)-HETE and 12(S)-HpETE to 3T3-L1 adipocytes was associated with the activation of various ER stress markers, including p-IRE1α, BiP, p-PERK, and XBP-1 [39]. ER stress could be attenuated through the addition of the 12/15-LO inhibitor, CDC [39]. Therefore, there are a number of pathways likely involved in the 12- and 15-LO activation and signaling in WAT.
Anti-inflammatory Roles of 12- and 15-LO in Adipose Tissue
Abundant evidence reviewed above emphasizes the pro-inflammatory role of the 12- and 15- lipoxygenase pathways in WAT, including in response to high-fat feeding [2]. Up-regulation of the 12/15-LO pathway has effects on insulin resistance, pancreatic β-cell function, and ER stress via paracrine and endocrine actions of the bioactive lipids and pro-inflammatory cytokines produced in WAT as a result of 12/15-LO activation. However, there is evidence that the 12- and 15-LO pathways may also generate metabolites that are key in the resolution of inflammation [84–86] (Figure 2). In particular, 12/15-LO may generate ω-6 PUFA metabolites such as lipoxins, or ω-3 PUFA metabolites such as maresins, resolvins and protectins [87,88]. The process may involve multiple LO isoforms and may therefore be para-cellular involving different cell types and complexly regulated [89,90].
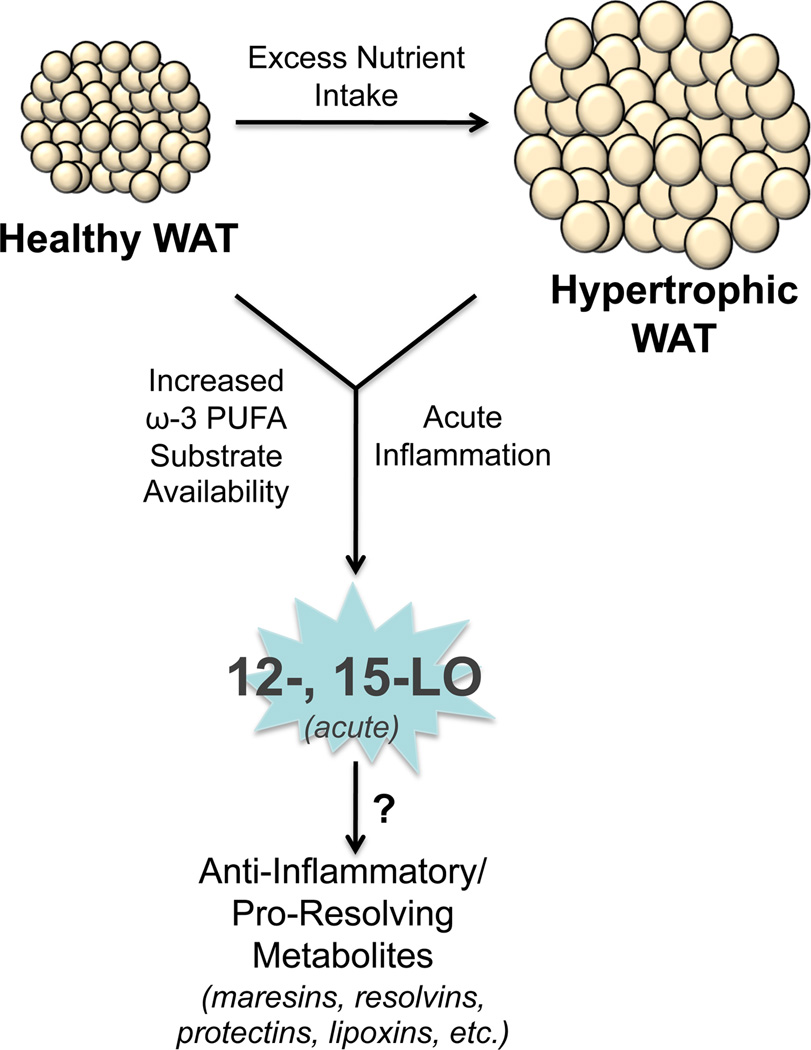
Figure 2.
The role of 12- and 15-LO in normal adipose tissue. Diets enriched in ω-3 fatty acids or acute inflammatory responses may activate the 12- and 15-LO enzymes in normal or inflamed hypertrophic WAT to generate anti-inflammatory or pro-resolving lipid metabolites, such as the maresins, resolvins, protectins, and lipoxins.
Obesity is a state of chronic inflammation resulting in impaired lipid partitioning by the adipocyte and production of pro-inflammatory cytokines and lipid metabolites. This dysfunction is likely the result of an inappropriate inflammatory response that remains uncontrolled due to intrinsic inability of the tissue for complete resolution of inflammation. The pro-resolving metabolites formed as a result of different lipoxygenases may therefore play beneficial effects for limiting inflammation in the WAT as a result of nutritional overload. In particular, resolvin E1 decreases T cell migration, reduces TNF-α and IFN-γ secretion, inhibits chemokine formation, and blocks IL-1-induced NFkB activation [91–93]. Importantly the receptor for resolvin E1 was identified as the G protein-coupled receptor ChemR23 which is expressed on monocytes, dendritic cells, and adipocytes [94]. Although there is no published evidence, it is tempting to speculate that resolvin E1 may have potentially important anti-inflammatory roles in chronically inflamed fat by reducing inflammatory cell infiltrate and limiting pro-inflammatory cytokine formation by adipocytes and macrophages.
The types and relative amounts of lipid metabolites generated in a tissue that expresses different 12- and 15-LO isoforms are dependent not only on 12- and 15-LO expression but also on substrate availability, local metabolite concentrations, and local partial oxygen pressure. Increased availability of the ω-3 vs. ω-6 fatty acids could lead to the predominant formation of lipid metabolites with anti-inflammatory properties. Interestingly, WAT is the major storage site for PUFAs in obese individuals [95]. Studies on effects of EPA and DHA on WAT showed convincingly that dietary supplementation with these ω-3 fatty acids have anti-inflammatory actions in WAT (Figure 2). DHA supplementation in the diets of high-fat diet-fed mice revealed that resolvin D1 and its precursor DHA lead to the resolution of adipose tissue inflammation by favoring an anti-inflammatory M2 phenotype in WAT macrophages and by blunting Th1 cytokine secretion (IL-6, MCP-1, and TNF-α) [96]. Moreover, in human interventional studies, supplementation with ω-3 PUFAs was associated with improved insulin sensitivity, improved lipid profiles, and reduced inflammation during the management of weight loss in overweight hyperinsulinemic women [97]. A recent study showed that the 17-hydroxydocosahexaenoic acid (17-HDHA), a metabolite generated by the action of 15-LO on DHA, is a more potent PPARγ agonist than DHA itself [98]. 17-HDHA along with protectin D1 and resolvin D1 were identified in WAT of leptin-deficient, obese ob/ob mice [99]. Interestingly, products of the 12- and 15-LO pathways were among the most abundant eicosanoids produced in WAT of genetically obese mice suggesting a key role of the latter in the inflamed WAT [99]. In addition to the substrate availability, nutritional overload seems to play a key role in regulation of the balance between the formation of pro- vs. anti-inflammatory 12- and 15-LO lipid products. The importance of substrate availability in the metabolite formation was recently emphasized in a paper by Merched et al. [100]. The authors showed that a high-fat diet prevents the formation and action of lipoxin A4 (LXA4) in ApoE−/− mice that overexpress 12/15-LO. While on chow diet the mice are protected from developing atherosclerosis, but when placed on a high-fat diet, they display a more severe atherosclerotic phenotype due to the inability of LXA4 to exert its anti-inflammatory action [100]. Also, a recent paper suggests that alternatively activated macrophages expressing 12/15-LO act as a sink for distinct soluble receptors for apoptotic cells via the oxidized phospholipids on plasma membranes therefore allowing the controlled phagocytosis of the apoptotic cells by the resident tissue macrophages and contributing to the maintenance of self-tolerance [101].
Collectively these studies suggest the complexity of the spatial and temporal regulation of the 12- and 15-LO pathways in response to nutritional overload and obesity. It is tempting to speculate that an early response to high-fat feeding in WAT results in the up-regulation of the 12- and 15-LO pathways and downstream formation of 12- and 15-HETEs that contribute to development of insulin resistance and local WAT inflammation via recruitment of immune cells and local production of pro-inflammatory cytokines. Also, a switch in the product formation of different LO isoforms may contribute to the downstream production of pro-inflammatory lipid mediators. This switch may be the result of changes in the substrate availability or local conditions such as changes in partial oxygen pressure. For example, a recent paper elegantly demonstrated that 5-LO may entirely switch its product from 5-HETE and leukotriene formation to 15-HETE production as a result of a mutation that mimics phosphorylation of a serine residue [102]; although not yet shown, this change could be achieved in vivo by the MAPK ERK1/2 [102]. It is well documented that ERK1/2 activation occurs as an early response to high-fat diet exposure or in insulin resistant states. Therefore this may be one of the mechanisms responsible for a switch in the eicosanoid metabolites in early stages of nutrient overload and impaired glucose homeostasis. Dietary supplementation with EPA and DHA may be therefore beneficial in obesity as it could lead to formation of the pro-resolving mediators of the 12- and 15-LO pathways, resulting in anti-inflammatory actions in WAT and an improved metabolic phenotype (Figure 2). While understanding the mechanisms that control the regulation of the 12- and 15-LO pathways require substantial future efforts, the contribution of this pathway to inflammation in WAT in obesity is well established in animal models and future studies may demonstrate its key importance in humans.
Conclusion
Much is known about the role that the 12- and 15-LOs and their products play in both health and disease states. Their specific contribution in the process of inflammation is becoming clear, and as inflammation plays a major role in the development of two of the great epidemics of our time, obesity and diabetes, we must learn more about how these enzymes might be manipulated in order to develop therapeutic agents [103,104]. We must also study how our Western diet increases inflammation, and what role 12- and 15-LO pathways may play in that process [105]. Identification of downstream signaling components of the 12- and 15-LO enzymes as well as the recent discovery of the GPCR for 12(S)-HETE will aid in our understanding of these LOs. Furthermore, the development and description of selective inhibitors of human reticulocyte 15-LO-1 and human platelet-type 12-LO provide exciting opportunities for future research into the function (and dysfunction) of these enzymes [44–46].
The evidence points towards a role for 12/15-LO activity in WAT in modulating chronic local inflammation and subsequent systemic metabolic decline in the obese state. However, little is known as to the role of 12- and 15-LOs in BAT. Indeed, in addition to WAT, platelet- and leukocyte-type 12-LO are expressed in BAT [19]. Unpublished observations from our lab also reveal that 12/15-LO expression is not increased in BAT when mice are fed a high-fat diet. Therefore, deciphering the mechanism of 12/15-LO activation in WAT and BAT as well as products generated in these fat depots by 12/15-LO will reveal insights into 12/15-LO function.
Though we have learned much in the last three decades regarding the mechanisms and effects of the 12- and 15-LOs and their products, much work still needs to be done in order to understand these enzymes and their role in health and disease. Future research needs to focus on the roles that the 12- and 15-LOs play in adipocyte and AT function, as these endocrine organs play a critical role in the development and maintenance of both obesity and insulin resistance. In the coming years we will see much of this science translated from the bench to the bedside, and a better understanding of these enzymes will serve as an important weapon in our armory when combating obesity and diabetes.
Highlights.
12- and 15-lipoxygenases are expressed in many tissues, including adipose tissue.
12- and 15-lipoxygenases can generate pro- and anti-inflammatory metabolites.
12-lipoxygenase activity in adipocytes is required for adipocyte differentiation.
12- and 15-lipoxygenases in obesity promote the onset of metabolic dysfunction.
Therapeutics against 12- and 15-lipoxygenases may treat metabolic dysfunction.
Acknowledgments
We would like to thank Swarup K. Chakrabarti for collaborative studies pertaining to the role of leukocyte-type 12-LO in rodent and human adipocytes. We would also like to acknowledge that the National Institutes of Health P01 HL55798 and R01 HL112605 grants and a National Institutes of Health NRSA Postdoctoral Research Fellowship F32 DK085716 supported many studies from our lab referenced in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
BKC, DCL, and ADD wrote the manuscript; JLN critically reviewed and updated the manuscript. All authors have approved the final article.
References
- 1.Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci. 2000;79(7):961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 2.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50(1):115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell VB, Maskrey B, Taylor GW. Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol. 2009;462:5–23. [PubMed] [Google Scholar]
- 4.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(9):1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 6.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107(10):1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Clemente M, Clària J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Curr Opin Clin Nutr Metab Care. 2011;14(4):347–353. doi: 10.1097/MCO.0b013e32834777fa. [DOI] [PubMed] [Google Scholar]
- 9.Reddy GR, Ueda N, Suzuki T, Yamamoto S, Ishimura K, Kawada N, et al. Characterization of arachidonate 12-lipoxygenase found in the liver of mongrel dog and its immunohistochemical localization in neutrophils. Tokushima J Exp Med. 1995;42(1–2):27–35. [PubMed] [Google Scholar]
- 10.Yokota S, Oda T, Fahimi HD. The role of 15-lipoxygenase in disruption of the peroxisomal membrane and in programmed degradation of peroxisomes in normal rat liver. J Histochem Cytochem. 2001;49(5):613–622. doi: 10.1177/002215540104900508. [DOI] [PubMed] [Google Scholar]
- 11.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 13.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54(9):1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 14.Hallenborg P, Jørgensen C, Petersen RK, Feddersen S, Araujo P, Markt P, et al. Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity. Mol Cell Biol. 2010;30(16):4077–4091. doi: 10.1128/MCB.01806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270(41):23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 16.Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VK, O'Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2001;276(12):9149–9157. doi: 10.1074/jbc.M009817200. [DOI] [PubMed] [Google Scholar]
- 17.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 18.Hausman GJ, Campion DR, Martin RJ. Search for the adipocyte precursor cell and factors that promote its differentiation. J Lipid Res. 1980;21(6):657–670. [PubMed] [Google Scholar]
- 19.Madsen L, Petersen RK, Sørensen MB, Jørgensen C, Hallenborg P, Pridal L, et al. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. Biochem J. 2003;375(Pt 3):539–549. doi: 10.1042/bj20030503. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity. 2009;17(9):1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am J Physiol Endocrinol Metab. 2011;300(1):E175–E187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, et al. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295(5):E1065–E1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nonogaki K, Fuller GM, Fuentes NL, Moser AH, Staprans I, Grunfeld C, et al. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology. 1995;136(5):2143–2149. doi: 10.1210/endo.136.5.7720663. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51(10):2929–2935. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 27.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, et al. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4(9):e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55(3):715–721. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobrian AD, Lieb DC, Ma Q, Lindsay JW, Cole BK, Ma K, et al. Differential expression and localization of 12/15 lipoxygenases in adipose tissue in human obese subjects. Biochem Biophys Res Commun. 2010;403(3–4):485–490. doi: 10.1016/j.bbrc.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horrillo R, González-Périz A, Martínez-Clemente M, López-Parra M, Ferré N, Titos E, et al. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184(7):3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 31.Boden G. Endoplasmic reticulum stress: another link between obesity and insulin resistance/inflammation? Diabetes. 2009;58(3):518–519. doi: 10.2337/db08-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29(1):42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 33.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57(9):2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93(11):4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsutsumi A, Motoshima H, Kondo T, Kawasaki S, Matsumura T, Hanatani S, et al. Caloric restriction decreases ER stress in liver and adipose tissue in ob/ob mice. Biochem Biophys Res Commun. 2011;404(1):339–344. doi: 10.1016/j.bbrc.2010.11.120. [DOI] [PubMed] [Google Scholar]
- 39.Cole BK, Kuhn NS, Green-Mitchell SM, Leone KA, Raab RM, Nadler JL, et al. 12/15-Lipoxygenase signaling in the endoplasmic reticulum stress response. Am J Physiol Endocrinol Metab. 2012;302(6):E654–E665. doi: 10.1152/ajpendo.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez-Clemente M, Ferré N, Titos E, Horrillo R, González-Périz A, Morán-Salvador E, et al. Disruption of the 12/15-lipoxygenase gene (Alox15) protects hyperlipidemic mice from nonalcoholic fatty liver disease. Hepatology. 2010;52(6):1980–1991. doi: 10.1002/hep.23928. [DOI] [PubMed] [Google Scholar]
- 42.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50(6):1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao L, Cuff CA, Moss E, Wille U, Cyrus T, Klein EA, et al. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J Biol Chem. 2002;277(38):35350–35356. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]
- 44.Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB, 2nd, et al. Discovery of potent and selective inhibitors of human platelet-type 12-lipoxygenase. J Med Chem. 2011;54(15):5485–5497. doi: 10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rai G, Jadhav A, Schultz L, Kenyon V, Leister W, Simeonov A, et al. Probe Reports from the NIH Molecular Libraries Program [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2010. Selective small molecule inhibitors of 12-human lipoxygenase (12-hLO). 2009 Nov 30 [Updated 2011 Mar 25] [PubMed] [Google Scholar]
- 46.Rai G, Kenyon V, Jadhav A, Schultz L, Armstrong M, Jameson JB, et al. Discovery of potent and selective inhibitors of human reticulocyte 15-lipoxygenase-1. J Med Chem. 2010;53(20):7392–7404. doi: 10.1021/jm1008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol. 1995;15(7):942–948. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- 48.Chen XS, Funk CD. Structure-function properties of human platelet 12-lipoxygenase: chimeric enzyme and in vitro mutagenesis studies. Faseb J. 1993;7(8):694–701. doi: 10.1096/fasebj.7.8.8500694. [DOI] [PubMed] [Google Scholar]
- 49.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci USA. 1997;94(12):6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn H, Borngraber S. Mammalian 15-lipoxygenases. Enzymatic properties and biological implications. Adv Exp Med Biol. 1999;447:5–28. [PubMed] [Google Scholar]
- 51.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 52.Fairfax BP, Vannberg FO, Radhakrishnan J, Hakonarson H, Keating BJ, Hill AV, et al. An integrated expression phenotype mapping approach defines common variants in LEP, ALOX15 and CAPNS1 associated with induction of IL-6. Hum Mol Genet. 2010;19(4):720–730. doi: 10.1093/hmg/ddp530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123(2):186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kühn H. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91(1):64–74. [PubMed] [Google Scholar]
- 55.Nassar GM, Morrow JD, Roberts LJ, 2nd, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269(44):27631–27634. [PubMed] [Google Scholar]
- 56.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci USA. 1992;89(1):217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48(3):486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- 58.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, et al. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA. 1993;90(11):4947–4951. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, et al. 12-lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int. 2001;59(4):1354–1362. doi: 10.1046/j.1523-1755.2001.0590041354.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res. 2003;92(9):992–1000. doi: 10.1161/01.RES.0000070881.65194.8F. [DOI] [PubMed] [Google Scholar]
- 61.Rydberg EK, Krettek A, Ullström C, Ekström K, Svensson P-A, Carlsson LMS, et al. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages. Arterioscler Thromb Vasc Biol. 2004;24(11):2040–2045. doi: 10.1161/01.ATV.0000144951.08072.0b. [DOI] [PubMed] [Google Scholar]
- 62.Limor R, Kaplan M, Sharon O, Knoll E, Naidich M, Weisinger G, et al. Aldosterone up-regulates 12- and 15-lipoxygenase expression and LDL oxidation in human vascular smooth muscle cells. J Cell Biochem. 2009;108(5):1203–1210. doi: 10.1002/jcb.22352. [DOI] [PubMed] [Google Scholar]
- 63.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39(9):2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collins JF, Hu Z, Ranganathan PN, Feng D, Garrick LM, Garrick MD, et al. Induction of arachidonate 12-lipoxygenase (Alox15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G948–G962. doi: 10.1152/ajpgi.00274.2007. [DOI] [PubMed] [Google Scholar]
- 65.Esteve E, Ricart W, Fernández-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32(Suppl 2):S362–S367. doi: 10.2337/dc09-S340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Law IKM, Xu A, Lam KSL, Berger T, Mak TW, Vanhoutte PM, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59(4):872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Lam KSL, Kraegen EW, Sweeney G, Zhang J, Tso AWK, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53(1):34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 68.Yan Q-W, Yang Q, Mody N, Graham TE, Hsu C-H, Xu Z, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56(10):2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 69.Xu Z-G, Miao L-N, Cui Y-C, Jia Y, Yuan H, Wu M. Angiotensin II type 1 receptor expression is increased via 12-lipoxygenase in high glucose-stimulated glomerular cells and type 2 diabetic glomeruli. Nephrol Dial Transplant. 2009;24(6):1744–1752. doi: 10.1093/ndt/gfn703. [DOI] [PubMed] [Google Scholar]
- 70.Abdel-Rahman EM, Abadir PM, Siragy HM. Regulation of renal 12(S)-hydroxyeicosatetraenoic acid in diabetes by angiotensin AT1 and AT2 receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1473–R1478. doi: 10.1152/ajpregu.90699.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Z-G, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, et al. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney reversal by angiotensin II type 1 receptor blockade. Circulation. 2005;111(15):1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- 72.Coon ME, Diegel M, Leshinsky N, Klaus SJ. Selective pharmacologic inhibition of murine and human IL-12-dependent Th1 differentiation and IL-12 signaling. J Immunol. 1999;163(12):6567–6574. [PubMed] [Google Scholar]
- 73.Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: Genetics, mechanisms, and implications for autoimmunity review for current allergy and asthma reports. Curr Allergy Asthma Rep. 2008;8(5):398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frangioudakis G, Garrard J, Raddatz K, Nadler JL, Mitchell TW, Schmitz-Peiffer C. Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology. 2010;151(9):4187–4196. doi: 10.1210/en.2010-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly A-K, et al. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286(39):33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu B, Khan WA, Hannun YA, Timar J, Taylor JD, Lundy S, et al. 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc Natl Acad Sci USA. 1995;92(20):9323–9327. doi: 10.1073/pnas.92.20.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szekeres CK, Tang K, Trikha M, Honn KV. Eicosanoid activation of extracellular signal-regulated kinase1/2 in human epidermoid carcinoma cells. J Biol Chem. 2000;275(49):38831–38841. doi: 10.1074/jbc.M002673200. [DOI] [PubMed] [Google Scholar]
- 78.Szekeres CK, Trikha M, Honn KV. 12(S)-HETE, pleiotropic functions, multiple signaling pathways. Adv Exp Med Biol. 2002;507:509–515. doi: 10.1007/978-1-4615-0193-0_78. [DOI] [PubMed] [Google Scholar]
- 79.Dobrian AD, Ma Q, Lindsay JW, Leone KA, Ma K, Coben J, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300(2):E410–E421. doi: 10.1152/ajpendo.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase products reduce insulin secretion and B-Cell viability in human islets. JCEM. 2010;95(2):887–893. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Natarajan R, Lanting L, Xu L, Nadler J. Role of specific isoforms of protein kinase C in angiotensin II and lipoxygenase action in rat adrenal glomerulosa cells. Mol Cell Endocrinol. 1994;101(1–2):59–66. doi: 10.1016/0303-7207(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 82.Wen Y, Scott S, Liu Y, Gonzales N, Nadler JL. Evidence that angiotensin II and lipoxygenase products activate C-Jun NH2-terminal kinase. Circ Res. 1997;81(5):651–655. doi: 10.1161/01.res.81.5.651. [DOI] [PubMed] [Google Scholar]
- 83.Wen Y, Gu J, Knaus UG, Thomas L, Gonzales N, Nadler JL. Evidence that 12-lipoxygenase product 12-hydroxyeicosatetraenoic acid activates p21-activated kinase. Biochem J. 2000;349(Pt 2):481–487. doi: 10.1042/0264-6021:3490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–S205. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serhan CN, Romano M. Lipoxin biosynthesis and actions: role of the human platelet LX-synthase. J Lipid Mediat Cell Signal. 1995;12(2–3):293–306. doi: 10.1016/0929-7855(95)00035-o. [DOI] [PubMed] [Google Scholar]
- 90.Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990;85(3):772–780. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280(52):43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 92.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 93.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278(44):43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 94.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lundbom J, Heikkinen S, Fielding B, Hakkarainen A, Taskinen MR, Lundbom N. PRESS echo time behavior of triglyceride resonances at 1.5T: detecting omega-3 fatty acids in adipose tissue in vivo. J Magn Reson. 2009;201(1):39–47. doi: 10.1016/j.jmr.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 96.Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187(10):5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 97.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, et al. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30(10):1535–1544. doi: 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
- 98.Morán-Salvador E, López-Parra M, García-Alonso V, Titos E, Martínez-Clemente M, González-Périz A, et al. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25(8):2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 99.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. Faseb J. 2009;23(6):1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics. 2011;4(1):12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 36(5):834–846. doi: 10.1016/j.immuni.2012.03.010. 25. [DOI] [PubMed] [Google Scholar]
- 102.Gilbert NC, Rui Z, Neau DB, Waight MT, Bartlett SG, Boeglin WE, et al. Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663. Faseb J. Apr 18; doi: 10.1096/fj.12-205286. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 104.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55(4):379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 105.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]