Abstract
Background and Purpose
This study was performed to test the hypothesis that spinal cord radiosensitivity is significantly modified by uniform versus laterally non-uniform dose distributions.
Materials and Methods
A uniform dose distribution was delivered to a 4.5–7.0cm length of cervical spinal cord in 22 mature Yucatan minipigs for comparison with a companion study in which a laterally non-uniform dose was given[1]. Pigs were allocated into four dose groups with mean maximum spinal cord doses of 17.5±0.1 Gy(n=7), 19.5±0.2 Gy(n=6), 22.0±0.1 Gy(n=5), and 24.1±0.2 Gy(n=4). The study endpoint was motor neurologic deficit determined by a change in gait within one year. Spinal cord sections were stained with a Luxol fast blue/periodic acid Schiff combination.
Results
Dose-response curves for uniform versus non-uniform spinal cord irradiation were nearly identical with ED50's (95% confidence interval) of 20.2 Gy(19.1–25.8) and 20.0 Gy(18.3–21.7), respectively. No neurologic change was observed for either dose distribution when the maximum spinal cord dose was ≤17.8 Gy while all animals experienced deficits at doses ≥21.8Gy.
Conclusion
No dose-volume effect was observed in pigs for the dose distributions studied and the endpoint of motor neurologic deficit; however, partial spinal cord irradiation resulted in less debilitating neurologic morbidity and histopathology.
Keywords: dose-volume effect, spinal cord tolerance, normal tissue tolerance, stereotactic spinal radiosurgery, swine
Introduction
Dose-volume effects are of great significance in radiation therapy and have been summarized for many organs by the Quantitative Analysis of Normal Tissue Effect in the Clinic (QUANTEC) collaboration[2]. Early efforts to investigate dose-volume effects in the spinal cord were limited to characterizing the influence of irradiated length on response[3–5]. Pioneering work in frame-based spinal radiosurgery at the University of Arizona[6], followed by the development of image-guidance and dose-shaping technologies, provided tools to localize and irradiate lesions of the spine while minimizing dose to the spinal cord but the effect of the resulting complex dose distributions on spinal cord tolerance was unknown. Since 2001, many studies performed in rats have demonstrated that spinal cord tolerance is modified by non-uniform dose distributions[7] including steep lateral dose gradients[8–10], longitudinal dose inhomogeneity[3, 11], and selective regional irradiation[9]. These studies were never repeated in a large animal. A general perception that dose-volume effects play a role in human spinal cord tolerance permeates the radiosurgery literature[12–15].
In this study, pigs received de novo single-session irradiation using a uniform dose distribution for comparison to a previous study in which the same cervical spinal cord segments received a steep lateral dose gradient[1]. Spinal cord tolerance to uniform irradiation has not previously been studied in a large animal under conditions relevant to clinical stereotactic body radiotherapy and the lateral dose-volume effect has not previously been studied in a model with spinal cord dimensions equivalent to humans. The inclusion of human clinical treatment parameters in this study may be responsible for the difference between these results and those of previous studies and may serve to refine models of normal tissue complication probability[16].
Methods and Materials
This study conformed to all national and local regulations regarding the use of animals for research and was approved by the Institutional Animal Care and Use Committee. A total of 50 female Yucatan minipigs were enrolled to study dose-volume effects in the cervical spinal cord. Pigs were randomly assigned to receive either uniform or partial-volume single-session irradiation to the same spinal cord segments. Twenty-two pigs received uniform irradiation and are described here. Twenty-six pigs were assigned to receive partial-volume irradiation and have been reported previously[1]. Two pigs served as unirradiated controls.
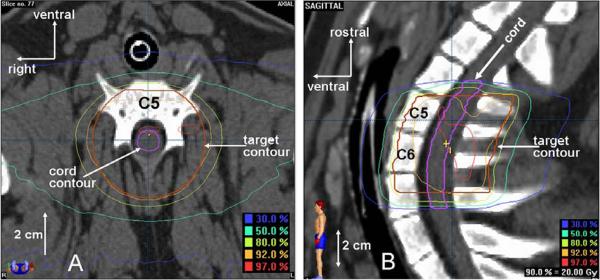
Animals that received uniform spinal cord irradiation were 42–102 weeks old and weighed approximately 35–60 kg when irradiated. Treatment parameters for all individual animals are presented in Table 1 including: a) prescription dose group, b) irradiated spinal cord length, c) irradiated spinal cord level, d) image-guidance/irradiation platform, e) maximum spinal cord dose, f) age, g) followup period, h) latency to response, and i) overall treatment time. All animals received a treatment planning CT scan with 0.75–1.5 mm thick slices and a 300–500 cm field of view. Treatment planning calculations were performed using either Brainscan 5.31 software (BrainLAB, AG, Feldkirchen) or Pinnacle3 8.0m (Philips Electronics N.V., Eindhoven). Radiation was delivered in a single session to a cylindrical target volume 4.5–7.0 cm in length and 5 cm in diameter that was centered on the spinal cord. In the rostral/caudal direction, the target volume was centered at the level of the sixth cervical vertebral body. Dose distributions in the axial and sagittal planes are shown in Figure 1. Treatment plans consisted of a series of 4–6 dynamically-shaped arcs or 12 conformal fields arranged with the goal of creating a uniform dose distribution through the spinal cord. The spinal cord volume was defined on CT images by contracting the thecal sac contour by 1.5mm in the axial plane. This method was based on CT/MRI fusion of two animals and is consistent with the method used in a companion study[1]. The spinal cord was contoured 5.5–6.5 mm beyond the irradiated volume in the rostral and caudal directions and the dose calculation grid resolution through the spinal cord ranged from 1.5–1.8 mm. Dose distributions were normalized to the global plan maximum and dose was prescribed to the 90% isodose line. Animals were stratified into 4 prescription dose groups as follows: 22 Gy (4), 20 Gy (5), 18 Gy (6), and 16 Gy (7). Treatment planning dose-volume histogram statistics for the spinal cord are summarized in Table 2 including: a) mean maximum point dose, b) mean percentage volume to receive ≥10 Gy, c) mean volume to receive ≥14 Gy, and d) mean maximum dose to 1 cc volume. For all procedures, animals were anesthetized with a mixture of Telazol® and xylazine and maintained on isoflurane. Animals were positioned supine in a body-length, vacuum-molded immobilization cushion that was individually molded for each pig and kept unchanged between simulation and irradiation. Image-guided localization was performed either using stereoscopic kilovoltage x-rays (Novalis Body X-ray 6D, BrainLAB AG) or conebeam computed tomography (CBCT) (XVI, Elekta AB, Stockholm). For stereoscopic image guidance, a pair of digital radiographs was exposed and automatically fused with digitally reconstructed radiographs (DRR's) generated from the pre-treatment CT scan to determine if any positioning adjustments were necessary. After visual evaluation of the fusion results, the treatment table was shifted until the actual position and required treatment position differed by less than 1 mm in the three primary axes. The image-guidance process was repeated following table adjustments to ensure that shifts were made correctly. The same general positioning and verification processes were followed for CBCT image guidance.
Table 1.
Dose and time parameters for individual irradiated animals.
| ID # | Rx Dose (Gy) | Irradiated Spinal Cord Length (cm) | Irradiated Spinal Cord Level | Image-Guidance / Irradiation Platform | Maximum Spinal Cord Dose (Gy) | Age at SRS (weeks) | Follow-up Period (weeks) | Latency (weeks ) | Overall Tx Time (min) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 4.5 | midC4–midC7 | X/N | 17.6 | 45 | 56 | NA* | 14 |
| 2 | 16 | 5.1 | midC4–midC7 | X/N | 17.6 | 43 | 55 | NA* | 10 |
| 3 | 16 | 6.8 | C5–C7 | X/N | 17.4 | 45 | 52 | NA* | 11 |
| 4 | 16 | 7.0 | C5–C7 | X/N | 17.4 | 45 | 53 | NA* | 14 |
| 5 | 16 | 7.0 | C5–C7 | X/N | 17.4 | 45 | 53 | NA* | 8 |
| 6 | 16 | 7.0 | C5–C7 | X/N | 17.6 | 45 | 53 | NA* | 10 |
| 7 | 16 | 7.0 | C5–C7 | X/N | 17.8 | 46 | 53 | NA* | 10 |
| 8 | 18 | 5.1 | C5–midC7 | X/N | 19.2 | 102 | 25 | 8 | 12 |
| 9 | 18 | 5.1 | C5–midC7 | X/N | 19.4 | 99 | 54 | NA* | 12 |
| 10 | 18 | 5.1 | C5–midC7 | X/N | 19.2 | 99 | 54 | NA* | 10 |
| 11 | 18 | 5.1 | midC4–midC7 | V/S | 19.7 | 46 | 54 | NA* | 22 |
| 12 | 18 | 5.1 | midC4–midC7 | V/S | 19.7 | 46 | 51 | NA* | 25 |
| 13 | 18 | 5.1 | midC4–midC7 | V/S | 19.7 | 47 | 52 | NA* | 25 |
| 14 | 20 | 5.1 | midC4–midC7 | X/N | 21.8 | 43 | 55 | 10 | 12 |
| 15 | 20 | 5.1 | midC4–midC7 | X/N | 22.0 | 42 | 14 | 9 | 10 |
| 16 | 20 | 5.1 | midC4–midC7 | V/S | 22.1 | 45 | 11 | 10 | 19 |
| 17 | 20 | 5.1 | midC4–midC7 | V/S | 21.9 | 47 | 14 | 13 | 17 |
| 18 | 20 | 5.1 | midC4–midC7 | V/S | 21.9 | 45 | 11 | 10 | 20 |
| 19 | 22 | 5.1 | midC4–midC7 | V/S | 24.0 | 46 | 9 | 9 | 26 |
| 20 | 22 | 5.1 | midC4–midC7 | V/S | 24.1 | 46 | 12 | 12 | 24 |
| 21 | 22 | 5.1 | midC4–midC7 | V/S | 24.1 | 45 | 11 | 11 | 23 |
| 22 | 22 | 5.1 | midC4–midC7 | V/S | 24.4 | 47 | 10 | 10 | 20 |
No motor neurologic deficits were observed in stated follow-up period.
C = “cervical”
X = “Stereoscopic X-ray”
N = “Novalis”
V = “Volumetric Cone Beam Computed Tomography”
S = “Synergy S”
Figure 1.
Dose distributions in the axial(A) and sagittal(B) planes.
Table 2.
Spinal cord dose-volume histogram statistics for animals receiving uniform irradiation.
| Rx Dose Group (Gy) | Mean Maximum Point Dose (Gy) | Mean Percent Volume >= 10 Gy | Mean Volume >=14 Gy (cc) | Mean Maximum Dose to 1 cc Volume (Gy) |
|---|---|---|---|---|
| 16 (n=7) | 17.5 ± 0.1 | 91 ± 3 | 4.12 ± 0.67 | 17.1 ± 0.0 |
| 18 (n=6) | 19.5 ± 0.2 | 94 ± 3 | 3.74 ± 0.13 | 19.0 ± 0.4 |
| 20 (n=5) | 22.0 ± 0.1 | 93 ± 4 | 3.87 ± 0.16 | 21.5 ± 0.2 |
| 22 (n=4) | 24.1 ± 0.2 | 92 ± 0 | 4.00 ± 0.21 | 23.7 ± 0.1 |
Irradiation was performed with either 6 MV (Novalis, BrainLAB AG) or 10 MV (Synergy S, Elekta AB) image-guided linear accelerators. Radiation from the Novalis was delivered through dynamically-shaped arcs at a rate of 800 monitor units per minute that equated to an approximate instantaneous dose rate of 5.0–6.3 Gy/min at the spinal cord, varying with the depth of overlying tissue. Radiation from the Synergy S was delivered through conformal fields at a rate of 500 monitor units per minute that equated to an approximate instantaneous dose rate of 3.8–4.4 Gy/min at the spinal cord. Overall delivery time (first beam on to last beam off) varied with prescription dose but the 20 Gy prescription was delivered in 10–20 minutes on the Novalis while the 22 Gy prescription was delivered in 20–26 minutes on the Synergy S.
After irradiation, animals were followed for 51–56 weeks or until a neurologic response was observed. The general health of animals was observed daily with attention toward unusual restlessness, vocalizing, loss of mobility, licking, biting, or guarding of a painful area, failure to groom, unkempt appearance, open sores, skin lesions, loss of appetite, and weight loss. Gait was observed approximately weekly with the animal walking freely in a large space. Response was defined as any study-related change in gait. Animals recognized to have a change in gait were evaluated by a veterinarian for symptoms indicative of pain and humanely killed. The cervical spinal cords of all study animals (irradiated plus controls) were removed and fixed in formalin before being sectioned and processed for embedding in paraffin. Five uniformly distributed axial sections through the irradiated volume of each animal were cut and stained with a Luxol fast blue/periodic acid Schiff combination and were examined for histology. The dose-related incidence of motor deficit was used as an endpoint to obtain quantal data that was then analyzed by probit analysis[4, 8] to establish a dose-response curve and to calculate the probability of a deficit at increasing dose levels with the associated 95% confidence bounds.
Results
Parameters such as prescription dose, maximum spinal cord dose, length of follow-up and latency until response are shown for individual animals in Table 1. Ten of twenty-two pigs developed motor neurologic changes while the neurologic status of twelve pigs remained normal throughout the study. Bilateral deficits were observed in the hind limbs either prior to or in coincidence with the forelimbs in nine of ten pigs. Only forelimb deficits were noted in pig #19 but this animal was humanely killed one day after changes were first noted. Motor deficits presented initially as general weakness and uncoordinated gait in all responders but the progression of deficits was variable. Pig #8 (19.2 Gy maximum spinal cord dose) experienced hind limb weakness 8 weeks after irradiation but fully recovered without intervention by 10 weeks only to relapse at 25 weeks. The response of pigs in the 20 Gy prescription group varied from transient weakness at 10 weeks after irradiation, followed by a complete recovery (pig #14), to rapid onset of weakness and immobility (pig #16) without recovery. All pigs in the 22 Gy prescription group experienced rapid onset of weakness and loss of coordination without recovery. The latent period for initial onset of motor deficit ranged from 8–13 weeks. No animal exhibited behavior indicative of pain throughout this study.
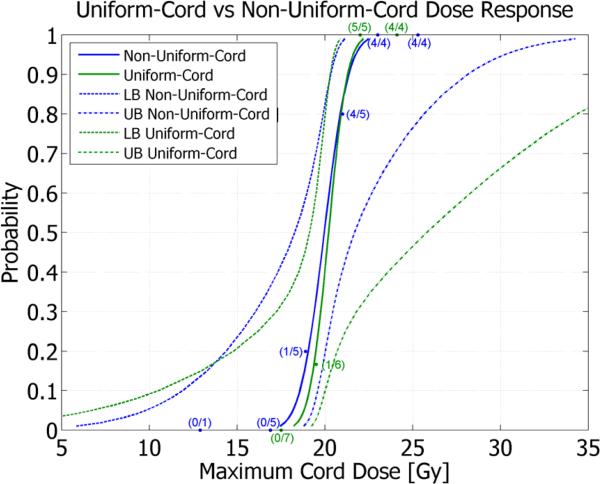
A clear dose-response relationship was observed for uniformly irradiated spinal cords, with no response when the maximum spinal cord dose was ≤17.8 Gy and 100% rate of motor neurologic deficits when the maximum spinal cord dose was ≥21.8 Gy. Calculated estimates of the maximum spinal cord doses resulting in 1, 5, 10, 50 and 90% probability of paralysis along with the associated 95% confidence bounds are shown in Supplementary File, Table 3. The dose-response curve for uniformly irradiated spinal cords has been overlayed with the dose-response curve from a previous study of non-uniformly irradiated spinal cords[1] and is presented in Figure 2. Observed response data points are superimposed on the dose-response curves and annotated to show the number of responders per number of animals at each dose point. The associated upper and lower 95% confidence bounds are also plotted (dashed lines) for each curve. The test for parallelism yielded a p-value of 0.577 indicating no significant difference between the two dose-response curves. The dose associated with a 50% incidence of paralysis (ED50) and 95% confidence interval (CI) due to uniform spinal cord irradiation was 20.2 Gy(19.1–25.8). A Pearson goodness-of-fit chi-square indicated (p=0.982) that the probit model provides an excellent fit.
Figure 2.
Dose-response curves with 95% confidence bounds for uniform versus non-uniform dose distributions. *”Lower Bound” and “Upper Bound” have been abbreviated as LB and UB, respectively.
General agreement was observed between the occurrence of neurologic change and the presence of histological lesions. All uniformly irradiated animals with motor deficits showed some degree of demyelination and focal white matter necrosis, seemingly randomly distributed in lateral, ventral and dorsal white matter tracts with sparing of the gray matter. Although the distribution of histologic damage differed from previously described non-uniformly irradiated spinal cords[1], the type of damage was the same. The only responder in the 18 Gy group (pig #8) with transient neurological changes in the hindlimbs was histologically characterized by extensive demyelination without frank white matter necrosis. In a few cases near the threshold dose for myelopathy, histology showed only some very small foci of demyelination. In addition to diffuse demyelination of the white matter, foci of white matter necrosis were observed increasingly with dose.
Discussion
This study was performed to test the hypothesis that spinal cord radiosensitivity is significantly modified by uniform versus laterally non-uniform dose distributions. Results of uniform spinal cord irradiation are reported here for comparison with a companion study in which a laterally non-uniform dose was delivered[1]. Dose-volume characteristics for pigs from the companion study are presented in Supplementary File, Table 4 for comparison with characteristics from the present study (Table 2). Despite vast differences in 10 Gy and 14 Gy volumes between studies, the dose-response curves for motor neurologic deficit are nearly identical suggesting that the maximum spinal cord point dose is the best predictor of response. The resulting ED50's (95% CI) are 20.2 Gy(19.1–25.8) and 20.0 Gy(18.3–21.7) for uniform and non-uniform irradiation, respectively.
Overall radiation delivery times varied among individual animals in every dose group of this study and the previous study that incorporated non-uniform spinal cord irradiation[1]. Overall delivery times are presented for all animals from both studies in Tables 1 and Supplementary File, Table 5, respectively. Pop et al.[17] investigated the impact of temporal dose distribution on the radiation tolerance of the rat spinal cord. An 192-Iridium brachytherapy source was stepped through catheters implanted lateral to the vertebral bodies to irradiate the spinal cord with varying overall treatment times and average dose rates. ED50 values were observed to increase by 2.1 Gy as the overall treatment time was increased from 4–8 to 28–37 minutes. Direct comparison of this rat data with the current pig study is confounded by considerable differences in study design and methods but the ED50 values derived from this pig study may have been influenced by varying degrees of sublethal damage repair that occurred due to differences in overall treatment time. The influence of sublethal damage repair is not readily apparent in this study.
Dose-response results from the present study are consistent with previous studies in mice[18], rats[3, 11] and guinea pigs[19] that also incorporated uniform spinal cord irradiation. All of these preclinical studies have reported steep dose-response curves with ED50 values ranging from 18.9–21.5 Gy. The only previous study in pigs reported a 37% greater ED50 value of 27.7(±0.6) Gy but our data suggests that the greater ED50 is almost certainly due to the low dose rate (0.2–0.3 Gy/min) used in that study[4].
van Luijk et. al.,[8] investigated the influence of laterally non-uniform dose distributions on spinal cord tolerance in rats. A 150 MeV proton beam was used to irradiate 50% of the lateral cross-section of the cervical spinal cord resulting in an extremely steep dose gradient (100% to <10% isodose) across the spinal cord. An ED50 (95% CI) of 30 Gy (26.3–31.3) was observed for paralysis compared to an ED50 of 20.4 Gy (19.6–21.1) for uniform cross-section irradiation. A more extensive followup study affirmed the lateral volume effect and demonstrated that the lateral white matter is much more radiosensitive than the central part of the white matter[9]. The laterally non-uniform dose distribution used in our companion pig study included a lateral dose gradient of 95% to 10% isodose across the spinal cord with the 50% isodose line bisecting the cord (similar to van Luijk, et. al.[8]); however, the diameter of the pig cervical spinal cord is approximately equivalent to humans (three times greater than for a rat). The reason for the lack of a demonstrable spinal cord sparing effect in pigs irradiated with steep lateral dose gradients is not clear, but the physical size of the spinal cord and/or the steepness of the dose gradient appear to be factors in the repair mechanism. Data from “length effect” studies suggests that migration of remyelinating cells from the unirradiated field edges is at least partially responsible for restoring the damaged glial cell population following irradiation[3, 11, 20]; however, the role of these cells in the development of white matter necrosis in unclear. If viable oligodendrocytes and their precursor cells can only migrate 2–7 mm from unirradiated tissue, as studies suggest[11, 20, 21], the diameter of the human spinal cord could be detrimental to remyelination and the encouraging results from rats may be muted.
Dose-volume effects in the spinal cord have been suggested in the human literature but not studied prospectively[12, 13, 15]. The group at Stanford University[15] provide the strongest evidence for clinical dose-volume effects in the spinal cord in a review of their experience treating hemangioblastomas with radiosurgery. Seventeen tumors were treated with single-session radiosurgery resulting in maximum spinal cord doses ranging from 17.8 to 30.9 Gy (median 22.7 Gy) yet neurologic toxicity was limited to two patients. In contrast, we observed 100% occurrence of motor neurologic deficits after partial irradiation of pig spinal cords with maximum point doses ≥21.1 Gy[1]. Multiple factors including irradiated length and axial dose gradient may have contributed to the contrasting rates of toxicity observed between the Stanford study and this pig study. A 5 cm length was treated in pigs while lengths are described as “short” in the Stanford series[15]. Many preclinical studies support the hypothesis that dose distribution is a better predictor of neurologic toxicity than dose-volume characteristics[3, 8, 9, 11]. For example, Bijl et. al.[11], observed no response when a dose ≤ 36 Gy was delivered to the full cross section of a 4 mm long spinal cord segment while a dose of 35 Gy to the lateral edge of the spinal cord resulted in 100% response when a 20 mm segment was irradiated[8].
Ideally, irradiation conditions would have been consistent for all pigs in this study but dose rate, radiation energy, and irradiated length varied slightly for spinal cords that were uniformly irradiated. None of these differences are believed to have influenced the study results. Dose rate has been demonstrated to modify spinal cord tolerance but only at much lower dose rates than used in this study. The ED50 for paralysis in the rat and mouse spinal cord appears to plateau between dose rates of 0.25 and 1.8 Gy/min and becomes insensitive to further increases[7]. The ED50 for paralysis in the pig is consistent with rats at dose rates between 0.2–0.3 Gy/min[4, 22] and the present study indicates that the ED50 for pigs is also consistent with rodents at higher dose rates of 5.0–6.3 Gy/min. Radiation dose response is not known to change between photon energy spectra from 6MV and 10MV linear accelerators. The irradiated length of spinal cord varied between 4.5 and 7.0 cm in the present study. As shown in Table 1, a length of 5.1 cm was irradiated in the majority of pigs in this study but 5 pigs in the 16 Gy prescription group were irradiated to a length of 6.8–7.0 cm to accommodate another companion study. Irradiated length has been shown to affect spinal cord tolerance but only for lengths < 1.6 cm[7]. There is no data to suggest that spinal cord radiosensitivity varies between irradiated lengths from 4.5–7.0 cm.
The follow-up period of the current study (51–56 weeks) is considered sufficient to observe the initial phase of radiation myelopathy as commonly reported for guinea pigs[19], rats[3, 11, 22], mice[18] and pigs[1, 4] (2–6 months). The latent period observed for the onset of motor deficits in the present study (8–13 weeks) is in good agreement with the companion study[1] Potentially more cases of radiation myelopathy would have been observed in a longer follow-up period; a previous spinal cord tolerance study[4] reported two late responding pigs at 65 and 75 weeks following irradiation to doses very close to ED50 but the maximum followup period was 56 weeks in the present study.
In conclusion, no dose-volume effect is observed in pigs for the dose distributions studied and the endpoint of motor neurologic deficit; however, partial spinal cord irradiation results in less debilitating neurologic and histopathologic morbidity. The maximum point dose to the spinal cord was the parameter that best predicted the risk of motor neurologic deficit in this study.
Supplementary Material
Acknowledgments
This project was funded entirely by the US National Institute of Neurological Disorders and Stroke, R01 NS049517.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification Paul Medin and Timothy Solberg teach in radiosurgery courses sponsored by BrainLAB AG. Ryan Foster and Timothy Solberg have received research funding from Elekta, Ltd. All remaining authors have no conflicts to report.
References
- [1].Medin PM, Foster RD, van der Kogel AJ, Sayre JW, McBride WH, Solberg TD. Spinal cord tolerance to single-fraction partial-volume irradiation: a Swine model. Int J Radiat Oncol Biol Phys. 2011;79:226–232. doi: 10.1016/j.ijrobp.2010.07.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) Int J Rad Onc Biol Phys. 2010;76:S1–S160. doi: 10.1016/j.ijrobp.2009.09.040. Various. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hopewell JW, Morris AD, Dixon-Brown A. The influence of field size on the late tolerance of the rat spinal cord to single doses of X rays. Br J Radiol. 1987;60:1099–1108. doi: 10.1259/0007-1285-60-719-1099. [DOI] [PubMed] [Google Scholar]
- [4].van den Aardweg GJ, Hopewell JW, Whitehouse EM. The radiation response of the cervical spinal cord of the pig: effects of changing the irradiated volume. Int J Radiat Oncol Biol Phys. 1995;31:51–55. doi: 10.1016/0360-3016(94)E0306-5. [DOI] [PubMed] [Google Scholar]
- [5].Powers BE, Thames HD, Gillette SM, Smith C, Beck ER, Gillette EL. Volume effects in the irradiated canine spinal cord: do they exist when the probability of injury is low? Radiother Oncol. 1998;46:297–306. doi: 10.1016/s0167-8140(97)00213-2. [DOI] [PubMed] [Google Scholar]
- [6].Hamilton AJ, Lulu BA, Fosmire H, Stea B, Cassady JR. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery. 1995;36:311–319. doi: 10.1227/00006123-199502000-00010. [DOI] [PubMed] [Google Scholar]
- [7].Medin PM, Boike TP. Spinal cord tolerance in the age of spinal radiosurgery: lessons from preclinical studies. Int J Radiat Oncol Biol Phys. 2011;79:1302–1309. doi: 10.1016/j.ijrobp.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Luijk P, Bijl HP, Coppes RP, et al. Techniques for precision irradiation of the lateral half of the rat cervical spinal cord using 150 MeV protons [corrected] Phys Med Biol. 2001;46:2857–2871. doi: 10.1088/0031-9155/46/11/307. [DOI] [PubMed] [Google Scholar]
- [9].Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van Der Kogel AJ. Regional differences in radiosensitivity across the rat cervical spinal cord. Int J Radiat Oncol Biol Phys. 2005;61:543–551. doi: 10.1016/j.ijrobp.2004.10.018. [DOI] [PubMed] [Google Scholar]
- [10].Philippens ME, Pop LA, Visser AG, van der Kogel AJ. Dose-volume effects in rat thoracolumbar spinal cord: the effects of nonuniform dose distribution. Int J Radiat Oncol Biol Phys. 2007;69:204–213. doi: 10.1016/j.ijrobp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- [11].Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van der Kogel AJ. Dose-volume effects in the rat cervical spinal cord after proton irradiation. Int J Radiat Oncol Biol Phys. 2002;52:205–211. doi: 10.1016/s0360-3016(01)02687-6. [DOI] [PubMed] [Google Scholar]
- [12].Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- [13].Gibbs IC, Patil C, Gerszten PC, Adler JR, Jr., Burton SA. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67–A72. doi: 10.1227/01.NEU.0000341628.98141.B6. [DOI] [PubMed] [Google Scholar]
- [14].Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- [15].Daly ME, Choi CY, Gibbs IC, et al. Tolerance of the spinal cord to stereotactic radiosurgery: insights from hemangioblastomas. Int J Radiat Oncol Biol Phys. 2011;80:213–220. doi: 10.1016/j.ijrobp.2010.01.040. [DOI] [PubMed] [Google Scholar]
- [16].Trott K-RD,W, Facoetti A, Hopewell J, Langendijk J, van Luijk P, Ottolenghi A, Smyth V. Biological mechanisms of normal tissue damage: Importance for the design on NTCP models. Radiother and Oncol. 2012 doi: 10.1016/j.radonc.2012.05.008. http://dx.doi.org/10.1016/j.radonc.2012.05.008. [DOI] [PubMed]
- [17].Pop LA, Millar WT, van der Plas M, van der Kogel AJ. Radiation tolerance of rat spinal cord to pulsed dose rate (PDR-) brachytherapy: the impact of differences in temporal dose distribution. Radiother Oncol. 2000;55:301–315. doi: 10.1016/s0167-8140(00)00205-x. [DOI] [PubMed] [Google Scholar]
- [18].Lo YC, McBride WH, Withers HR. The effect of single doses of radiation on mouse spinal cord. Int J Radiat Oncol Biol Phys. 1992;22:57–63. doi: 10.1016/0360-3016(92)90982-n. [DOI] [PubMed] [Google Scholar]
- [19].Knowles JF. The radiosensitivity of the guinea-pig spinal cord to X-rays: the effect of retreatment at one year and the effect of age at the time of irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1983;44:433–442. doi: 10.1080/09553008314551411. [DOI] [PubMed] [Google Scholar]
- [20].Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van der Kogel AJ. Unexpected changes of rat cervical spinal cord tolerance caused by inhomogeneous dose distributions. Int J Radiat Oncol Biol Phys. 2003;57:274–281. doi: 10.1016/s0360-3016(03)00529-7. [DOI] [PubMed] [Google Scholar]
- [21].Chari DM, Blakemore WF. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–313. [PubMed] [Google Scholar]
- [22].Scalliet P, Landuyt W, Van der Schueren E. Repair kinetics as a determining factor for late tolerance of central nervous system to low dose rate irradiation. Radiother and Oncol. 1989;14:345–353. doi: 10.1016/0167-8140(89)90147-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.