Abstract
Objectives
To evaluate the effect of metformin and pioglitazone on insulin resistance, ovulation and hyperandrogenism in women with PCOS.
Methods
100 patients of age 18–30 years were included in this randomised double blind trial for treatment with either metformin or pioglitazone for a period of 6 months.
Results
Administration of metformin and pioglitazone for 6 months revealed that 50 % of the patients achieved menstrual cyclicity. A decline in F–G grading for hirsutism within the both the groups was observed. The lipid profile also showed a decrease in total cholesterol, an increase in HDL-C, a decrease in VLDL-C levels but more so in the pioglitazone group. HOMA-IR declined more than 50–55 % with pioglitazone and 15 % with metformin. Thus, pioglitazone may be a better treatment option as far as protection from tendency to development of diabetes is conscerned. The rise in serum SHBG levels and decline in free androgen index and L/H ratio are more remarkable with pioglitazone (P < 0.05). Ovulation was restored in 44.2 and 56 % of patients on metformin and pioglitazone, respectively.
Conclusion
Pioglitazone may be a new alternative for use in women with PCOS, providing more metabolic and reproductive benefits and possibly protection from developing diabetes and cardiovascular problem.
Keywords: Pioglitazone metformin, Polycystic ovarian disease, Insulin resistance, Hyperandrogenism, Ovulation
Introduction
Polycystic ovarian syndrome is a heterogenous collection of signs and symptoms, which when gathered together, form a spectrum of a disorder with a mild presentation in some and a severe disturbance of reproductive, endocrine and metabolic function in others. Polycystic ovarian syndrome is characterized by 1. Anovulation (irregular menstrual periods). 2. Hyperandrogenism, 3. Insulin resistance, 4. Obesity and 5. Inappropriate gonadotrophin secretion (↑ LH/FSH ratio).
Several pieces of data support the hypothesis that insulin resistance and hyperinsulinemia may play a pathogenic role in this syndrome:
At the central level, insulin seems to be involved in the dysregulation of Luteinizing hormone (LH) secretion.
At the peripheral level, insulin promotes ovarian androgen secretion by enhancing cytochrome P450 C17 activity and appears to affect the normal follicular growth.
Insulin also decreases—serum–sex hormone-binding globulin synthesis (SHBG) by the liver, thus increasing free androgen levels.
Insulin also potentiates in vivo ACTH-stimulated adrenal androgen production in women with PCOS.
Long-term sequelae of PCOS [1]:
Cardiovascular disease: (a) increased risk of atherosclerosis, (b) dyslipidaemia, (c) increased risk of hypertension, (d) increased risk of ischaemic heart disease.
Type 2 diabetes: the prevalence of diabetes in women with PCOS is ~11 %.
Endometrial and breast cancer: women with PCOS have increased levels of testosterone which is a precursor for Oestrogen. Thus, they are exposed to unopposed Oestrogen.
Insulin has a powerful mitogenic influence on various tissues-like endometrium and breast epithelium, and this proliferative affect may contribute to the appearance of oncogenes and transformation of benign to malignant tissue.
Study Design
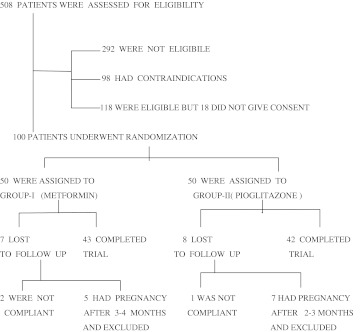
This is a randomized, double-blinded, comparative study conducted at Gandhi Hospital—a tertiary care centre and a teaching hospital for graduation and post-graduation in medicine at Hyderabad, Andhra Pradesh, India. One-hundred PCOS patients in the age group of 18–30 years were enrolled and randomly allocated to two groups and given the following treatment modalities for a period of 6 months. Prior approval from the Institutional Ethics & Review Committee Gandhi Hospital—Secunderabad was obtained for the study, Flowchart 1 [2, 3].
Flowchart 1.
Group I: Metformin—500 mg bid, Group II: Thiazolidinediones—Pioglitazone (15 OD). Each group consisted of 50 patients. The sample size was calculated before the study, on the basis of a previous study [4], using different parameters, and the maximum sample size was derived using the formula:
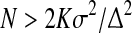 |
where N is the sample size, K = 7.8 (derived from the table of two-sided test of α = 0.05 (probability of type 1 error) and β = 0.02 (probability of type 2 error, giving power of the study as 80 %); σ the standard deviation (of the selected parameters) taken from the previous study; and ∆ the minimum difference between baseline and post-treatment means from the previous study. The minimum sample size required was 25–30. In this study, 50 patients were included in each group to make allowance for dropouts.
Methods
Diagnostic Criteria [5]
At a recent joint European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) consensus meeting, a definition of PCOS was agreed—the presence of two out of the following three criteria after the exclusion of other criteria: (1) oligo and/or anovulation, (2) hyperandrogenism (clinical and/or biochemical), and (3) polycystic ovaries, (12 or more follicles of 2–9 mm in diameter or ovarian volume >10 cc).
Exclusion Criteria
(1) Pregnancy and nursing, (2) Significant liver impairment, (3) Significant renal impairment, (4) Neoplastic disease, (5) Cardiovascular diseases, (6) Cushing’s disease, (7) Hypothyroidism, (8) Hyperprolactinemia, and (9) Any drug intake-like Anti-diabetic (or) Oestrogen and progesterone.
The following parameters are studied for each group:
Clinical
Menstrual cycle irregularity defined according to (Van Hoof et al.)
Virilisation—grading is done as per Ferriman–Gallwey (F–G) score.
Virilisation symptoms include: ↑ in body hair and facial hair, deepening of voice, male pattern baldness, clitoral enlargement, acne and acanthosis nigricans (discoloration of skin under arms, breasts and groins).
After taking a written consent from the patients, studies are conducted during the early follicular phase of spontaneous or induced (Medroxy progesterone acetate) menstrual cycles (i.e. Days 3–7)
On the first visit—A medical and Gynaecological examination is done, and patient is advised a standard carbohydrate diet for 3 days and after 10–12 h fasting overnight, blood samples have been collected for investigations, as follows:
Baseline Investigations
1. CBP, ESR, 2. Liver function tests, 3. Serum creatinine and urea, 4. TSH, 5. Serum prolactin, 6. Lipid profile, 7. Oral GTT with 75 g glucose with corresponding insulin levels to look for IR and hyperinsulinemia, 8. Serum testosterone, 9. Sex hormone-binding globulin (SHBG), 10. LH/FSH ratio and 11.USG of abdomen and pelvis—to look for enlarged ovaries with multiple cysts and ovulation study (PCO is bilaterally enlarged ovaries 2–5 times containing at least 7–10 micro cysts <5 mm in diameter with thick, white covering).
The treatment is given over a period of 6 months with follow up visits every month. On each visit, compliance with the treatment has been checked with a questionnaire about the side effects and a subjective evaluation of the tolerability of the drugs administered.
After 6 months, again an examination to evaluate the changes in the baseline findings, both clinical and biochemical, is done.
During the study, therapies not interfering with the parameter under evaluation were permitted in both the groups
Standard statistical methods, i.e. Z-test-based probability were derived by comparing the means and P < 0.05 was taken as statistically significant. This was calculated for all the variables and compared as post-treatment versus baseline between the groups.
The power of the study for different variables was found to be 79.3–100 % except F–G grading for hirsutism (5 %) and serum testosterone (5.6 %) (power calculation done with the help of standard statistical tools).
The confidence-interval for each mean variable is also calculated, with a confidence-level of 95 %.
Results
In the Group I, 43 (86 %) and out of the 50 patients and in the Group II, 42 (84 %), out of completed the trial (Table 1).
Table 1.
Comparison of baseline values of Groups I and II (all baseline values were identical, and any differences among them were statistically insignificant)
| Variables | Group I | Group II | P values |
|---|---|---|---|
| F–G grading | 15.9 ± 5.89 | 14.32 ± 5.29 | <0.05 |
| Total cholesterol (mg/dl) | 184.86 ± 14.43 | 188.39 ± 10.34 | <0.05 |
| HDL-C (mg/dl) | 38.33 ± 5.6 | 38.50 ± 2.06 | <0.05 |
| VLDL-C (mg/dl) | 38.28 ± 3.1 | 22.63 ± 2.6 | <0.05 |
| Fasting insulin (μU/ml) | 43 ± 7.4 | 42.5 ± 8.7 | <0.05 |
| Post-glucose insulin (μU/ml) | 150 ± 9.2 | 150 ± 9.2 | <0.05 |
| HOMA-IR (Fasting) | 16.54 ± 4.06 | 15.69 ± 3.1 | <0.05 |
| HOMA-IR (post-glucose) | 103.107 ± 8.3 | 103.172 ± 6.5 | <0.05 |
| Testosterone (nmol/l) | 1.97 ± 0.27 | 1.89 ± 0.34 | <0.05 |
| SHBG (nmol/l) | 47.07 ± 15.06 | 30.43 ± 10.6 | <0.05 |
| FAI | 12.81 ± 3.97 | 13.46 ± 4.1 | <0.05 |
| LH (mIU/ml) | 4.2 ± 1.3 | 6.2 ± 4.1 | <0.05 |
| FSH (mIU/ml) | 8.59 ± 1.6 | 6.34 ± 1.2 | <0.05 |
| LH/FSH | 0.696 ± 0.1 | 0.978 ± 0.27 | <0.05 |
Clinical Characteristics
Menstrual Cycles
In the Group I, all the 43 patients had irregular menstrual cycles at the beginning of the study and at the end of the study 20 (46 %), reported having regular cycles (CI 31–62 %).
In Group II, 41 patients had irregular cycles at the beginning, and at the end of the study 24 (58 %) reported having regular cycles at the end of the study (CI 42–73 %).
Virilisation
In Group I out of 43 patients, 33 presented with hirsutism (F–G score > 8) at the start. At the end of the study, all of them had lesser F–G score, and one among them had reported with her hirsutism as being completely resolved. In Group II out of the 42 patients, 28 presented with hirsutism (F–G score > 8) at the start. At the end of the study, all had a lesser F–G score and three among them reported their hirsutism as having been resolved completely.
Metabolic and Hormonal Parameters
Total Cholesterol
In Group I out of 43 patients, ten were found to have Serum cholesterol levels >200 at baseline and at the end of the treatment four had S. cholesterol >200. In Group II out of 42 patients, seven had a S. cholesterol of >200, and at the end of treatment two had S. cholesterol >200. In both the groups, S. cholesterol levels show a decline (P < 0.05), but the decrease is more significant in Group II, when compared with Group I (P < 0.05)
HDL-C
In both the groups, there is an increase in HDL levels (statistically significant versus baseline), more in Group II than in Group I. The average increase is 17 % in Group I as compared to 64 % in Group II.
VLDL-C
There is a trend of decrease in VLDL levels in both groups, the decrease being more in Group II (32 %)than in Group I (15 %)—statistically significant when both groups are compared.
Insulin Levels
In Group I 24 out of 43 (about 56 %) patients and in Group II 30 out of 42 (about 71 %) patients were Hyper-insulinemic (fasting insulin levels >40 μU/ml), and the rest were normo-insulinemic (fasting insulin levels <40 μU/ml).
Insulin Levels and Insulin Resistance
In Group II, the fall in fasting insulin level is more (by 50.2 %) from an average of 42.5 to 21.4 μU/ml than in Group I (by 15.2 %) from an average of 43 to 36.05 μU/ml, after treatment for 6 months (statistically significant versus Group I). Similarly in Group II the fall in insulin level is more (48.1 %) than in Group I (11.3 %) after glucose load was given. The difference between the two groups is statistically significant (P < 0.05).
HOMA-IR is an Indicator of Insulin Resistance
A decrease in HOMA-IR is seen in both the groups (P < 0.05 versus baseline), but the decrease in insulin resistance is greater in Group II (53–55 %) than in Group I (15–20 %), i.e. statistically significant versus Group.
Testosterone
In both the groups, there is a fall in testosterone levels but statistically insignificant.
Sex Hormone-Binding Globulins (SHBG)
In both the groups, there is a rise in SHBG, (statistically significant versus baseline). In Group I, the mean increase in SHBG was 6.34 nmol/l (20.8 %), but in Group II, mean rise in SHBG was more, i.e. .33.41 nmol/l (70.9 %) and this difference is statistically significant(P < 0.05).
Free Androgen Index (FAI)
In Group I, average fall in FAI was 1.4 (22.6 %), but in Group II, it was 2.0 (47 %). In both the groups, FAI decreased, but in Group II, the decrease was more, and this is statistically significant (P < 0.05) versus Group I.
Luteinizing Hormone (LH)
LH values on an average decreased by 2.29 mIU/ml in Group I (statistically insignificant) and by 4.59 mIU/ml in Group II (statistically significant P < 0.05) versus baseline values. This effect in Group II is also statistically significant (P < 0.05) versus Group I.
LH/FSH Ratio
Both the Groups showed a decrease in LH/FSH ratio. The fall in LH/FSH ratio was more in Group II, than in Group I. This fall in Group II was statistically significant P < 0.05 versus Group I.
Ovulation
In Group I, out of 43 anovulatory patients 19 (44.2 %) had their ovulation restored (CI 29–59 %). In Group II, out of 41 anovulatory patients 23 (56 %) had ovulatory Table 1 cycles after treatment (CI 40.9–71.3 %). Thus, pioglitazone shows an equally effective success rate in restoration of ovulation when compared to metformin.
Side Effects
There were no major side effects which resulted in discontinuation of treatment in both the groups. In Group I, only mild gastrointestinal side effects are reported in Table 2. In Group II, mild peripheral oedema in 40 %; muscle cramping in 11 % were reported. There were no major changes in hepatic parameters after 6 months of its administration. Thus, pioglitazone is a safe drug and does not share any of its unsafe ‘class effects’.
Table 2.
Comparison of post-treatment values of Groups I and II
| Variables | Group I | Group II | Z-score | P values | Power of the study |
|---|---|---|---|---|---|
| F–G grading | 7.82 ± 4.2 | 7.89 ± 5.52 | 0.0656 | >0.05 | 5 % |
| Total cholesterol (mg/dl) | 173.00 ± 12.12 | 149.45 ± 10.6 | 9.542 | <0.05 | 100 % |
| HDL-C (mg/dl) | 45.32 ± 3.98 | 63.02 ± 4.22 | 19.889 | <0.05 | 100 % |
| VLDL-C (mg/dl) | 19.1 ± 1.33 | 25.9 ± 1.66 | 20.905 | <0.05 | 100 % |
| Fasting insulin (μU/ml) | 36.05 ± 5.7 | 21.4 ± 3.2 | 14.66 | <0.05 | 100 % |
| Post-glucose insulin (μU/ml) | 133 ± 15.6 | 80 ± 12.3 | 17.4 | <0.05 | 100 % |
| Fasting HOMA-IR | 12.531 ± 1.2 | 7.5 ± 5.9 | 5.4 | <0.05 | 100 % |
| Post-glucose HOMA-IR | 86.963 ± 13.6 | 47.745 ± 10.1 | 15.1 | <0.05 | 100 % |
| Testosterone (nmol/l) | 1.78 ± 0.56 | 1.81 ± 0.64 | 0.2 | >0.05 | 5.6 % |
| SHBG (nmol/l) | 36.77 ± 8.7 | 80.48 ± 16.21 | 15.45 | <0.05 | 100 % |
| FAI | 11.17 ± 2.43 | 8.22 ± 2.87 | 5.11 | <0.05 | 99.9 % |
| LH (mIU/ml) | 4.8 ± 2.6 | 2.2 ± 0.8 | 6.2 | <0.05 | 100 % |
| FSH (mIU/ml) | 7.37 ± 1.3 | 9.10 ± 1.4 | 5.904 | <0.05 | 100 % |
Discussion
Administration of both metformin and pioglitazone for 6 months revealed that nearly 50 % of the patients achieved regular menstrual cycles. A decline in F–G grading for hirsutism in both the groups was observed, and both drugs were equally effective. The lipid profile also showed a decrease in total cholesterol, an increase in HDL-C, and a decrease in VLDL-C levels in both the groups (statistically significant).
This study also reveals that on an average, 64 % of PCOS patients were hyperinsulinemic (fasting insulin effects >40 μU/ml) supporting the hypothesis [6] that the underlying pathogenic feature of PCOS may be insulin resistance in a majority of cases. Administration of both metformin and pioglitazone showed a decrease of insulin resistance as shown by a decline in HOMA-IR found after the administration of both metformin and pioglitazone [7]. However, a remarkable decline of more than 50–55 % was observed with pioglitazone as compared to metformin where the decline in HOMA-IR was 15–20 %. Thus, pioglitazone is certainly a better insulin sensitizer when compared with metformin.
The fall in testosterone levels (not statistically significant), the rise in serum SHBG levels (P < 0.05, in both the groups) and the resultant decline in FAI (P < 0.05, in both groups) were also observed. A fall in LH is seen in both the groups but (P < 0.05 with pioglitazone-post-treatment versus baseline).
Pioglitazone has both anti-inflammatory and anti-arteriosclerotic properties and may be useful for reducing the cardiovascular risks associated with PCOS. Buchanan et al. showed that administration of pioglitazone reduced the incidence of diabetes mellitus by more than 50 % and protection from diabetes in women with PCOS persisted even after medication was stopped.
During the study period there was no major change in liver enzymes as reported in some previous studies. The incidents of serious acute hepatic failure following the administration of troglitazone or cardiac failure following rosiglitazone administration was not observed with administration of pioglitazone administration for 6 months.
In this study, 36 % of patients were normoinsulinemic and 64 % were hyperinsulinemic at baseline but the effect of these drugs was not analysed in relation to the different insulin secretions. In a previous study, Romualdi et al. all have clearly demonstrated a marked decrease of LH levels and LH/FSH ratio in both normoinsulinemic and hyperinsulinemic PCOS patients, reaching statistical significance in the group after pioglitazone administration. However, the magnitude of decrease was similar, irrespective of insulin secretion, suggesting a mechanism of action for this drug unrelated to pretreatment circulating insulin levels. Troglitazone was found to repress combined stimulation by LH and insulin of de novo androgen biosynthesis by porcine thecal cells in vitro thus providing a hypothesis that excessive androgen levels in PCOS can be reduced by direct action of thiazolidinediones on ovarian function. This androgen-lowering effect may be due to inhibition of P450c17 and 3β-hydroxysteroid dehydrogenase, the two important enzymes in human androgen synthesis.
Some other studies [8] have demonstrated the decrease in LH and LH/FSH ratio only in obese PCOS patients.
Nevertheless, in PCOS women treated with thiazolidinediones, these drugs have to be stopped as soon as the patient is pregnant as these drugs are classified under Category C drugs by FDA and they present teratogenic risks as PPARγ is important for embryonic development, and thiazolidinediones can affect foetal maturation. However, metformin being a Category B drug can be administered in pregnancy as it reduces pregnancy losses which are frequently observed in 30–50 % PCOS women during the first trimester.
Conclusion
Pioglitazone may open up as a possible new treatment in patients with PCOS, since it can restore menstrual cyclicity, achieve a better ovulatory rate, improve clinical signs of hyperandrogenism, and may prevent or delay the onset of Type 2 diabetes and other long-term sequelae.
References
- 1.Brettenhaler N, De Geyter C, Huber PR et al. Effect of insulin sensitizer pioglitazone on IR, hyperandrogenism and ovulatory dysfunction in women with PCOS. J Clin Endocrinol Metab. 2004;89(8):3835–40. doi:10.1210/jc.2003-031737. [DOI] [PubMed]
- 2.Schulz KF, Altman DG, Moher D et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115(5):1063–70. [DOI] [PubMed]
- 3.Moher D, Hopewell S, Schulz KF et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trial. J Clin Epi. 2010; 63(8):834–40. [DOI] [PubMed]
- 4.Romualdi D, Guido M, Ciampelli M, et al. Selective effects of pioglitazone on insulin and androgen abnormalitiesin normo- and hyperinsulinaemic obese patients with polycystic ovary syndrome. Hum Reprod. 2003;18(6):1210–1218. doi: 10.1093/humrep/deg264. [DOI] [PubMed] [Google Scholar]
- 5.Froment P, Touraine P. Thiazolidinediones and fertility in polycystic ovary syndrome (PCOS). PPAR Res. 2006;2006:73986. Published online 14 Dec 2006. doi:10.1155/PPAR/2006/73986. [DOI] [PMC free article] [PubMed]
- 6.Ciampelli M, Lanzone A. Insulin and polycystic ovary syndrome: a new look to an old subject. Gynecol Endocrinol. 1998;12:277–92 [ISI] [Medline]. [DOI] [PubMed]
- 7.Cho LW, Kilpatrick ES, Holding S et al. Comparison of metformin, orlistat and pioglitazone in treatment of polycystic ovarian syndrome. Society for Endocrinology Annual Meeting 2006 (University of Hull, UK), (Dept. of Clinical Chemistry, Hull Royal Infirmary, Hull, UK). Endocrinology Abstracts (2006) 12 P113.
- 8.Ortega-Gonzalez C, Luna S, et al. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin resistant women with PCOS. J Clin Endocrinol Metab. 2005;90(3):1360–1365. doi: 10.1210/jc.2004-1965. [DOI] [PubMed] [Google Scholar]