Abstract
Electroconvulsive therapy (ECT) is a potent therapy in severe treatment-refractory depression. Although commonly applied in psychiatric clinical routine since decades, the exact neurobiological mechanism regarding its efficacy remains unclear. Results from preclinical and clinical studies emphasize a crucial involvement of the serotonin-1A receptor (5-HT1A) in the mode of action of antidepressant treatment. This includes associations between treatment response and changes in 5-HT1A function and density by antidepressants. Further, alterations of the 5-HT1A receptor are consistently reported in depression. To elucidate the effect of ECT on 5-HT1A receptor binding, 12 subjects with severe treatment-resistant major depression underwent three positron emission tomography (PET) measurements using the highly selective radioligand [carbonyl-11C]WAY100635, twice before (test–retest variability) and once after 10.08±2.35 ECT sessions. Ten patients (∼83%) were responders to ECT. The voxel-wise comparison of the 5-HT1A receptor binding (BPND) before and after ECT revealed a widespread reduction in cortical and subcortical regions (P<0.05 corrected), except for the occipital cortex and the cerebellum. Strongest reductions were found in regions consistently reported to be altered in major depression and involved in emotion regulation, such as the subgenual part of the anterior cingulate cortex (−27.5%), the orbitofrontal cortex (−30.1%), the amygdala (−31.8%), the hippocampus (−30.6%) and the insula (−28.9%). No significant change was found in the raphe nuclei. There was no significant difference in receptor binding in any region comparing the first two PET scans conducted before ECT. This PET study proposes a global involvement of the postsynaptic 5-HT1A receptor binding in the effect of ECT.
Keywords: antidepressant, [carbonyl-11C]WAY100635, electroconvulsive therapy, major depression, positron emission tomography, serotonin-1A receptor
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are the first line treatment for major depression. Still, up to 60% of the treated patients fail to achieve full symptomatic remission after an adequate trial with SSRIs,1, 2 while 30% are even rated as treatment-refractory.3 Therapy-refractory depression is defined by the failure of at least two trials with antidepressants of a different substance class for a minimum period of 1 month and in a sufficiently high dosage (equivalent to 150 mg of tricyclic antidepressants).4, 5 For these patients electroconvulsive therapy (ECT) is a rapidly acting, highly effective treatment option,4, 5, 6, 7, 8 as ECT leads to a significantly greater reduction of the Hamilton Rating Scale for Depression (HAM-D) than treatment with SSRIs in patients with major depressive episodes.9 Moreover, ECT has been shown to be more effective in delusional depression, psychosis and catatonic conditions.10 ECT is frequently referred to as a more precarious and risky treatment compared with antidepressants, as it is performed under short anesthesia while generating a generalized epileptic seizure11 and may be accompanied by cognitive impairment, such as deficits in working memory during the early post-treatment period.12 Although ECT represents an effective treatment option, when patients fail to respond sufficiently to SSRI or other antidepressants, studies emphasize that ECT have an even better response rate when conducted on subjects who did not yet achieve a state of treatment resistance.13, 14
ECT was successfully used in psychiatry since more than 70 years, however, its therapeutic mechanism of action is unclear. Neuroimaging studies reported unspecific changes in the brain glucose metabolism and cerebral blood flow (rCBF). Preclinical investigations using autoradiography with [14C]2-deoxyglucose in rats undergoing electroconvulsive shocks (ECS) demonstrated a rapid reduction of the brain glucose metabolism after ECS in the nucleus accumbens.15 Positron emission tomography (PET) studies with [18F]fluorodeoxyglucose revealed a significantly reduced postictal glucose utilization in depressive patients.16, 17, 18, 19 Both glucose metabolism and rCBF, visualized using the radioligands [18F]fluorodeoxyglucose and [15O]H2O, respectively, decreased in frontal cortical areas after 6–11 bilateral ECT sessions in patients.20 Likewise Prohovnik et al.21 noted that bilateral ECT results in bilateral frontal CBF reduction, where right unilateral treatment leads to a greater decrease of CBF in the right hemisphere. These results could not be directly related to the therapeutic efficacy of ECT, as acute effects of the generalized seizure itself on glucose metabolism and CBF could not be excluded. Furthermore, a proposed correlation between the reduction in HAM-D scores and cerebral metabolic rates of glucose could not be confirmed.19 Regarding regional CBF, Takano et al. showed a decrease in the medial frontal cortex and the anterior cingulate cortex (ACC) in six depressive patients after ECT using [15O]H2O, while rCBF increased in subcortical regions, such as the thalamus.20 However, both PET methods provide rather unspecific topological data, thus there is an urgent need to investigate specific changes in neurotransmission and dedicated molecular targets with PET in patients treated with ECT.
The serotonergic neurotransmitter system has been subject to intense research in the last decades regarding the pathogenesis and treatment of affective and anxiety disorders.2, 22 The effectiveness of pharmacological treatments targeting key structures of the serotonergic system, mainly the serotonin transporter and distinct serotonin receptors, supports the assumption that altered serotonin neurotransmission is causally associated with the manifestation of clinical symptoms.23, 24 Moreover, serotonergic alterations have been repeatedly described in animal models of depression25 and in clinical studies with depressed patients.26, 27 In recent years, the serotonin-1A receptor subtype (5-HT1A) has been extensively investigated with respect to affective disorders as it represents a main inhibitory serotonergic receptor of at least 16 known receptor subtypes.28, 29 The presynaptic 5-HT1A receptor that regulates autoinhibition of serotonergic firing and serotonin release is localized on serotonergic neurons of the midbrain raphe region. The postsynaptic 5-HT1A receptor that mediates inhibition of excitation by serotonin is mainly expressed on glutamatergic and GABAergic neurons.29, 30 The 5-HT1A receptor density is highest in the hippocampus, the cingulate cortex (especially in the subgenual part of the anterior cingulate), the orbitofrontal cortex (OFC), the insula, the amygdala, and in the midbrain raphe region,30 which implies that the regional distribution of the 5-HT1A concentrates in the brain areas with strong involvement in emotional and affective processes.31 Postmortem data32 and pharmacological challenge studies33 pointed toward altered 5-HT1A receptor density in depression. PET studies using the highly specific radioligand [carbonyl-11C]WAY100635 confirmed alterations of the 5-HT1A receptor binding in patients suffering from depression34, 35 and anxiety disorders.22, 36, 37 Most human PET studies have reported a widespread reduction of 5-HT1A receptor binding in patients compared to healthy individuals,28, 38 with exception of the studies published by Parsey et al.39 In a cross-sectional study Parsey et al.39 showed higher 5-HT1A receptor binding in antidepressant-naïve depressive subjects compared with both patients previously exposed to antidepressants and healthy controls across investigated brain regions. This reduction of the 5-HT1A BP induced by medication is consistent with the results of our longitudinal PET study in anxiety patients, demonstrating a further reduction of 5-HT1A receptor binding potential (BPND) in the hippocampus, the subgenual part of the ACC and the posterior cingulate cortex after 12 weeks of treatment with the SSRI escitalopram.2
According to the findings described above, several preclinical studies focused on the influence of ECT on the serotonergic system. Goodwin et al.40 showed a reduction of 5-HT1A receptor-mediated effects after repeated ECS and after repeated administration of the antidepressant drugs zimeldine and desimpramine in rats, underlining the involvement of 5-HT1A receptors in the mechanisms of action of both treatment approaches. After administration of six ECS, microiontophoretic application of serotonin markedly enhanced the responsiveness of hippocampal pyramidal neurons in rats compared with controls undergoing subconvulsive shocks.41 Moreover, these effects were associated with postsynaptic 5-HT1A receptors as neuronal responsiveness to 5-methoxydimethyltryptamine, a direct agonist of postsynaptic 5-HT1A receptors, was increased in ECS-treated animals.41 Further preclinical experiments using 8-OH-DPAT suggested reduced mRNA expression and postsynaptic-binding site densities of the 5-HT1A receptor in the hippocampus.33, 42 These results point toward specific regional effects of ECS on 5-HT1A receptors.42, 43, 44
Taken together, numerous preclinical findings show considerable effects of ECS on serotonin receptors. Also, several studies in humans have demonstrated the involvement of the 5-HT1A receptor in depression and antidepressant drug treatment. Therefore, this study aims to determine the influence of ECT on 5-HT1A receptor binding in treatment-resistant patients suffering from major depression using PET and the radioligand [carbonyl-11C]WAY100635.
Materials and methods
Subjects and study design
Altogether, 18 subjects with severe unipolar depression were included in this ECT study after failure of treatment with at least two adequate trials with antidepressants of different pharmacological classes.5 The Structured Clinical Interviews for DSM-IV (SCID) and the 17-item HAM-D (HAM-D ⩾23) were used for diagnosis and estimation of severity of illness. The participants were recruited through the Department of Psychiatry and Psychotherapy, Medical University of Vienna, comprising the only ECT center in the area of Vienna. Subjects had to fulfill the inclusion criteria for ECT, including an internistic and anesthesiological approval (electrocardiography, thoracic X-ray, laboratory measurements and physical examination), as well as no concomitant major neurological illness, current substance abuse or a history of mania, schizophrenia or schizoaffective disorder. Treatment with drugs targeting directly the 5-HT1A receptor, for example, Aripiprazol, Risperidone, Ziprasidone, Clozapine, Chlorpromazine, Amitryptyline, Nefazodone, Trazodone, Buspirone, Pindolol (Ki<1000 nmol),45 within 1 month prior inclusion was an exclusion criterion. For ethical reasons, antidepressant, antipsychotic and mood-stabilizing medication had to be in steady state for at least 10 days before the baseline PET measurement (PET1) and was continued during the ECT period. Mean time ±s.d. of steady state was 25.5±19.3 days. Benzodiazepines were given in variable dosages and therefore steady-state conditions were not fulfilled. An overview of psychotropic drugs is given in Table 1. Due to technical reasons (PET data reconstruction) two patients had to be excluded from the analysis, while four more patients were dropouts because not all three planned PET scans could be conducted adequately (phobic reaction, failure of radioligand synthesis). Therefore, 12 patients, including 8 women, with a mean age±s.d. of 47.83±11.12 years were enrolled in the final analysis. All subjects provided written informed consent after detailed explanation of the study by an experienced psychiatrist. The participants were reimbursed for participation. The study was approved by the Ethics Committee of the Medical University of Vienna and the General Hospital of Vienna.
Table 1. Medication: antidepressant, antipsychotic, mood-stabilizing and tranquilizing medication given during study duration.
| Patients ID |
Antidepressants |
Antipsychotics | Benzodiazepines | Mood-stabilizers | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSRI | SNRI | NDRI | NAssA | |||||||
| 1 | — | — | — | — | Olanzapine | — | Lorazepam | Zolpidem | — | — |
| 2 | Citalopram | Venlafaxine | — | Mirtazapine | — | — | Alprazolam | Zolpidem | Lamotrigine | — |
| 3 | — | — | — | Mirtazapine | Prothipendyl | — | Lorazepam | Zolpidem | — | — |
| 4 | — | — | — | Mirtazapine | Prothipendyl | — | Lorazepam | — | — | — |
| 5 | — | Venlafaxine | — | — | Prothipendyl | Amysulpride | Lorazepam | Zolpidem | — | — |
| 6 | — | Milnacipran | — | — | Prothipendyl | — | Lorazepam | — | Lamotrigine | Trileptal |
| 7 | — | Duloxetine | — | Mirtazapine | — | — | — | — | Pregabalin | — |
| 8 | Fluoxetine | — | — | — | — | — | Lorazepam | Triazolam | — | — |
| 9 | Escitalopram | — | Bupropion | — | Prothipendyl | — | Lorazepam | Alprazolam | — | — |
| 10 | — | Duloxetine | Bupropion | — | — | — | — | — | — | — |
| 11 | Fluoxetine | — | — | Mirtazapine | Olanzapine | — | Zolpidem | — | — | — |
| 12 | — | Duloxetine | — | Mirtazapine | — | — | — | — | — | — |
Abbreviations: NAssA, noradrenergic and specific serotonergic antidepressant; NDRI, norepinephrine-dopamine reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptale inhibitor.
The doses were chosen individually by experienced psychiatrists and never exceeded the recommended maximum. The medication was in steady-state over 10 days before the baseline positron emission tomography (PET) measurement (PET 1) and was left unchanged till the end of the study except for benzodiazepines, which could be provided in low doses as needed by the patients.
In this longitudinal PET study, the patients underwent three PET measurements using the highly selective and specific radioligand for the 5-HT1A receptors, [carbonyl-11C]WAY100635.2, 35 Two PET scans (PET1 and PET2) were carried out before the first ECT session within 1 week, that is, during steady state of the prescribed medication to determine the test–retest variability under medication. The third PET scan (PET3) was conducted within 7 days after completion of the ECT series. To assess the effect of ECT on depression severity, HAM-D scaling was applied repetitively, that is, at baselines before the start of ECT (PET1 and PET2) and after completion of ECT (PET3) (see Table 2).
Table 2. Demographic and treatment data of the study sample given before (PET1, PET2) and after ECT (PET3).
| Demographic and treatment data in 12 patients | Minimum | Maximum | Mean | s.d. | T | P |
|---|---|---|---|---|---|---|
| Age (years) | 22 | 63 | 47.83 | 11.12 | — | — |
| Sex (M/F) | 4M, 8F | — | — | — | — | — |
| Total ECT sessions | 4 | 13 | 10.08 | 2.35 | — | — |
| Unilateral ECT sessions (n=12/12 patients) | 4 | 12 | 6.58 | 2.61 | — | — |
| Bilateral ECT sessions (n=8/12 patients) | 0 | 8 | 3.5 | 3.06 | — | — |
| HAM-D before ECT (day of PET1) | 23 | 36 | 28.53 | 4.24 | ||
| HAM-D before ECT (day of PET2) | 17 | 32 | 24.75 | 3.96 | — | — |
| HAM-D after ECT (day of PET3) | 2 | 14 | 7.17 | 3.95 | — | — |
| HAM-D before vs after ECT | — | — | 17.58 | 6.65 | 9.16 | <0.01 |
Abbreviations: ECT, electroconvulsive therapy; HAM-D, Hamilton Rating Scale for Depression; PET, positron emission tomography.
A significant reduction of HAM-D values was found after ECT, testifying for an overall successful treatment response. In some patients the HAM-D17 scores obtained at screening visit (⩾23 for meeting the inclusion criteria) were higher than those at PET2, pointing toward a subtle therapeutic effect of the involved medical care.
Electroconvulsive therapy
ECT was conducted using the Thymatron® System IV device (Somatics, LLC., Lake Bluff, IL, USA) according to the standard operating procedures of the Department of Psychiatry and Psychotherapy, based on international guidelines and consensus statements for ECT treatment.8, 9 Briefly, the patients were anesthesized (methohexital) and given muscle relaxants (succinylcholine) before each of the ECT stimuli, which were carried out three times a week. Five ECT were provided unilaterally using an electrode placement in the right frontotemporal position. A bilateral treatment approach was used in patients with minimal or no improvement of the depressive symptoms, according to the HAM-D score, from the sixth ECT session onward (n=8). Seizure duration was determined routinely by electroencephalography, whereas the stimulus intensity was chosen according to the titration at the first treatment.46 At the first treatment of each patient, repeated stimuli of increasing intensity were administered until a seizure occurred, where the lowest stimulus intensity able to induce a seizure was defined as the threshold. In the following treatments the charge was set at three times the seizure threshold. The intensity was further elevated in the absence of seizure activity or in case of inadequate seizures. In our sample, the minimum of administered ECT was four and the maximum was 13 sessions (see Table 2 for details).
Positron emission tomography
All PET measurements were carried out at the Department of Nuclear Medicine, Medical University of Vienna, using a Siemens Biograph 64 TruePoint PET-CT scanner (Siemens Medical, Erlangen, Germany) as described previously.22 Briefly, the patients' heads were placed into the scanner parallel to the orbitomeatal line with a laser beam system. Head movement was minimized by a polyurethane molded cushion and straps around the forehead and chin. The protocol started with a computed tomography attenuation scan, followed by three-dimensional dynamic emission measurement and simultaneous injection of the radioligand [carbonyl-11C]WAY100635 (mean injected dose±s.d.=224.82±47.62 MBq). The synthesis of the radioligand was carried out as published previously.47 Scans lasted for 90 min, acquired in list-mode and reconstructed into 23 consecutive frames (15 × 1 min, 5 × 5 min, 1 × 10 min, 2 × 20 min). For image reconstruction a Siemens standard algorithm was used (TrueX,48 ‘HD-PET option'), which includes modeling of the scanner's point spread function. Such point spread function reconstruction methods have been shown to improve neuroreceptor quantification through reduction of partial volume effects.49 The final images comprised a uniform spatial resolution of 2 mm full-width at half-maximum 1 cm next to the center of the field of view (matrix 128 × 128, 109 slices).
Data preprocessing and 5-HT1A quantification
PET scans were corrected for head motion and normalized to the stereotactic space defined by the Montreal Neurological Institute with SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) using a study- and tracer-specific template.50, 51, 52 The normalization procedure was optimized similar to a recently proposed method for longitudinal data analysis.53 For each patient, the second and third PET scans were individually coregistered to the first one. The mean of the three PET scans then served as a template to align all three scans in a second coregistration step. Afterwards, the coregistered scans were averaged again and the mean image was used to estimate the normalization transformation matrix, which was finally applied to the individual (coregistered) PET scans. SPM8 standard parameters were applied for all preprocessing steps, except for the realignment (quality=1) and spatial normalization (affine regularization=average-sized template).
Quantification of the 5-HT1A BPND54 was done in PMOD 3.3 (PMOD Technologies, Zurich, Switzerland) as described previously.55 Briefly, the multilinear reference tissue model 2 (MRTM256) was applied to obtain voxel-wise 5-HT1A receptor BPND. Here, the clearance rate of the radiotracer from the reference region to plasma (k2′) was calculated from the insula and cerebellum (that is, receptor rich and poor region56, 57). These regions of interest were taken from an automated anatomical labeling-based atlas,52, 58 whereas the cerebellar gray matter (excluding vermis) served as reference region due to negligible-specific receptor binding.59
Statistical analysis
To evaluate the difference in 5-HT1A binding across time, we conducted a voxel-wise repeated measures ANOVA in SPM8 with subjects and time (that is, PET scans 1, 2 and 3) as between- and within-subject factors, respectively. Following an overall F-test, subsequent post-hoc t-tests were carried out between the three PET scans. Hence, we aimed to assess both test–retest variability in this clinical population during steady-state medication (PET1 vs PET2) and ECT-induced effects on 5-HT1A binding (PET2 vs PET3). To test for potential confounders, further interaction analyses were carried out between treatment-induced effects on 5-HT1A BPND (PET2 vs PET3) and sex, ECT mode (uni/bilateral), treatment outcome (remitter/responder/non-responder) and anticonvulsive medication. Furthermore, we investigated the effects of the covariates age and the number of ECT sessions. Finally, voxel-wise correlation was used to investigate the association between changes in 5-HT1A binding and HAM-D scores before and after ECT, as well as the prediction of treatment-induced changes in HAM-D scores by baseline 5-HT1A binding. Similarly, the influence of steady-state time on changes in 5-HT1A binding was evaluated by correlation analysis. All statistical tests were evaluated at P<0.05 corrected for multiple comparisons with the false discovery rate (FDR) at voxel level. Regions of no interest (for example, skull and cerebral blood vessels) were excluded from the statistical analyses. Absolute and relative changes in 5-HT1A BPND between PET scans were calculated as PET3 − PET2 and, (PET3 − PET2)/PET2, respectively. To evaluate hemispheric differences in 5-HT1A BPND changes, maps representing absolute changes were axially flipped and together with the original ones used within a paired-samples t-test in SPM8.
Results
We found a significant reduction of HAM-D scores after ECT (P<0.01, see Table 2), emphasizing the efficacy of ECT in severe unipolar depression. Ten patients (83.3%) were considered as treatment responders, given a reduction of the HAM-D score corresponding to the baseline value divided by two. Three of the latter had a full remission of the symptoms with a HAM-D score lower than seven. On the other hand, two patients failed to show a sufficient response to ECT. The evaluation of the 5-HT1A BPND across time showed a significant main effect of the ECT in virtually all cortical areas and subcortically within the hippocampus–amygdala region (F2,22>6.75, P<0.05 FDR corrected). The PET measurements before ECT (PET1 vs PET2, Figures 1a and b) showed no significant differences in 5-HT1A binding in any brain region (P>0.05 FDR corrected). In contrast, we found significant changes when comparing 5-HT1A BPND before and after electroconvulsive treatment (PET2 vs PET3, Figures 1b and c). Specifically, post-hoc t-test revealed a large interconnected cluster of 436 cm3 with significant reductions in 5-HT1A receptor binding comprising almost the entire cortex (P<0.05 FDR corrected, Figure 2). These reductions reached peak differences beyond 25% in, for example, the ACC including its subgenual part (sgACC), the OFC, insula, the hippocampus and amygdala (see Table 3 and Supplementary Material for details). The direct comparison between PET measurements 1 vs 3 showed similar but slightly stronger reductions in 5-HT1A BPND. There were no significant increases in 5-HT1A BPND after electroconvulsive treatment in any brain region. Evaluation of potential confounding variables showed neither significant interactions between changes in 5-HT1A binding and sex, uni-/bilateral ECT, anticonvulsive medication or treatment outcome nor influence of the covariates age and number of ECT sessions. Investigating lateralization of 5-HT1A BPND changes showed no significant differences between left and right hemisphere. Also, there were no significant correlations between the magnitude of treatment-induced 5-HT1A BPND change and changes in HAM-D scores. The prediction of HAM-D changes by baseline 5-HT1A BPND was not significant. Similarly, steady-state time of medications did not correlate with changes in 5-HT1A binding (all P>0.05 FDR corrected).
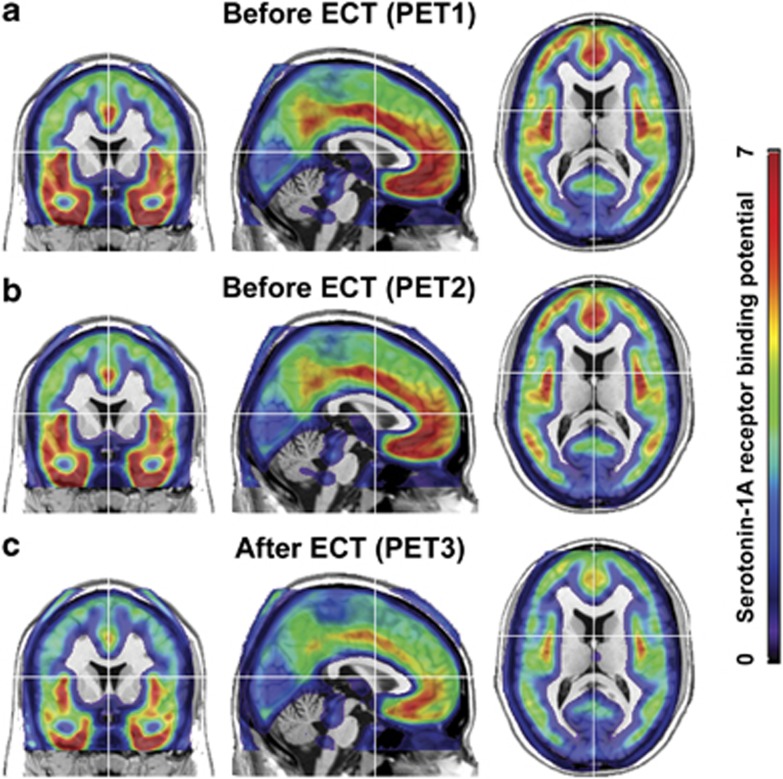
Figure 1.
Average serotonin-1A receptor (5-HT1A) binding potential (BPND) of patients with major depressive disorder (n=12) at baselines before electroconvulsive therapy (ECT), that is, positron emission tomography (PET)1 (a) and PET2 (b), and after ECT, that is, PET3 (c). Note that 5-HT1A BPND is virtually identical between the baselines (PET1, PET2) before treatment, whereas after ECT a reduction in receptor binding can be observed almost across the entire cortex. For visualization, values below 0.5 are not shown.
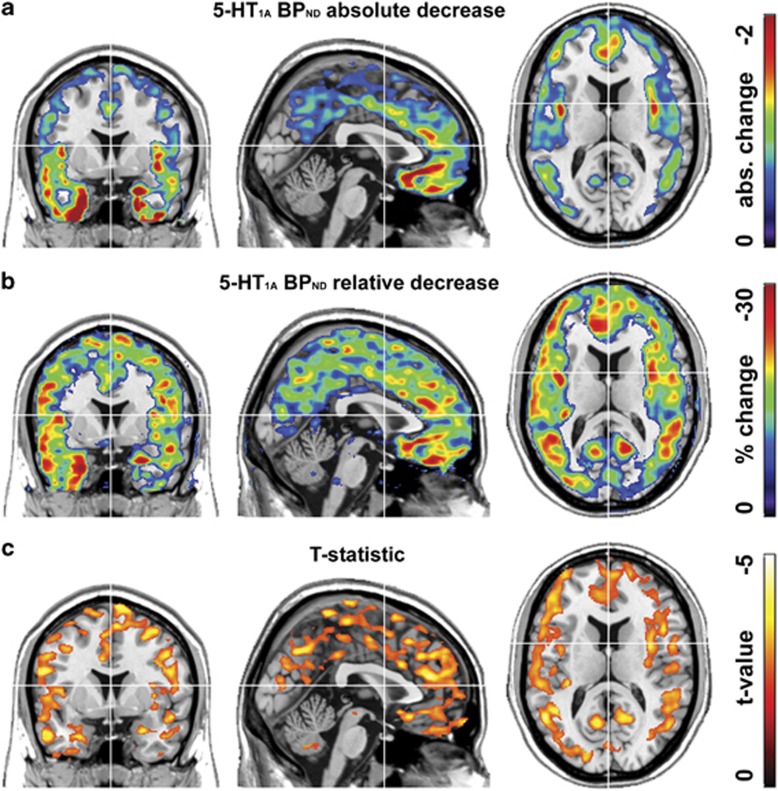
Figure 2.
Changes in serotonin-1A receptor (5-HT1A) binding potentials (BPND) before (PET2) vs after electroconvulsive therapy (PET3). Shown are absolute (a) and relative (b) differences in 5-HT1A BPND as well as the post-hoc t-test (c) of the repeated measures ANOVA (t>2.31, P<0.05 false discovery rate (FDR) corrected). For visualization, values below 0.5 and 5% are not included in a and b, respectively.
Table 3. 5-HT1A BPND for selected ROI taken from an AAL-based atlas52, 58.
| Region |
MNI coordinates (mm) |
5-HT1A
binding potential |
5-HT1A
before vs after ECT |
|||||
|---|---|---|---|---|---|---|---|---|
|
PET1 |
PET2 |
PET3 |
PET2 vs PET3 |
|
||||
| x | y | z | before ECT | before ECT | after ECT | Peak t-value | % Change | |
| Anterior cingulate L | −2 | 40 | 20 | 5.9±1 | 5.9±1.4 | 4.3±1.1 | 4.28* | −25.9±21.7 |
| Anterior cingulate R | 2 | 40 | 20 | 5.7±0.9 | 5.7±1.2 | 4.1±1.1 | 4.58* | −26.2±23.9 |
| Subgenual L | −2 | 36 | −10 | 8.5±2.5 | 7.7±2.3 | 5.8±2.2 | 2.69* | −23.1±26.8 |
| Subgenual R | 6 | 36 | −8 | 4.4±1 | 4.3±1 | 3±1.1 | 3.77* | −27.5±27.3 |
| Median cingulate L | −2 | −36 | 38 | 4±0.8 | 4.1±1.1 | 3.2±0.8 | 3.55* | −19.7±22.7 |
| Median cingulate R | 4 | 36 | 34 | 4.8±1 | 4.6±1.3 | 3.4±0.9 | 4.54* | −23.6±19.7 |
| Posterior cingulate L | −8 | −54 | 10 | 2.7±0.6 | 2.6±0.6 | 2±0.5 | 3.59* | −20.9±17.4 |
| Posterior cingulate R | 6 | −46 | 28 | 2.2±0.6 | 2.2±0.7 | 1.7±0.5 | 4.05* | −20.7±23.5 |
| Amygdala L | −26 | 4 | −26 | 6.2±1.2 | 6.3±1.7 | 4.1±1.3 | 3.91* | −31.8±26.6 |
| Amygdala R | 26 | 4 | −24 | 6.4±1.6 | 5.6±1.7 | 4±1.3 | 2.9* | −26.1±26 |
| Caput hippocampus L | −24 | −6 | −22 | 8.5±2.6 | 8.8±3.2 | 5.8±2.2 | 3.03* | −30.6±30.8 |
| Caput hippocampus R | 26 | −2 | −24 | 5.4±1 | 4.9±1.1 | 3.5±1.4 | 2.86* | −27.8±24.4 |
| Superior frontal orbital L | −12 | 66 | −12 | 5.5±1.4 | 5.9±1.8 | 4±1.5 | 4.17* | −30.1±28.2 |
| Superior frontal orbital R | 22 | 68 | −6 | 5.6±0.9 | 5.5±1.1 | 4.1±1.3 | 4.77* | −26.4±19.3 |
| Insula L | −40 | 20 | −10 | 4.3±1.3 | 4.3±1.2 | 3.3±1.1 | 4.44* | −24.6±14.1 |
| Insula R | 44 | −12 | 2 | 4.8±0.9 | 5±1.4 | 3.4±1 | 4.72* | −28.9±24.5 |
| Dorsal raphe nucleus | −2 | −28 | −8 | 1.2±0.4 | 1.1±0.5 | 1±0.3 | 1.15 | −5.7±26.4 |
Abbreviations: BPND, binding potential; ECT, electroconvulsive therapy; 5-HT1A, serotonin-1A receptor; MNI, Montreal Neurological Institute; ROI, regions of interest.
5-HT1A BPND values are given as mean±s.d. at baseline before ECT (PET1, PET2) and after ECT. Following voxel-wise post-hoc t-test of the repeated measures ANOVA, peak t-values (*t>2.31, P<0.05 false discovery rate (FDR) corrected) and relative changes (%) are obtained when comparing 5-HT1A binding before (PET2) and after ECT (PET3). For further detail see Supplementary table S1.
Discussion
The main finding of this longitudinal PET study is the overall decrease of the 5-HT1A receptor BPND induced by ECT in cortical areas as well as in the hippocampus–amygdala region. The treatment response rate of ECT based on HAM-D scores in this study sample was ∼83%, which is higher than the commonly reported ∼60% response rate in the literature.60 In a study with 80 depressed patients, response rates of ECT were up to 80% 1–2 days after the treatment course, and they decreased to 65% 1 week after treatment termination.46 This is consistent with our results as the final study evaluation was completed mostly 1 day after the last ECT session.
Regarding the main PET results of our ECT study in patients with MDD, the 5-HT1A BPND reduction was in the range between ∼20 and ∼30% in most brain areas (see Table 3 and Supplementary Table for details). The highest reductions in 5-HT1A BPND were found in the hippocampus (−30.6%), amygdala (−31.8%), OFC (−30.1%), ACC (−26.2%) and insula (−28.9%). Interestingly, the effect size is similar to the decrease of 5-HT1A receptor BPND in the hippocampus (−28.2%) and subgenual part of the ACC (−25.4%) after treatment with SSRIs, revealed by PET in patients with anxiety disorders.2 Furthermore, regarding the topology, our ECT results coincide with the pharmacological treatment studies using PET and [carbonyl-11C]WAY100635,2 and also with the consistently reported alterations of the hippocampus, amygdala, ACC and the OFC in major depression. As for the amygdala, a brain area essential to the processing of emotion, a high number of neuroimaging studies using functional magnetic resonance imaging demonstrated a hyperactivation of this subcortical structure in the presence of negatively affected pictures, for example, fearful faces, in depressive patients compared with healthy controls.61 This insight is equally mirrored in findings provided from structural magnetic resonance imaging studies, pointing toward an increased amygdala volume in depressive patients.62 Moreover, the supposed key role of the amygdala in the pathogenesis of psychiatric disorders is further substantiated by molecular imaging data showing alterations of this region in patients suffering from major depression, anxiety disorders and bipolar disorder.28, 63, 64, 65 Voxel-based morphometry studies revealed smaller ACC volumes in patients with major depression, which is in line with the frequently hypothesized impaired top–down regulation of frontal cortical areas on limbic structures in affective disorders, supposedly resulting in the frequently described disinhibition of the amygdala.66 Accordingly, alterations in glucose metabolism and CBF were found within the amygdala and more pronounced in the sgACC in depression,67 which is in turn decreased by various treatments as reported by Hamani et al.68 In remitted patients exposed to tryptophan depletion-induced depressive relapse, decreased brain metabolism was revealed in the OFC compared with patients administrated placebo.69 In addition, widespread reductions of 5-HT1A receptor BPND were found in cortico–limbic brain areas, including the amygdala, the ACC and the OFC in depressive patients.28, 38, 70
On the basis of the predominantly described reduced 5-HT1A receptor binding accompanying psychiatric disorders,34, 71 one might presume that treatment response should consequently walk along with a ‘normalization', hence an increase of 5-HT1A receptor BPND. However, PET studies of pharmacological treatment response have not confirmed this premise.2, 36, 70 The 5-HT1A receptor BPND was shown to be significantly reduced in anxiety disorders22 and an even further reduction in receptor BPND after 12 weeks of escitalopram treatment in limbic (hippocampus) and cortical brain regions (posterior cingulate cortex, subgenual part of the ACC) was revealed in a longitudinal PET.2 This finding is in concert with our results, showing a global decrease of postsynaptic 5-HT1A receptor binding in virtually the entire cortex after ECT, despite the fact that one could expect an increase. Besides, there is a lack of clarity with regard to antidepressant treatment effects on presynaptic 5-HT1A receptors.70, 72 However, on a system level in a pharmacological treatment study we found that SSRIs change the interplay between pre- and postsynaptic 5-HT1A receptor BPND, indicating that the re-adjustment between presynaptic 5-HT1A receptor-mediated serotonergic firing and the postsynaptic 5-HT1A receptor-mediated inhibition by serotonin might be an important mechanism of action.55 Accordingly, one could presume that—taking into consideration the involvement of numerous complex mechanisms and presumably different transmitter systems—successful ECT leads to a sort of ‘reset' of the brain resulting in a new arrangement of neuronal networks on a molecular level, reflected in a reduction of 5-HT1A heteroreceptors in serotonergic projection areas.55
The significant reductions of postsynaptic 5-HT1A receptor binding in cortical regions demonstrated in our ECT study match preclinical results showing decreased 5-HT1A receptor densities in the hippocampal region CA4 after ECS in rats.42 However, our findings are in contrast to the enhanced 5-HT1A receptor binding and serotonergic neurotransmission after ECS in pyramidal cells of the hippocampus and the dentate gyrus.41, 42 Likewise, regarding serotonin-2A (5-HT2A) receptors, ECS was consistently shown to increase 5-HT2A receptor densities and mRNA expression in rodents,42 whereas in a PET study using [18F]setoperone in non-human primates, ECS leads to a downregulation of 5-HT2A receptors in several cortical regions lasting up to 1 week post-treatment.73 Yatham et al. equally demonstrated a widespread reduction of 5-HT2A receptor binding in 15 patients suffering from major depression after ECT, with peak changes in the parahippocampal gyrus and the medial prefrontal cortex.74 These discrepancies might be ascribed to species differences in such complex neuronal networks.
To the best of our knowledge, only one PET study so far investigated the 5-HT1A receptor binding using [carbonyl-11C]WAY100635 in depressed patients undergoing ECT.75 Applying a region of interest analysis approach, they found no effect of ECT on 5-HT1A receptor binding in a group of nine patients. Although Saijo et al. administered a series of 6–7 bilateral ECTs, we started with unilateral ECT and switched to bilateral ECT in 8 of 12 patients due to insufficient treatment response. However, the total number of ECT was significantly higher (10.08±2.35, mean±s.d.) in our sample. The effect size regarding 5-HT1A receptor BPND reduction might increase with the number of ECT sessions in accordance with cumulative clinical effects in ECT series. In both studies pharmacological treatment was continued during study participation. Furthermore, there was a predominance of men (6 males of 9 subjects) in the study sample of Saijo et al., whereas in our study more women (4 males of 12 subjects) were included. One distinction concerns the population group, investigating Caucasian patients in our study and Asian patients in Japan. From a methodological point of view concerning PET data analysis, our study focussed on whole-brain voxel-wise analysis. This includes advantages over region of interest-based assessment due to its independence of choice, size, definition and location of a limited number of regions. On the other hand, voxel-wise analyses require correction for multiple comparisons, where region of interest-based evaluation may represent a reasonable alternative because of increased signal-to-noise ratio. Analysis procedures were similar in the two studies in terms of reference region (cerebellum gray matter excluding cerebellar vermis) and the applied model (SRTM vs MRTM2). The relatively small sample sizes might be a critical point in both studies as well as the exposure to pharmacological antidepressant treatment during the study. Despite this limitation, a discontinuation of drugs was inacceptable in view of the clinical severity of the depressive symptoms and therefore ethical reasons. We cannot exclude differences in the interaction between ECT and pharmacological treatment that might affect 5-HT1A receptor binding, given the differences in medication between both studies.
We found no correlation between the treatment outcome (HAM-D scores) and the magnitude of changes in 5-HT1A receptor binding in this sample with high response rate. This suggests a dose-independent or suprathreshold effect of ECT on 5-HT1A receptor binding. This finding is in line with a study showing no association of HAM-D scores and changes in the brain glucose metabolism after ECT in major depression.19
In sum, this study shows a significant global decrease of 5-HT1A receptor binding in major depression after completion of ECT with considerable changes in areas having a major role in affective processes in the human brain, such as the hippocampus, amygdala, cingulate and orbitofrontal cortices. Altered function of these areas represents a consistent finding in depression in psychiatric research. The results of the present PET study imply that ECT might act upon similar molecular mechanisms, particularly the 5-HT1A receptor, involved in the mode of action of pharmacological antidepressant treatments.
Acknowledgments
This research was funded by grants from the Austrian National Bank (OeNB 13219) to R. Frey and the Brain and Behavior Research Foundation, USA (NARSAD, http://bbrfoundation.org/) to R. Lanzenberger. A Hahn is recipient of a DOC-fellowship of the Austrian Academy of Sciences (OeAW) at the Department of Psychiatry and Psychotherapy. We thank Hofer-Irmler Irmgard, MD, Spindelegger Christoph, MD, and Höflich Anna, MD for clinical and scientific support. We thank the PET team at the Department of Nuclear Medicine for technical support. We are especially grateful to Professor Dudczak Robert, MD, head of the Department of Nuclear Medicine, and Professor Kletter Kurt, MD, for their generous support.
Without any relevance to this work, S Kasper declares that he has received grant/research support from Eli Lilly, Lundbeck A/S, Bristol-Myers Squibb, Servier, Sepracor, GlaxoSmithKline, Organon, and has served as a consultant or on advisory boards for AstraZeneca, Austrian Sick Found, Bristol-Myers Squibb, GlaxoSmithKline, Eli Lily, Lundbeck A/S, Pfizer, Organon, Sepracor, Janssen, and Novartis, and has served on speakers' bureaus for AstraZeneca, Eli Lilly, Lundbeck A/S, Servier, Sepracor and Janssen. R Lanzenberger received travel grants and conference speaker honoraria from AstraZeneca and Lundbeck A/S. M Mitterhauser and W Wadsak received speaker honoraria from Bayer. R Frey declares that he has received grant/research support from Bristol-Myers Squibb, AstraZeneca, Sandoz, Eli Lilly, and Janssen.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Parts of this study were presented at the 24th ECNP congress, Paris, France.
Supplementary Material
References
- Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry. 2011;72:e18. doi: 10.4088/JCP.8133tx4c. [DOI] [PubMed] [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, et al. Influence of escitalopram treatment on 5-HT1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry. 2008;14:1040–1050. doi: 10.1038/mp.2008.35. [DOI] [PubMed] [Google Scholar]
- Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Br J Psychiatry. 2002;181:284–294. doi: 10.1192/bjp.181.4.284. [DOI] [PubMed] [Google Scholar]
- Husain SS, Kevan IM, Linnell R, Scott AI. Electroconvulsive therapy in depressive illness that has not responded to drug treatment. J Affect Disord. 2004;83:121–126. doi: 10.1016/j.jad.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bschor T, Pfennig A, Whybrow PC, Angst J, Versiani M, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders in primary care. World J Biol Psychiatry. 2007;8:67–104. doi: 10.1080/15622970701227829. [DOI] [PubMed] [Google Scholar]
- Schosser A, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, et al. European Group for the Study of Resistant Depression (GSRD) - where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol. 2012;22:453–468. doi: 10.1016/j.euroneuro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Frey R, Schreinzer D, Heiden A, Kasper S. Use of electroconvulsive therapy in psychiatry] Nervenarzt. 2001;72:661–676. doi: 10.1007/s001150170045. [DOI] [PubMed] [Google Scholar]
- Abrams R. Stimulus titration and ECT dosing. J ECT. 2002;18:3–9. doi: 10.1097/00124509-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Folkerts HW, Michael N, Tolle R, Schonauer K, Mucke S, Schulze-Monking H. Electroconvulsive therapy vs paroxetine in treatment-resistant depression—a randomized study. Acta Psychiatr Scand. 1997;96:334–342. doi: 10.1111/j.1600-0447.1997.tb09926.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Whybrow PC, Angst J, Versiani M, Moller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: acute and continuation treatment of major depressive disorder. World J Biol Psychiatry. 2002;3:5–43. doi: 10.3109/15622970209150599. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157 (4 Suppl:1–45. [PubMed] [Google Scholar]
- Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68:568–577. doi: 10.1016/j.biopsych.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Decina P, Kerr B, Malitz S. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. J Clin Psychopharmacol. 1990;10:96–104. doi: 10.1097/00004714-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Lock T, McCulloch J. Local cerebral glucose utilization after chronic electroconvulsive shock: implications for the mode of action of electroconvulsive therapy. J Psychopharmacol. 1991;5:111–119. doi: 10.1177/026988119100500204. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell C, Sackeim HA, et al. Decreased regional brain metabolism after ECT. Am J Psychiatry. 2001;158:305–308. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- Ackermann RF, Engel J, Baxter L. Positron emission tomography and autoradiographic studies of glucose utilization following electroconvulsive seizures in humans and ratsa. Annals N Y Acad Sci. 1986;462:263–269. doi: 10.1111/j.1749-6632.1986.tb51260.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Kuhl DE, Phelps ME. Patterns of human local cerebral glucose metabolism during epileptic seizures. Science. 1982;218:64–66. doi: 10.1126/science.6981843. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Clark CC, Zis AP. A preliminary study of the effects of electroconvulsive therapy on regional brain glucose metabolism in patients with major depression. J ECT. 2000;16:171–176. doi: 10.1097/00124509-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Bellar S, Mullani N, Jould L, Dewey S. Effects of electroconvulsive therapy on brain glucose metabolism: a preliminary study. Convuls Ther. 1988;4:199–205. [PubMed] [Google Scholar]
- Prohovnik I, Sackeim HA, Decina P, Malitz S. Acute reductions of regional cerebral blood flow following electroconvulsive therapy. Interactions with modality and time. Ann N Y Acad Sci. 1986;462:249–262. doi: 10.1111/j.1749-6632.1986.tb51259.x. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Best J, Nijhout HF, Reed M. Bursts and the efficacy of selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2011;44 (Suppl 1:S76–S83. doi: 10.1055/s-0031-1273697. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, McKinney WT., Jr Depressive disorders: toward a unified hypothesis. Science. 1973;182:20–29. doi: 10.1126/science.182.4107.20. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Sternberg DE. Serotonergic function in depression: prolactin response to intravenous tryptophan in depressed patients and healthy subjects. Arch Gen Psychiatry. 1984;41:398–402. doi: 10.1001/archpsyc.1984.01790150088012. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42:2039–2057. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- Hornung JP.The Neuroanatomy of the Serotonergic SystemIn: Müller CP, Jacobs BL (eds). Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; London, UK, 2010, pp 51–64. [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology. 1991;5:219–229. [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang Y-y, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. Attenuation by electroconvulsive shock and antidepressant drugs of the 5-HT1A receptor-mediated hypothermia and serotonin syndrome produced by 8-OH-DPAT in the rat. Psychopharmacology (Berl) 1987;91:500–505. doi: 10.1007/BF00216018. [DOI] [PubMed] [Google Scholar]
- de Montigny C. Electroconvulsive shock treatments enhance responsiveness of forebrain neurons to serotonin. J Pharmacol Exp Ther. 1984;228:230–234. [PubMed] [Google Scholar]
- Burnet PW, Sharp T, LeCorre SM, Harrison PJ. Expression of 5-HT receptors and the 5-HT transporter in rat brain after electroconvulsive shock. Neurosci Lett. 1999;277:79–82. doi: 10.1016/s0304-3940(99)00857-5. [DOI] [PubMed] [Google Scholar]
- Blier P, Bouchard C. Effect of repeated electroconvulsive shocks on serotonergic neurons. Eur J Pharmacol. 1992;211:365–373. doi: 10.1016/0014-2999(92)90394-j. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Amano T, Hayakawa H, Yamawaki S, Sasa M. Enhancement of serotonin1A receptor function following repeated electroconvulsive shock in young rat hippocampal neurons in vitro. Int J Neuropsychopharmacol. 1999;2:101–104. doi: 10.1017/S1461145799001467. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- Wadsak W, Mien LK, Ettlinger DE, Lanzenberger RR, Haeusler D, Dudczak R, et al. Simple and fully automated preparation of [carbonyl-11C]WAY-100635. Radiochim Acta. 2007;95:417–442. [Google Scholar]
- Casey M.Point spread function reconstruction in PET. Siemens Molecular Imaging 2007; (http://www.medical.siemens.com/siemens/it_IT/gg_nm_FBAs/ files/whtpap/wp_07_btruep_psf_reconstruction.pdf).
- Varrone A, Sjoholm N, Eriksson L, Gulyas B, Halldin C, Farde L. Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging. 2009;36:1639–1650. doi: 10.1007/s00259-009-1156-3. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kapur S, Houle S, DaSilva J, Owczarek B, Brown GM, et al. Prefrontal cortex 5-HT2 receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry. 1999;156:1029–1034. doi: 10.1176/ajp.156.7.1029. [DOI] [PubMed] [Google Scholar]
- Fink M, Wadsak W, Savli M, Stein P, Moser U, Hahn A, et al. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage. 2009;45:598–605. doi: 10.1016/j.neuroimage.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Stein P, Savli M, Wadsak W, Mitterhauser M, Fink M, Spindelegger C, et al. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur J Nucl Med Mol Imaging. 2008;35:2159–2168. doi: 10.1007/s00259-008-0850-x. [DOI] [PubMed] [Google Scholar]
- Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, et al. Changes in brain size during the menstrual cycle. PLoS One. 2011;6:e14655. doi: 10.1371/journal.pone.0014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien L-K, et al. Escitalopram enhances the association of serotonin-1A autoreceptors to heteroreceptors in anxiety disorders. J Neuroscience. 2010;30:14482–14489. doi: 10.1523/JNEUROSCI.2409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, et al. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Abrams R.Electroconvulsive therapy (ECT) practice in Metropolitan New York community hospitals Psychol Med 2002321323–1324.author reply 1324–1326. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord. 2006;94:219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Ichimiya T, Takano A, Ando T, Okubo Y. Decreased 5-HT1A receptor binding in amygdala of schizophrenia. Biol Psychiatry. 2004;55:439–444. doi: 10.1016/j.biopsych.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Wadsak W, Spindelegger C, Mitterhauser M, Akimova E, Mien L-K, et al. Cortisol plasma levels in social anxiety disorder patients correlate with serotonin-1A receptor binding in limbic brain regions. Int J Neuropsychopharmacol. 2010;13:1129–1143. doi: 10.1017/S1461145710000581. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JSD, Simpson N, Huang Y-y, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Raemaekers MA, Ruigrok AN, Hermans EJ, Kenemans JL, Baas JM. Prefrontal mechanisms of fear reduction after threat offset. Biol Psychiatry. 2010;68:1031–1038. doi: 10.1016/j.biopsych.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion—induced depressive relapse. Arch Gen Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1a receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, et al. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [11C]WAY-100635. Synapse. 2007;61:523–530. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome EM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive shock decreases binding to 5-HT2 receptors in nonhuman primates: An in vivo positron emission tomography study with [18F]setoperone. Biol Psychiatry. 2005;57:1004–1010. doi: 10.1016/j.biopsych.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Lam RW, Zis AP, Stoessl AJ, Sossi V, et al. Effect of electroconvulsive therapy on brain 5-HT receptors in major depression. Br J Psychiatry. 2010;196:474–479. doi: 10.1192/bjp.bp.109.069567. [DOI] [PubMed] [Google Scholar]
- Saijo T, Takano A, Suhara T, Arakawa R, Okumura M, Ichimiya T, et al. Effect of electroconvulsive therapy on 5-HT1A receptor binding in patients with depression: a PET study with [11C]WAY 100635. Int J Neuropsychopharmacol. 2010;13:785–791. doi: 10.1017/S1461145709991209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.