Abstract
It is increasingly recognized that we need a better understanding of how mental disorders such as depression alter the brain's functional connections to improve both early diagnosis and therapy. A new holistic approach has been used to investigate functional connectivity changes in the brains of patients suffering from major depression using resting-state functional magnetic resonance imaging (fMRI) data. A canonical template of connectivity in 90 different brain regions was constructed from healthy control subjects and this identified a six-community structure with each network corresponding to a different functional system. This template was compared with functional networks derived from fMRI scans of both first-episode and longer-term, drug resistant, patients suffering from severe depression. The greatest change in both groups of depressed patients was uncoupling of the so-called ‘hate circuit' involving the superior frontal gyrus, insula and putamen. Other major changes occurred in circuits related to risk and action responses, reward and emotion, attention and memory processing. A voxel-based morphometry analysis was also carried out but this revealed no evidence in the depressed patients for altered gray or white matter densities in the regions showing altered functional connectivity. This is the first evidence for the involvement of the ‘hate circuit' in depression and suggests a potential reappraisal of the key neural circuitry involved. We have hypothesized that this may reflect reduced cognitive control over negative feelings toward both self and others.
Keywords: fMRI, functional connectivity, hate circuit, major depression, mental disorder, voxel-based morphometry
Introduction
At the brain circuit level, most of what we understand about depression and its biological abnormalities during the resting state comes from functional magnetic resonance imaging (fMRI) studies targeting changes in a small number of the brain regions, as recently reviewed in Raichle1 and Zhang and Raichle.2 These studies have suggested the involvement of a default mode network including the dorsal anterior cingulate cortex and some subcortical areas such as amygdala and thalamus.3, 4, 5, 6, 7 However, the conclusions drawn from these studies are based on either seed-based analysis or independent component analysis and are questionable in spite of the wide and successful application of such methods in the analysis of resting-state fMRI data.8, 9, 10, 11, 12, 13 Seed-based analysis is a hypothesis-driven approach, which means the foci (seeds) of the disorder must be specified a priori. It is therefore a biased approach lacking a global and independent view.14 With the independent component analysis approach, it is assumed that the human brain is composed of independent components, whereas in reality, different parts of the human brain undoubtedly work in a coordinated manner. Hence, given the complexity and multiple causes of depression together with variability between individuals, a novel, unbiased approach is urgently called for which identifies pathway changes in a holistic manner.
In the current paper, we have adopted such a new holistic approach aiming to unambiguously identify the key connections, which are modified in the brains of depressed patients. To achieve this, we have first applied a canonical template constructed for the whole brain based upon data from a large number of healthy individuals. Using a community discovery algorithm we have developed previously,15 six discrete interconnected communities were recovered from this template, each corresponding to a functional system in the human brain: a default mode network that has been reported extensively in the literature,11, 12 an attention network consisting of frontal and parietal areas, the auditory system, the visual system, the sensory-motor areas and finally a subcortical network. Corresponding functional maps for patients suffering from severe depression with or without pharmacological treatments (first-episode major depressive disorder (FEMDD) patients and resistant major depressive disorder (RMDD) patients) were then constructed using a similar approach enabling us to carry out a thorough comparison between the brains of healthy and depressed individuals. Voxel-based morphometry analysis was also carried out to assess whether gray or white matter volume changes occurred in those brain regions of depressed patients showing altered functional connectivity.
The most significant differences between both FEMDD and RMDD patients and healthy populations occurred in intercommunity rather than intracommunity links. The greatest change was in a circuit associated with feelings of hate, comprising the superior frontal gyrus, insula and putamen,16 where these regions were uncoupled in depressed patients in both hemispheres. The other main circuits identified that were significantly affected in depressed patients included those involved with risk-taking and action, emotion and reward, attention and memory. This is the first time changes in the functional connectivity of the so-called ‘hate circuit' have been identified in the brains of depressed patients.
Subjects and methods
Subjects
Fifteen treatment-naive adult patients with FEMDD (seven females, eight males; mean age 28.27±7.45 years; mean education 12.13±3.60 years) and 24 treatment-RMDD patients (16 females, 8 males; mean age 27.83±7.86 years; mean education 11.92±3.56 years) were recruited from inpatient or outpatient departments of the Second Xiangya Hospital of Central South University in Changsha, Hunan Province, China. The mean illness duration for patients with FEMDD was 21.10±24.67 months, and the mean score on the 17-item version of Hamilton Rating Scale for Depression17, 18 was 26.07±6.51. The mean illness duration for patients with RMDD was 34.00±45.14 months, and the mean score on the 17-item version of Hamilton Rating Scale for Depression was 24.29±3.70. None of the patients had comorbidities with other disorders. Detailed treatments for RMDD patients can be found in Supplementary Table S1.
All RMDD patients were taking antidepressants at the time of the MRI scan and treatment resistance was defined as non-responsiveness to at least two adequate trials in terms of dosage, duration (6 weeks for each trial), and use of different classes of antidepressants consistent with previous studies.19, 20 Non-responsiveness was defined as a <50% reduction in HRSD score21 after a treatment at a minimum dose of 150 mg per day of imipramine equivalents (dose converted using a conversion table22) for 6 weeks.
Thirty-seven age, gender and education duration matched healthy control subjects (14 females, 23 males; mean age 28.22±6.47 years; mean education 13.32±3.29 years) were also recruited (gender matching: χ2 test=4.873, P=0.087; age matching: F=0.037, P=0.964 and education matching: F=1.205, P=0.306).
All patients met the following inclusion criteria: (I) current MDD attack as assessed by two experienced psychiatrists using the Structural Clinical Interview for DSM-IV; (II) 18–45 years of age; (III) right-handed Han Chinese; (IV) Hamilton Rating Scale for Depression scores of at least 17; (V) treatment-naive adult patients with FEMDD had not taken any medication before MRI scan.
Patients and healthy controls were excluded if they had any of the following: (I) a history of neurological diseases or other serious physical diseases; (II) a history of electroconvulsive therapy; (III) history of substance (that is drugs, alcohol and other psychoactive substance) abuse; (IV) comorbidities with other disorders (no evidence for schizoaffective disorder or Axis II, personality disorders and mental retardation); (V) any contraindications for MRI.
This study was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University, Hunan, China. Written informed consent was obtained from all subjects.
Imaging acquisitions and data preprocessing
All functional imaging data were acquired using a 1.5-T GE Signa Twinspeed scanner (General Electric Medical System, Milwaukee, WI, USA) at the Second Xiangya Hospital of Central South University in Changsha, Hunan Province, China. A total of 180 volumes of echo planar images were obtained axially (repetition time, 2000 ms; echo time, 40 ms; slices, 20; thickness, 5 mm; gap, 1 mm; field of view, 24 × 24 mm2; resolution, 64 × 64; flip angle, 90°).
Before functional images preprocessing, the first 10 volumes were discarded to allow for scanner stabilization and the subjects' adaptation to the environment. fMRI data preprocessing was then conducted by SPM8 (University College London, UK; http://www.fil.ion.ucl.ac.uk/spm) and DPARSF (Data Processing Assistant for resting-state fMRI).23 Briefly, the remaining functional scans were first corrected for within-scan acquisition time differences between slices, and then realigned to the middle volume to correct for interscan head motions. Subsequently, the functional scans were spatially normalized to a standard template (Montreal Neurological Institute) and resampled to 3 × 3 × 3 mm3. After normalization, the Blood Oxygenation Level Dependent (BOLD) signal of each voxel was first detrended to abandon linear trend and then passed through a band-pass filter (0.01–0.08 Hz) to reduce low-frequency drift and high-frequency physiological noise. Finally, nuisance covariates including head motion parameters, global mean signals, white matter signals and cerebrospinal fluid signals were regressed out from the Blood Oxygenation Level Dependent signals.
High-resolution whole-brain volume T1-weighted images were acquired sagittally with a 3D spoiled gradient echo pulse sequence (repetition time, 12.1 ms; echo time, 4.2 ms; flip angle, 15° field of view=240 × 240 mm2; acquisition matrix, 256 × 256; thickness, 1.8 mm; number of excitations, 2; 172 slices.) All T1-weighted structural data were processed with VBM5 toolbox (Structural Brain Mapping Group, Jena, Germany; http://dbm.neuro.unijena.de/vbm) based on the SPM software package. After modulate normalizing, the images were segmented into gray matter, white matter and the cerebrospinal fluid. These segmented images were smoothed using a 12-mm full width at half maximum (FWHM) Gaussian kernel. An automated anatomical labeling atlas24 was used to parcellate the brain into 90 regions of interest (ROIs) (45 in each hemisphere). The names of the ROIs and their corresponding abbreviations are listed in Table 1. Volumes of the gray matter and the white matter for each ROI were extracted. Two sample t-tests with Bonferroni correction were then used to assess whether there were significant volume differences between patients and normal controls.
Table 1. The names and abbreviations of the regions of interest (ROIs).
| Regions | Abbr. | Regions | Abbr. |
|---|---|---|---|
| Amygdala | AMYG | Orbitofrontal cortex (middle) | ORBmid |
| Angular gyrus | ANG | Orbitofrontal cortex (superior) | ORBsup |
| Anterior cingulate gyrus | ACG | Pallidum | PAL |
| Calcarine cortex | CAL | Paracentral lobule | PCL |
| Caudate | CAU | Parahippocampal gyrus | PHG |
| Cuneus | CUN | Postcentral gyrus | PoCG |
| Fusiform gyrus | FFG | Posterior cingulate gyrus | PCG |
| Heschl gyrus | HES | Precentral gyrus | PreCG |
| Hippocampus | HIP | Precuneus | PCUN |
| Inferior occipital gyrus | IOG | Putamen | PUT |
| Inferior frontal gyrus (opercula) | IFGoperc | Rectus gyrus | REC |
| Inferior frontal gyrus (triangular) | IFGtriang | Rolandic operculum | ROL |
| Inferior parietal lobule | IPL | Superior occipital gyrus | SOG |
| Inferior temporal gyrus | ITG | Superior frontal gyrus (dorsal) | SFGdor |
| Insula | INS | Superior frontal gyrus (medial) | SFGmed |
| Lingual gyrus | LING | Superior parietal gyrus | SPG |
| Middle cingulate gyrus | MCG | Superior temporal gyrus | STG |
| Middle occipital gyrus | MOG | Supplementary motor area | SMA |
| Middle frontal gyrus | MFG | Supramarginal gyrus | SMG |
| Middle temporal gyrus | MTG | Temporal pole (middle) | TPOmid |
| Olfactory | OLF | Temporal pole (superior) | TPOsup |
| Orbitofrontal cortex (inferior) | ORBinf | Thalamus | THA |
| Orbitofrontal cortex (medial) | ORBmed |
Construction of whole-brain functional network
After data preprocessing, the time series were extracted in each ROI by averaging the signals of all voxels within that region. Pearson correlation coefficients between all pairs of ROIs were first calculated. Significant correlations were detected with a P-value <0.01. A 90 × 90 correlation matrix was obtained for each subject. However, significant correlation between two ROIs may be spurious, that is, a by-product of the correlations of the two ROIs with a third region. To find out whether the correlation for the two ROIs is genuine, the third ROI should be kept constant. Statistically, this problem can be tackled by means of a partial correlation test. In such a test, the effects of the third ROI upon the relation between the other two ROIs are eliminated. By calculating partial correlation coefficients between all pairs of ROIs with all the remaining ROIs being controlling variables, a 90 × 90 connection matrix was obtained for each subject with a P-value <0.01. The population level network can be obtained by summarizing all individual networks in FEMDD, RMDD and Healthy subject groups, respectively, and thresholding them into binarized matrices with matched and reasonable sparsity values (defined as the total number of edges in a network divided by the maximum number of possible edges). In the analysis of this paper, the sparsities of the normal, FEMDD and RMDD networks were 2.74, 2.77 and 2.64%, respectively.
Community mining algorithm
A network community generally refers to a group of vertices within which the connecting links are dense but sparse in between. In this study, a community structure of the functional network of the brain corresponds to groups of brain regions that have similar functions and dense functional connectivity with each other. Our former developed community mining algorithm described in Yang et al.15 tries to explore the notion of network modularity by means of understanding the dynamics of the network, which can naturally reflect the intrinsic properties of the network with modularity structure and exhibit local mixing behaviors. Based on large deviation theory,15 this algorithm sheds light on the fundamental significance of the network communities and the intrinsic relationships between the modularity and the characteristics of the network. See Supplementary Information for more details.
Results
Canonical template
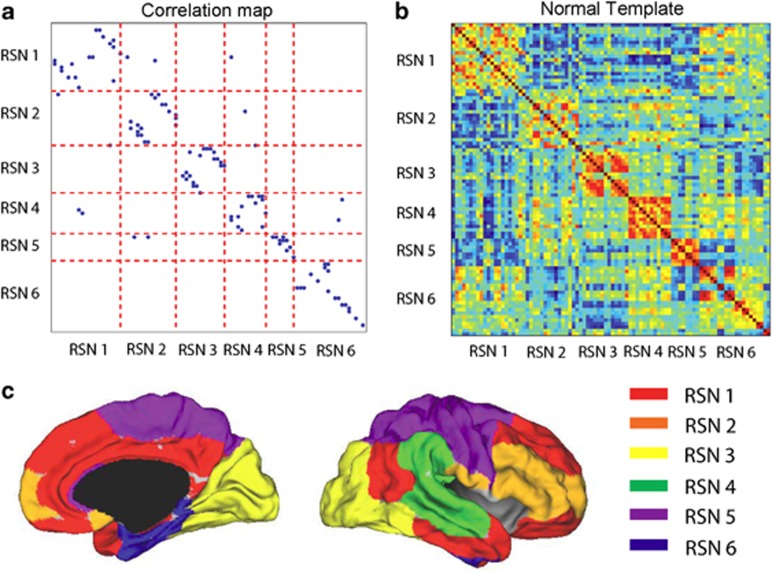
The six-community structure constructed for the whole brain from 37 healthy subjects is shown in Figure 1a. Each dot represents a significant link between the two brain regions, with their names listed in Table 1. For clarity, only one dot is plotted for any two linked regions and it is easy to see the existence of the six communities in the whole brain. We have observed this same structure in an even larger population of around 400 people (data from Cambridge USA and Beijing publicly available in Biswal et al.,25 results not shown). Figure 1b is the actual correlation matrix for a randomly selected individual, showing again the clear community structure. The six communities correspond to six resting-state networks (RSN), which can be identified in terms of broad functions and can be classified as a default mode network (RSN1), an attention network (RSN2), a visual recognition network (RSN3), an auditory network (RSN4), sensory-motor areas (RSN5) and a subcortical network (RSN6). Figure 1c shows the medial and lateral views of the cortical surface mapping of the six-community structure.
Figure 1.
(a) Community structure of the normal template. (b) The correlation coefficient matrix of the Blood Oxygenation Level Dependent (BOLD) signals from 90 regions of interest (ROIs) of one randomly selected subject. (c) (Left) Medial view of the surface of the brain. (Right) The lateral view of the surface of the brain. Different colors represent different communities.
FEMDD and RMDD patients
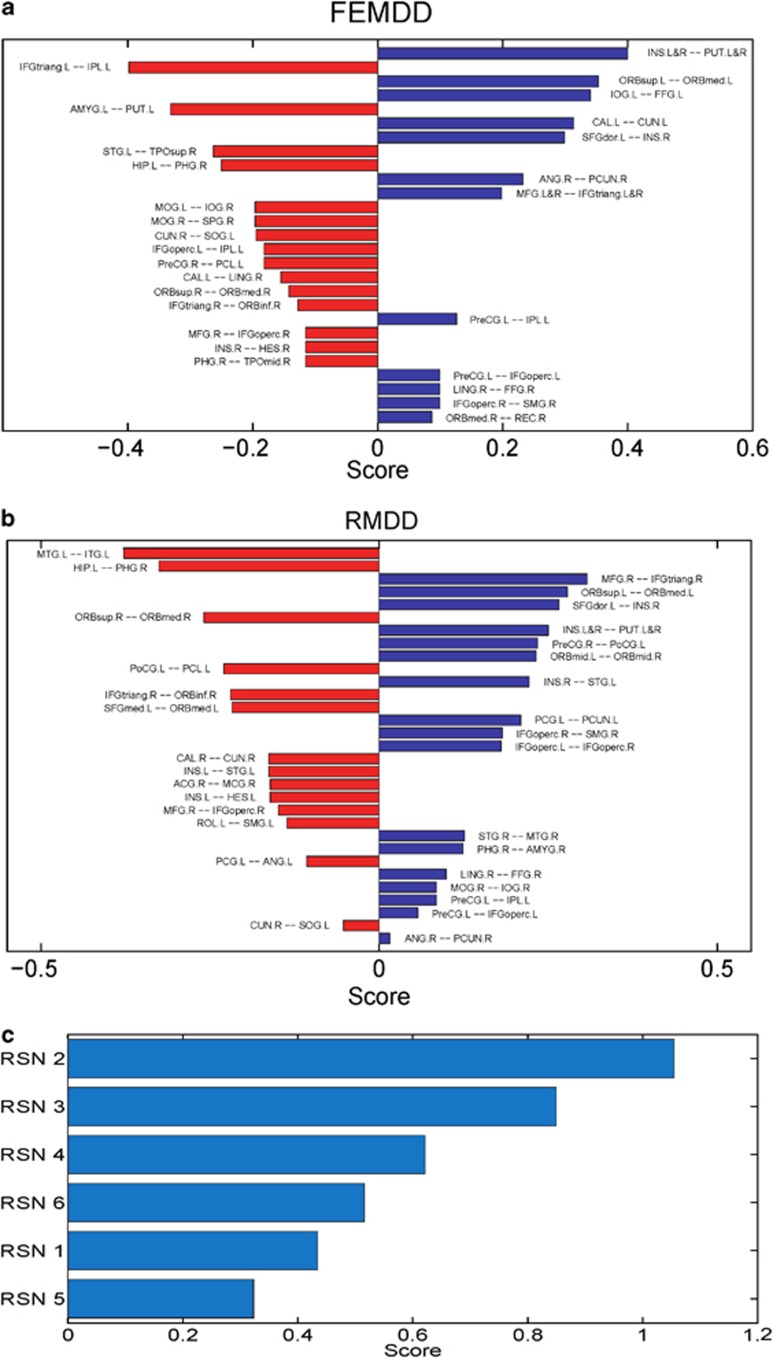
For both the 15 FEMDD patients and 24 RMDD patients, functional maps were constructed and compared with those for the healthy subject group. Comparing the FEMDD network with the canonical template from healthy subjects, there are 97 common links that appear in both networks, 14 links that appear in healthy subjects but are absent in the FEMDD network and 15 links that appear in the FEMDD network but cannot be found in the canonical template. For the RMDD and healthy subject networks, there are 93 common links, 14 links that only appear in the RMDD network and 18 links that appear in the healthy subjects only. In order to rank the significance of the change for each link, a score is defined as follows for each particular link:
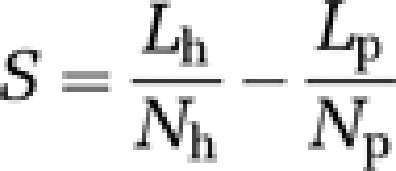 |
where s is the score for a particular link, Lp is the number of this link present in the individual networks of depressives, Np is the total number of patients, Lh is the number of this link present in the individual networks of normal controls and Nh is the total number of healthy controls. Scores for different links in the two hemispheres between the FEMDD network and the canonical template are shown in Figure 2a and those between the RMDD network and canonical template in Figure 2b. The summations of the scores for links within each RSN in both FEMDD and RMDD networks are shown in Figure 2c. To better visualize the changes, Figures 3a and b show the altered connections for the two patient groups but without differentiating between brain hemispheres. Scores for the links altered in both hemispheres are combined to aid clarity. Figures 4a and b do the same but to illustrate the similarities and differences between changes in the two patient groups. It can be seen from Figures 2, 3 and 4 that the strongest evidence for reduced connectivity compared with control subjects in both FEMDD and RMDD is that between the insula and putamen in both brain hemispheres (s=0.4 and 0.25 for FEMDD and RMDD, respectively). Additionally, the link between the left superior frontal gyrus and the right insula is also reduced (s=0.2991 and 0.2658). Thus, the links between the three main components of the ‘hate circuit' have become largely uncoupled. Connections to the left inferior frontal gyrus (opercular) from the left precentral gyrus (s=0.0991 and 0.0574) and those from the right inferior frontal gyrus (opercular) to the right supramarginal gyrus (s=0.0991 and 0.1824) are also reduced together with those from left precentral gyrus to the left inferior parietal lobe (s=0.1261 and 0.0845). These four regions comprise a risk/action circuit. In what we have designated as an emotion/reward circuit, the connection from the superior to the inferior orbitofrontal cortex, is considerably weakened in the left hemisphere of depressed patients (s=0.3532 and 0.2782) but correspondingly strengthened in the right hemisphere (s=−0.1423 and −0.259). There is also a weakening of the connection between the right lingual gyrus and right fusiform gyrus in the visual recognition circuit (s=0.0991 and 0.0991) and between the right angular gyrus and right precuneus in the default circuit (s=0.2324 and 0.0158). In both groups of depressed patients, strengthened connections were also found within a number of circuits, most notably between the left hippocampus and right parahippocampal gyrus in the subcortical network (s=−0.2505 and −0.3255) but also between the right inferior frontal gyrus (triangular) and right inferior orbitofrontal cortex (s=−0.1279 and −0.2196) and between the right medial frontal gyrus and right inferior frontal gyrus (triangular) (s=0.1658 and 0.3074) in the attention circuit. Finally, the connection between the right cuneus and left superior occipital gyrus in the visual recognition circuit (s=−0.1946 and −0.0529) was strengthened.
Figure 2.
(a) Bar plot of the scores of the first-episode major depressive disorder (FEMDD) network compared with the normal template. (b) Bar plot of the scores of the resistant major depressive disorder (RMDD) network compared with the normal template. In both (a, b), red bars represent the links appeared in patients' network while disappeared in normal template, blue bars are vise verse. (c) Summarized scores for the six communities.
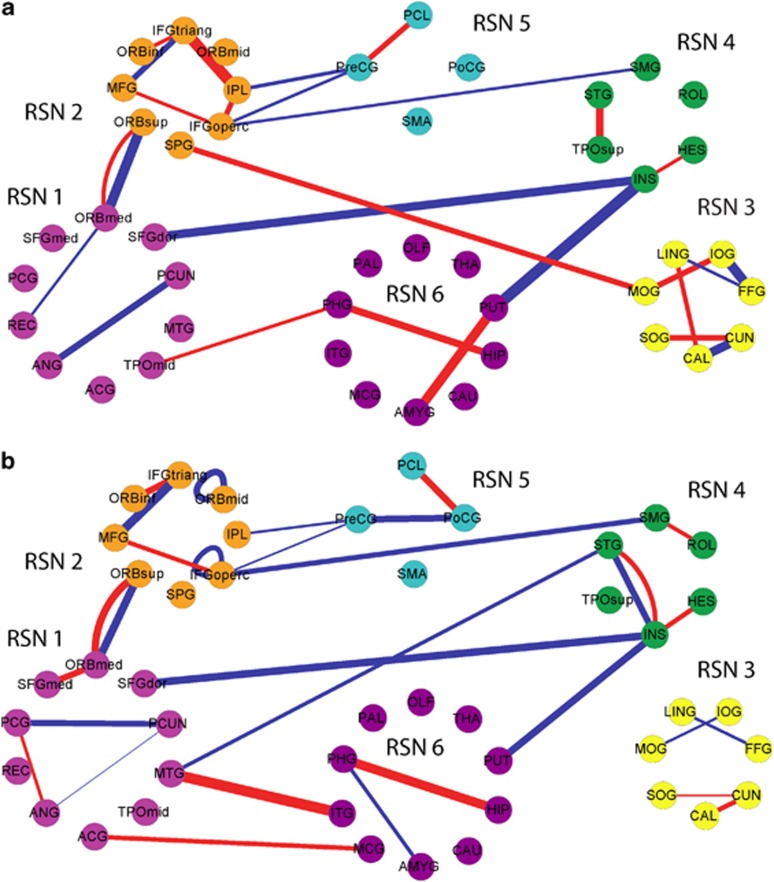
Figure 3.
(a) Functional network structure with different links of normal template and first-episode major depressive disorder (FEMDD) patients. (b) Functional network structure with different links of normal template and resistant major depressive disorder (RMDD) patients. In both (a, b), red lines are links that appear in depression network only while blue lines are links that appear in normal template only. The widths of the lines are proportional to the scores.
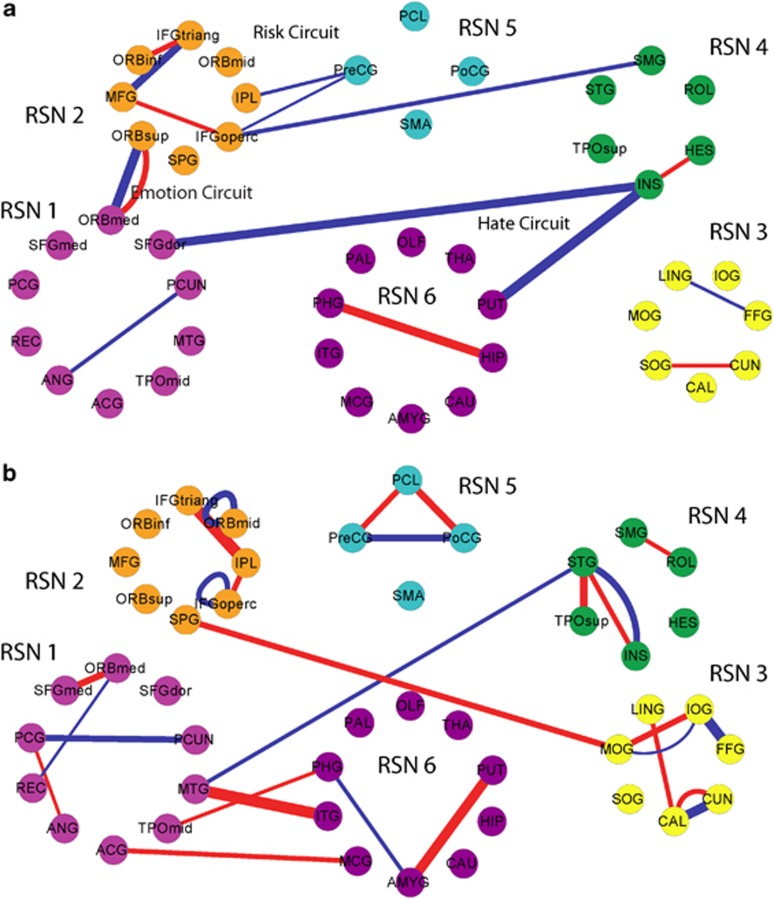
Figure 4.
(a) The common links of the first-episode major depressive disorder (FEMDD) and resistant major depressive disorder (RMDD) networks. (b) Different links of the FEMDD and RMDD networks. In both (a, b), red lines are links that appear in depression network only while blue lines are links that appear in normal template only. The widths of the lines are proportional to the scores.
With the large number of individual connections analyzed between 90 different brain structures, the changes for individual links could fail to be significant after making corrections for multiple comparisons. We therefore also carried out a permutation analysis on total scores for different circuits (the summation of the scores for all altered links within a circuit) to assess the significance of changes in depressed patients. A comparison of the total scores for the main intercommunity connection changes was 0.61 (P=0.003) for the hate circuit (superior frontal gyrus, insula and putamen), 0.32 (P=0.026) for the risk/action circuit (inferior frontal gyrus (opercular), precentral gyrus, superior medial gyrus and inferior parietal lobule) and 0.52 (P=1.76e–4) for the emotion/reward circuit (superior and medial orbitofrontal cortex). The main intracommunity circuit changes were in the attentional circuit (right inferior frontal gyrus (triangular), right inferior orbitofrontal cortex and medial frontal gyrus) (s=0.4, P=0.007) and between the hippocampus and parahippocampal gyrus (s=0.29, P=0.005). The overall change in the visual circuit (cuneus, superior occipital gyrus, angular gyrus and fusiform gyrus) just failed to achieve significance (s=0.23, P=0.06).
Voxel-based morphometry analysis revealed no significant (P>0.05, t-test with Bonferroni correction) gray or white matter volume reductions in these pathways. (See Supplementary Tables S2–S4 for details of the gray/white matter volumes of the ROIs involved in these pathways for both patients and normal controls are listed.)
Discussion
The holistic approach adopted here to identify altered functional circuits in the brains of depressed patients has proved to be very informative. The approach is completely different from existing methods: seed-based analysis and independent component analysis—and makes no assumptions about which circuits might be altered or that brain regions are independent of one another. Furthermore, our approach has identified the so-called ‘hate circuit', as the one showing the largest change in both FEMDD and RMDD, although similar major changes also occurred in the emotion and risk/action circuits. This involvement of the ‘hate circuit' has not, to the best of our knowledge, been found in previous studies. Interestingly, some of the main circuitry identified by other studies using an a priori seed-based approach, such as the links related to the amygdala and cingulate cortex,4, 5, 6, 7 was not found to be altered consistently in both patient groups. A link with the amygdala was present in RMDD, but absent in FEMDD, while the cingulate link was absent in RMDD but present in FEMDD.
Overall, our voxel-based morphometry analysis revealed no significant gray or white matter changes in any of the brain regions, showing connectivity changes in depressed patients. It therefore seems unlikely that observed changes were simply caused by reduced tissue volumes.
Although the current approach has only been applied to one of the major brain disorders, depression, it is clear that it could be easily applied to other forms of psychiatric, developmental or neurodegenerative disorders and provide information on how each of these disorders are characterized by a specific subset of functional connectivity changes as well as helping to identify possible common traits across, for example, affective or learning and memory disorders.
It could be argued that the changes in functional circuit we have identified are simply a reflection of altered coherent activities (both positive and negative correlations) among the brain regions in the resting state and that they might not be predictive of altered responsivity to internal or external stimuli promoting behavioral responses. For example, the ‘hate circuit' might lose its coherency in the patients in a resting state, but regain this coherency and function normally in response to appropriate stimuli. This is certainly an issue for all resting-state studies requiring further investigation although, as we will discuss below, there are some interesting parallels between our current findings and previous studies, showing stimulus-evoked changes in these same circuits in depressed patients.
So what might be the significance of the uncoupling we have found bilaterally in the so-called ‘hate circuit' of depressed patients? This circuit is associated with feelings of hate because it has been reported that the superior frontal gyrus, insula and putamen are the three main brain regions showing altered activation when individuals view people who they hate,16 although interestingly they are also affected similarly by seeing people you love, or have loved but recently been rejected by.16, 26, 27, 28 The insula region is also reported to be involved in feelings of disgust as well as other emotions29 and a recent fMRI study has shown enhanced responses in the insula to faces expressing disgust.30 A relationship between the different components of the ‘hate circuit' and various psychiatric and neurodegenerative diseases such as schizophrenia,31 Huntington's disease32, 33 and depression30 has already been reported.
A recent meta-analysis of changes in brain activation during depression shows that the superior frontal gyrus, insula and putamen are consistently affected.34 This meta-analysis reports that the superior frontal gyrus shows increased activation in depressed patients as well as enhanced activation in response to positive emotional stimuli and decreased activation in response to negative emotional stimuli. The insula exhibits decreased basal activity and responses to both positive and negative emotional stimuli in depressed patients. The putamen on the other hand shows decreased responses to positive emotional stimuli and increased ones to negative emotional stimuli. The differential patterns of changes in these three structures are consistent with our finding that in depressed patients they have become functionally uncoupled both in the resting state and during exposure to emotional stimuli, although the latter needs to be confirmed in further experiments. Interestingly, the meta-analysis also reports that with SSRI treatments, all three structures tend to show decreased activity, which might suggest reestablishment of the coupling between them leads to more coordination between them.
Depression is associated with reduced size of the putamen,35 although we did not find any evidence for this in our depressed patients. Elevated dopamine D2 receptor binding36 and increased oxidative stress37 have also been reported in the putamen of depressed patients, and stroke patients with damage to this region can show depressive symptoms.38 Altered functioning of the insular cortex has been found in a number of psychiatric disorders, including depression.39 Depression is associated with a disturbed sense of interoceptive awareness and the insula appears to be important in this respect.40 The superior frontal gyrus has also been reported to be reduced in size in depressed patients41 although we did not find this in our patients.
Depression is often characterized by intense self-loathing, and while it is associated with anhedonia, there is no obvious indication that depressives are less prone to hate others. One possibility is that the uncoupling of this circuit could be associated with impaired ability to control and learn from social or other situations that provoke feelings of hate toward self or others. This in turn could lead to an inability to deal appropriately with feelings of hate and an increased likelihood of both uncontrolled self-loathing and withdrawal from social interactions. Depressed patients also have problems in controlling negative thoughts and so a potential hypothesis is that the functional uncoupling in this circuit may be contributing to impaired cognitive control over pervasive internal feelings of self-loathing or hatred toward others and/or external circumstances. Indeed, reduced cognitive control over emotions has been proposed as one of the important factors in depression.42
The ‘hate circuit' may be involved in the control of other behaviors influenced by depression. The same brain regions, for example, also appear to be involved in feelings of self-awareness. A recent experiment using real-time fMRI training has reported that feedback enhanced metacognitive awareness, in terms of turning attention toward or away from their own thoughts, results in increased activation of the prefrontal cortex, insula and putamen.43 Depressed patients also have problems in controlling their thoughts and perhaps feedback training on meta-cognitive awareness might actually be a potential therapeutic approach for restoring functional coupling in this circuit.
Other significantly affected circuits in depressed patients were those associated with risk and action, reward and emotion, attention and memory processing. The risk/action circuitry comprises the inferior frontal gyrus and its connections with the precentral gyrus in the left hemisphere and supramarginal gyrus in the right hemisphere. The inferior frontal gyrus is particularly associated with response inhibition44, 45 and its activation has been found to be positively correlated with perception of and taking higher risks in a variety of experimental contexts.46, 47, 48 These pathways together with the left precentral gyrus connection to the inferior parietal lobe are all reduced in strength in depressed patients and are part of the ‘mirror' system involved in imitating the actions of others and also responding to self-movements.49, 50 They are also involved in aspects of semantic processing, which can also be impaired in depressed patients.51 In this context, it is interesting to note that connectivity between the angular gyrus and cuneus is weakened in our depressed patients, which might contribute to impaired semantic processing.52 The inferior parietal lobe has also been shown to be important for the perception of emotions during presentation of facial stimuli.53 This finding, coupled with the observed weakening of connections between the fusiform and lingual gyri provides a neural basis for impaired face emotion processing in depressed patients. Indeed, altered responsiveness to emotional facial stimuli in depressives has been taken as one of the biomarkers for early diagnosis.54
A meta-analysis of the brain regions affected in depressed patients has shown the inferior frontal gyrus to be influenced both in terms of basal activity and responses to affective stimuli.34 Overall therefore one possibility is that, as with the ‘hate circuit', the altered coupling in the risk/action circuit may also reflect reduced cognitive control via the frontal cortex over a range of adaptive responses to emotional stimuli.
The part of an emotion and reward circuit significantly altered in depressed patients comprises the links between the superior and medial regions of the orbitofrontal cortex, and is known to be closely related to psychiatric and developmental disorders such as schizophrenia,55 obsessive compulsive disorder56 and autism.57 Neuroimaging studies have found that the reward value, the expected reward value, and even the subjective pleasantness of foods and other reinforcers are also represented in the orbitofrontal cortex.58, 59 Interestingly, there is a clear difference between the effects of depression on right and left brain hemispheres in relation to functional coupling in this pathway. Our results show that it is increased in strength in the right hemisphere and decreased in the left. There is evidence that the right orbitofrontal cortex is more activated by punishment, whereas the left is activated more by positive rewards.60 This may therefore possibly reflect increased responsivity to negative stimuli (right hemisphere) and decreased responses to positive stimuli (left) typically found in depressed patients. Since there are also strengthened connections between the right inferior frontal gyrus and the right inferior orbitofrontal cortex, and also the medial frontal gyrus in depressed patients in the attention circuit, this might also reflect an increased attentional bias toward negative stimuli.
Another main pathway affected in both groups of depressed patients was that between the hippocampus and parahippocampal gyrus. This may reflect aspects of impaired memory functions in depressed patients. Interestingly, there is increasing evidence that neurogenesis in the hippocampus is important for learning and there may be a link between depression and reduced neurogenesis in the hippocampus.61, 62 Loss of hippocampal neurons is found in some depressive individuals and correlates with impaired memory and dysthymic mood. Antidepressant drugs that increase serotonin levels in the brain may also help by stimulating neurogenesis and increasing the total mass of the hippocampus thereby helping to restore mood and memory dysfunction.63
The main focus of this study was to identify functional pathways altered in both FEMDD and RMDD patients who were currently suffering from severe depression in order to try and help establish which ones are most strongly linked with depression per se. There were clearly a large number of differences between the patient groups, which emphasizes the importance of not basing analyses of potential brain correlates of depression on a single type of patient group. However, it must be emphasized that the RMDD group had only been drug-treatment free for 24 h compared with at least 2 weeks for the FEMDD group and so some of the differences observed in the two groups could be drug related although obviously in the REMDD group their severe depression symptoms had not ameliorated significantly in response to these drugs. It is clear that it would need a more detailed investigation on drug-free patients in the two groups to establish which differences between the two patient groups could be of potential relevance. However, as already mentioned above, it is of particular interest at this stage that alterations in amygdala and cingulate connectivity, which have often been considered to be central to neural circuitry involved in depression, are different in these two patient groups despite both having similar current levels of severe depression.
Acknowledgments
SG was supported by The National Natural Science Foundation of China (NSFC) (10901049) and excellent talent of Hunan Normal University (ET11001) and Key Laboratory of Computational and Stochastic Mathematics and Its Application of Hunan Province (11K038). TG was supported by the China Scholarship Council (CSC). ZL was supported by research grants from the NSFC (81071092), the National Basic Research Program of China (2007CB512300), and the 11th Five-Year Key Program for Science and Technology Development of China (2007BAI17B05). JF is a Royal Society Wolfson Research Merit Award holder, partially supported by an EU Grant BION, a UK EPSRC grant and National Centre for Mathematics and Interdisciplinary Sciences (NCMIS) in Chinese Academy of Sciences.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Raichle M. The brain's dark energy. Sci Am Mag. 2010;302:44–49. doi: 10.1038/scientificamerican0310-44. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola JH, Jauhiainen J, Hyvärinen A, Tervonen O. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Human Brain Mapping. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci. 2009;106:13040. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci. 2003;100:253. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Liu J, Feng J.On the spectral characterization and scalable mining of network communities Knowledge and Data Engineering, IEEE Transactions Ondoi: 10.1109/TKDE.2010.233(in press). [DOI]
- Zeki S, Romaya JP. Neural correlates of hate. PLoS One. 2008;3:e3556. doi: 10.1371/journal.pone.0003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psychiatry Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Furtado CP, Maller JJ, Fitzgerald PB. A magnetic resonance imaging study of the entorhinal cortex in treatment-resistant depression. Psychiatry Res. 2008;163:133–142. doi: 10.1016/j.pscychresns.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- Nierenberg A, Amsterdam J. Treatment-resistant depression: definition and treatment approaches. Discussion. J Clin Psychiatry. 1990;51:39–50. [PubMed] [Google Scholar]
- Iidaka T, Nakajima T, Suzuki Y, Okazaki A, Maehara T, Shiraishi H. Quantitative regional cerebral blood flow measured by Tc-99m HMPAO SPECT in mood disorder. Psychiatry Res. 1997;68:143–154. doi: 10.1016/s0925-4927(96)02969-1. [DOI] [PubMed] [Google Scholar]
- Yan C, Zang Y. DPARSF: a MATLAB toolbox for ‘pipeline' data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci. 2010;107:4734. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D. Reward, addiction, and emotion regulation systems associated with rejection in love. J Neurophysiol. 2010;104:51. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Chen YH, Dammers J, Boers F, Leiberg S, Edgar JC, Roberts TPL, et al. The temporal dynamics of insula activity to disgust and happy facial expressions: a magnetoencephalography study. Neuroimage. 2009;47:1921–1928. doi: 10.1016/j.neuroimage.2009.04.093. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, El-Hage W, Dalgleish T, Radua J, Gohier B, Phillips ML. Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. J Psychiatric Res. 2010;44:894–902. doi: 10.1016/j.jpsychires.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff N, et al. Elevated putamen D2 receptor binding potential in major depression with motor retardation: an [11C] raclopride positron emission tomography study. Am J Psychiatry. 2006;163:1594. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- Michel TM, Camara S, Tatschner T, Frangou S, Sheldrick AJ, Riederer P, et al. Increased xanthine oxidase in the thalamus and putamen in depression. World J Biol Psychiatry. 2010;11:314–320. doi: 10.3109/15622970802123695. [DOI] [PubMed] [Google Scholar]
- Vataja R, Leppavuori A, Pohjasvaara T, Mantyla R, Aronen HJ, Salonen O, et al. Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci. 2004;16:156. doi: 10.1176/jnp.16.2.156. [DOI] [PubMed] [Google Scholar]
- Craig A. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed ‘material me'. World J Biol Psychiatry. 2010;11:538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Leung KK, Lee T, Wong M, Li L, Yip P, Khong PL. Neural correlates of attention biases of people with major depressive disorder: a voxel-based morphometric study. Psychol Med. 2009;39:1097–1106. doi: 10.1017/S0033291708004546. [DOI] [PubMed] [Google Scholar]
- Ebmeier K, Rose E, Steele D. Cognitive impairment and fMRI in major depression. Neurotox Res. 2006;10:87–92. doi: 10.1007/BF03033237. [DOI] [PubMed] [Google Scholar]
- McCaig RG, Dixon M, Keramatian K, Liu I, Christoff K. Improved modulation of rostrolateral prefrontal cortex using real-time fMRI training and meta-cognitive awareness. Neuroimage. 2010;55:1298–1305. doi: 10.1016/j.neuroimage.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29:12574. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Gianotti LRR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Théoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci. 2007;27:6212. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macuga KL, Frey SH. Selective responses in right inferior frontal and supramarginal gyri differentiate between observed movements of oneself vs. another. Neuropsychologia. 2011;49:1202–1207. doi: 10.1016/j.neuropsychologia.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Deldin P. Review of brain functioning in depression for semantic processing and verbal fluency. Int J Psychophysiol. 2010;75:77–85. doi: 10.1016/j.ijpsycho.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Price C, Wise R, Frackowiak R. Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: a positron-emission tomography study in normal human subjects. Neurosci Lett. 1994;182:25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, et al. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Marquand A, Ehlis A, Dresler T, Kittel-Schneider S, Jarczok T, et al. Integrating neurobiological markers of depression. Arch Gen Psychiatry. 2010;68:361–368. doi: 10.1001/archgenpsychiatry.2010.178. [DOI] [PubMed] [Google Scholar]
- Larquet M, Coricelli G, Opolczynski G, Thibaut F. Impaired decision making in schizophrenia and orbitofrontal cortex lesion patients. Schizophr Res. 2010;116:266–273. doi: 10.1016/j.schres.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Rolls E, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Minkel J.Brain pathway may underlie depression: a crescent of electrical activity spotted in rats may allow researchers to map the depressed brain Sci Am Mind News ; http://www.sciam.com/article.cfm , 2007
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.