Abstract
The purpose of this study was to evaluate whether berberine (BER) administration could attenuate depression- and anxiety-like behaviors and increase corticotrophin-releasing factor (CRF) and tyrosine hydroxylase (TH) expression following chronic morphine withdrawal in rats. Male rats were exposed to chronic, intermittent, escalating morphine (10~50 mg/kg) for 10 days. After the last morphine injection, depression- and anxiety-like beahvior associated with morphine discontinuation persisted for at least three days during withdrawal without any change in ambulatory activity. Daily BER administration significantly decreased immobility in the forced swimming test and increased open-arm exploration in the elevated plus maze test. BER administration also significantly blocked the increase in hypothalamic CRF expression and TH expression in the locus coeruleus (LC) and the decrease in hippocampal brain-derived neurotrophic factor (BDNF) mRNA expression. Taken together, these findings demonstrated that BER administration significantly reduced morphine withdrawal-associated behaviors following discontinuation of repeated morphine administration in rats, possibly through modulation of hypothalamic CRF and the central noradrenergic system. BER may be a useful agent for treating or alleviating complex withdrawal symptoms and preventing morphine use relapses.
Keywords: Berberine, Corticotrophin-releasing factor, Depression, Morphine, Tyrosine hydroxylase
INTRODUCTION
Morphine, a potent pain reliever, is widely used to treat moderate to severe pain and a number of other pathological conditions. The continuous use of morphine causes drug craving, tolerance to opiate analgesia, and withdrawal syndrome, particularly in terms of relapse into drug-seeking behavior when the drug is discontinued [1]. Therefore, morphine abuse and subsequent withdrawal cause a negative emotional state and psychiatric side effects, including depression and anxiety [2]. Many studies have demonstrated that morphine withdrawal causes depression- and anxiety-related disorders in humans and corresponding behavioral responses in animals [3,4]. Importantly, depression and anxiety that occur during morphine abstinence often lead to relapse to morphine use in humans [5]. Over the past several decades, several antidepressant classes [i.e., monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants (TCAs)] have been developed and clinically used to treat psychiatric side-effects, including depression and anxiety, following morphine withdrawal [2]. However, most antidepressants are not very effective against the wide variety of complex depression symptoms, and most are associated with serious side-effects such as drowsiness, dryness of the mouth, headache, nausea, and sexual dysfunction [6,7]. Therefore, development of new alternative drugs or therapies is urgently needed.
In the present study, we explored the pharmacological activity of berberine (BER), an isoquinoline alkaloid derived from Korean traditional medicinal herbs, such as Berberis, Hydrastis Canadensis, Coptidis Rhizoma and Cortex Phellodendri [8]. BER is widely reported to improve multiple physiological actions and produce a variety of biological effects in the central nervous system (CNS). BER has antioxidant, anticoagulant, antitumor, antiviral and anti-inflammatory activities, suggesting its potential value in medicinal use [9]. BER significantly reduces the total duration of immobility in the forced swimming test (FST) and tail-suspension test (TST) [10], and ameliorates anxiety-related behavior through activation of the serotonergic system in mice [11]. Although a brief report was published on the antidepressant effects of BER, unresolved questions remain regarding the mechanisms underlying BER's effect as a therapeutic intervention for treating depression- and anxiety-like behaviors closely associated with morphine abstinence and development of morphine dependence after chronic BER prior to every morphine treatment.
The aim of the present study was to investigate the effects of BER on depression- and anxiety-like behaviors in rats exposed to repeated morphine administration and withdrawal due to morphine discontinuation using the FST and elevated plus maze (EPM) test. We also aimed to identify the underlying mechanism to elucidate how these behavioral effects were associated with the central noradrenergic system in the brain.
METHODS
Animals
Adult male Sprague-Dawley (SD) rats weighing 260~280 g were obtained from Samtako Animal Co. (Seoul, Korea). The rats were housed in a limited access rodent facility with up to five rats per polycarbonate cage. The room controls were set to maintain the temperature at 22±2℃ and the relative humidity at 55±15%. Cages were lit by artificial light for 12 h each day. Sterilized drinking water and standard chow diet were supplied ad libitum to each cage during the experiments. The animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised in 1996, and were approved by the Kyung Hee University Institutional Animal Care and Use Committee. All animal experiments began at least 7 days after the animals arrived.
The morphine treatment and experimental groups
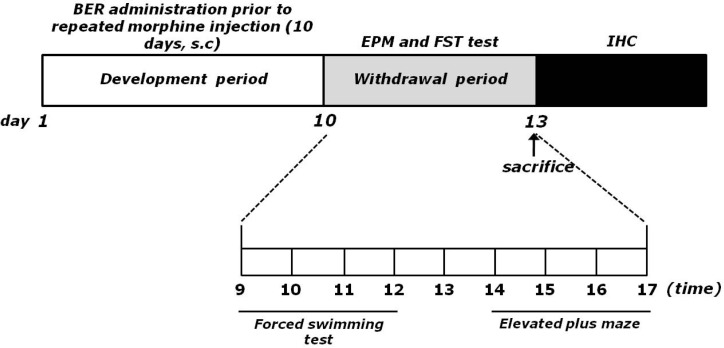
The withdrawal group following repeated morphine administration was given morphine (dose ranging from 10 to 50 mg/kg-body weight, s.c., MOR group, n=6) twice a day for 10 consecutive days. Animals received two daily applications of increasing doses of morphine according the following treatment schedule: days 1 and 2, 2×10 mg/kg; days 3 and 4, 2×20 mg/kg; days 5 and 6, 2×30 mg/kg; days 7 and 8, 2×40 mg/kg; days 9 and 10, 2×50 mg/kg, as described previously [12]. No drugs were injected within 72 h after the last morphine injection and behavioral responses were tested during this period. The vehicle-treated rats (as a negative control of the addiction withdrawal model development) were administered with saline (0.9% NaCl, s.c.) instead of morphine in the same way (SAL group, n=6). The BER-treated groups were divided as follows: 10 mg/kg BER plus morphine-treated group (BER10, n=6), 20 mg/kg BER plus morphine-treated group (BER20, n=6), 50 mg/kg BER plus morphine-treated group (BER50, n=6), and 10 mg/kg fluoxetine (FLX) plus morphine-treated group (FLX, n=6). FLX, a selective serotonin reuptake inhibitor, was used as positive control. BER and FLX were purchased from Sigma Chemical Co. (St. Louise, MO, USA). The rats were intraperitoneally administration with BER and FLX 30 min prior to the injection of morphine for 10 consecutive days, as the development phase. BER and FLX dissolved in 0.9% physiological saline solution before use. All drugs were freshly prepared right before every experiment. Behavior testing for depression- and anxiety-like behavior was done 72 h after the last morphine injection. The entire experimental schedules of all drug administration and behavioral examinations are shown in Fig. 1.
Fig. 1.
Experimental schedule of morphine withdrawal-induced depression- and anxiety-like behaviors in rats.
Forced swimming test (FST)
FST, a representative behavioral test for depression, is frequently used to evaluate the activities of potential antidepressant drugs in rodent models. Forced immersion of rats in water for an extended period produces a characteristic behavior of immobility. The antidepressant treatments decrease the immobility behavior accompanying with an increase in the escape responses such as climbing and swimming. A transparent Plexiglas cylinder (20 cm diameter×50 cm height) was filled up to a depth of 30 cm with water at 25℃. At this depth, rats could not touch the bottom of the cylinder with their tails or hind limbs. On day 1, the rats in all groups were trained for 15 min by placing them in the water-filled cylinder. On day 2, animals were subjected to 5 min of forced swim, and escape behaviors (climbing and swimming) were determined. The duration of immobility was scored during the 5 min test period. Climbing was defined as upward-directed movements of the forepaws the side of the swim chamber and swimming was considered as movements throughout the swim chamber including crossing into another quadrant. Immobility behavior was calculated as the length of time in which the animal did not show escape responses (e.g., total time of the test minus time spent in climbing and swimming behaviors). The animals' behavior was continuously recorded throughout the testing session with an overhead video camera. After the test, the rat was removed from the tank, dried with a towel and placed back in its home cage. The water in the swim tank was changed between rats.
Elevated plus maze test (EPM)
The EPM test is a widely used behavioral test to assess anxiogenic or anxiolytic effects of pharmacological agents. Animals conduct anxiety-like behaviors usually show the reductions both in the number of entries and in the time spent in the open arms, along with an increase in the amount of time spent in the closed arms in the EPM. The elevated plus test was conducted. This apparatus consisted of two open arms (50×10 cm each), two closed arms (50×10×20 cm each) and a central platform (10×10 cm), arranged in a way such that the two arms of each type were opposite to each other. The maze was made from black Plexiglas and elevated 50 cm above the floor. Exploration of the open arms was encouraged by testing under indirect dim light (2×60 W).
At the beginning of each trial, animals were placed at the centre of the maze, facing a closed arm. During a 5-min test period, the following parameters were recorded: a) number of open arm entries, b) number of closed arm entries, c) time spend in open arms, and d) time spent in closed arms. Entry by an animal into an arm was defined as the condition in which the animal has placed its four paws in that arm. The maze was cleaned with alcohol after each rat had been tested. The behavior in the maze was recorded using a video camera mounted on the ceiling above the center of the maze and relayed to the S-MART program (PanLab, Barcelona, Spain). Anxiety reduction, indicated by open arm exploration in the EPM, was defined as an increase in the numbers of entries into the open arms relative to total entries into either open or closed arm, and an increase in the proportion of time spent in the open arms relative to total spending time in either open or closed arm. Total arm entries were also used as indicators of changes in locomotor activities of the rats.
Immunohistochemistry of corticotrophin-releasing factor (CRF) and tyrosine hydroxylase (TH)
For immunohistochemical studies, the animals were deeply anesthetized with sodium pentobarbital (80 mg/kg, by intraperitoneal injection) and perfused through the ascending aorta with normal saline (0.9%) followed by 300 ml (per rat) of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed over-night, and cryoprotected with 20% sucrose in 0.1 M PBS at 4℃. Coronal sections 30 µm thick were cut through the hypothalamus and locus coeruleus (LC) using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany). The sections were obtained according to the rat atlas of Paxinos and Watson [13]. The sections were immunostained for CRF and TH expression using the avidin-biotin-peroxidase complex (ABC) method. Briefly, the sections were incubated with primary goat anti-CRF antibody (1:500 dilution; Santa Cruz Biotechnology Inc., California, CA, USA) and sheep anti-TH antibody (1:2,000 dilution; Chemicon International Inc., Temecular, CA, USA) for 72 h at 4℃. The sections were incubated for 120 min at room temperature with biotinylated rabbit anti-goat IgG secondary antibody (for the anti-CRF antibody) and biotinylated goat anti-sheep IgG secondary antibody (for the anti-TH antibody). The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunoreactivity, the sections were incubated for 90 min in ABC reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3'-diaminobenzidine (DAB; Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Finally, the tissues were washed in PBS, followed by a brief rinse in distilled water, and mounted individually onto slides. Images were captured using the AxioVision 3.0 imaging system (Carl Zeiss, Inc., Oberkochen, Germany) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 200× magnification, and the numbers of cells within 100×100-µm2 grids were counted by observers blinded to the experimental groups. Counting immunepositive cells was performed in at least three different hypothalamus or LC sections per rat brain. Stained sections were randomly chosen from equal levels serial sections along the rostral-caudal axis. The cells were obtained according to the stereotactic atlas of Paxinos and Watson [13]. The brightness and contrast between images were not adjusted to exclude any possibility of subjective selection of immunoreactive cells.
Total RNA preparation and RT-PCR analysis
The expression levels of BDNF mRNA was determined by the reverse transcription-polymerase chain reaction (RT-PCR). The brain hippocampus was isolated from three rats per group. After decapitation, the brain was quickly removed and stored at -80℃ until use. The total RNA was prepared from the brain tissue using a TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier's instruction. Complementary DNA was first synthesized from total RNA using reverse transcriptase (Takara Co., Shiga, Japan). PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). The operating conditions were as follows: for glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 30 cycles of denaturation at 95℃ for 30 sec, annealing at 58℃ for 30 sec, and extension at 72℃ for 30 sec; for BDNF, 27 cycles of denaturation at 95℃ for 30 sec, annealing at 57℃ for 30 sec, and extension at 72℃ for 30 sec. All primer was designed using published mRNA sequences of those cytokines and a primer designing software, Primer 3, offered by the Whitehead Institute for Biomedical Research (Cambridge, MA, USA; www.genome.wi.mit.edu) on the website. The following sequences were used: for GAPDH (409 bp), (forward) 5'-ATC CCA TCA CCA TCT TCC AG-3' and (reverse) 5'-CCT GCT TCA CCA CCT TCT TG-3'; for BDNF (153 bp), (forward) 5'-CAG GGG CAT AGA CAA AAG-3' and (reverse) 5'-CTT CCC CTT TTA ATG GTC-3'. The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide. The density of each band was quantified using an image-analyzing system (i-Max™, CoreBio System Co., Seoul, Korea). The expression level was compared each other by calculating the relative density of target band, such as BDNF, to that of GAPDH.
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. Results in figures are expressed as mean±standard error of means (SE). Differences within or between normally distributed data were analyzed by analysis of variance (ANOVA) using SPSS (Version 13.0; SPSS, Inc., Chicago, IL, USA) followed by Tukey's post-hoc test. Statistical significance was set at p<0.05.
RESULTS
Effect of BER on morphine-induced depression-like behavior
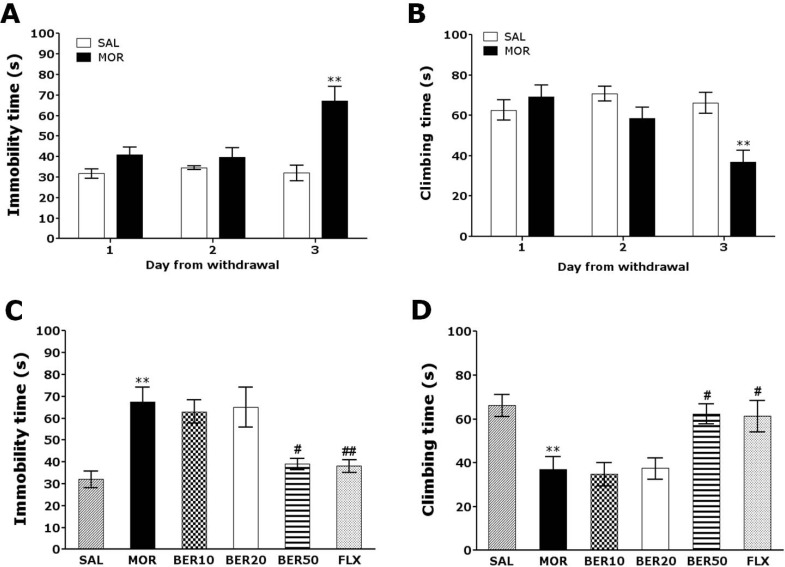
Following withdrawal from repeated morphine exposure, rats exhibited a marked depression phenotype characterized by increased immobility time during the FST as compared with saline-treated controls (SAL group). Rats were subjected to the FST 72 h after the last injection of morphine or saline (Fig. 2A and B). Immediately after the last morphine administration (days 1 and 2), an increase in immobility and decrease in climbing behavior were observed in the experimental group as compared with the SAL group. Furthermore, on day 3 following withdrawal from repeated morphine administration, rats showed a significant increase in immobility and a decrease in climbing time during the FST as compared with the SAL group (Student's t-test, p<0.01). Following withdrawal from morphine administration, the depression phenomenon persisted for at least three days (i.e., increased immobility without any effect on ambulatory activity).
Fig. 2.
Changes in immobility time (A) and climbing time (B) in the forced swimming test after withdrawal from repeated saline or morphine administration. Rats were placed in plastic cylinders, and their behavior was recorded for 5 min at 24, 48, and 72 h following the last saline or morphine injection. The effect of BER on immobility time (C) and climbing time (D) in forced swimming test during morphine withdrawal is shown. **p<0.01 vs. SAL group; #p<0.05, ##p<0.01 vs. MOR group.
The effects of BER administration prior to morphine injection were evaluated during the withdrawal period by the FST. Rats in the BER50 group had significantly decreased immobility time during the 5 min in the FST compared with those in the MOR group (p<0.05; Fig. 2C), indicating that 50 mg/kg BER administration decreased depression-like behavior. We next focused on "climbing behavior" [14]. Rats in the BER50 group had significantly restored climbing behavior during the 5 min in the FST compared with those in the MOR group (p<0.05; Fig. 2D). This finding indicated that BER administration significantly reduced depression-like despair behavior. However, withdrawal from repeated morphine exposure did not lead to significant differences in swimming behavior among groups in the FST (data not shown). Immobility and climbing behavior in the BER50 group were comparable to those observed in the FLX group.
Effect of BER on morphine-induced anxiety-like behavior
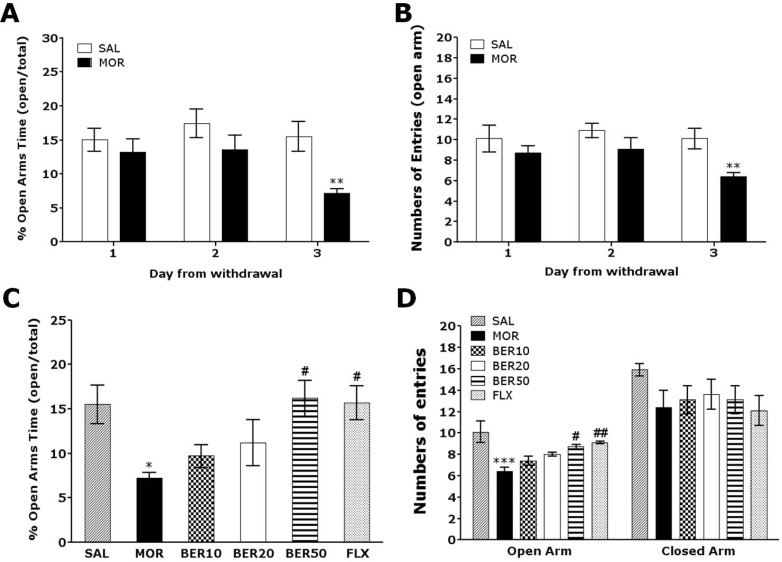
Anxiety expressed as a decrease in open-arm exploration in the EPM test was analyzed. Rats were challenged in the EPM test 72 h after the last injection of morphine or saline (Fig. 3). Rats displayed showed a significant decrease in both the percentage of time spent (Student's t-test, p<0.01) and the number of entries (Student's t-test, p<0.01) into the open arms of the maze 72 h after the last morphine injection compared with the SAL group. Conversely, statistical analysis revealed that the number of entries into the closed arms was not effected by withdrawal from repeated morphine exposure, as there were no significant differences among the groups (p=0.214; data not shown).
Fig. 3.
Changes in anxiety like behavior in the elevated plus maze after withdrawal from repeated saline or morphine administration. Rats were placed in the center of the maze facing an enclosed arm, and their behavior was recorded for 5 min at 24, 48 and 72 h following the last saline or morphine injection. The percentage of time spent in open-arm exploration (A) and the number of entries into open-arms (B) in the elevated plus maze are shown. The effect of BER on the percentage of time spent in open-arm exploration (C) and the number of entries into open arms (D) during morphine withdrawal are presented. *p<0.05, **p<0.01, ***p<0.001 vs. SAL group; #p<0.05, ##p<0.01 vs. MOR group.
The effects of BER administration prior to morphine injection were evaluated during the withdrawal period using the EPM test. Rats in the BER50 group spent significantly more time in the open arms of the maze compared with those in the MOR group (p<0.05; Fig. 3C). Similarly, rats in BER50 group also had a significantly greater number of entries into the open arms of the maze compared with those in the MOR group (p<0.01; Fig. 3D). These finding indicate that 50 mg/kg BER administration significantly ameliorated anxiety-like despair behavior. Because no significant differences in the number of closed-arm entries was observed among groups in the EPM test, we suggest that the observed anxiety-like behaviors in morphine-treated rats could not be attributed to differences in locomotor activity. Furthermore, the percentage of time spent and number of entries into the open arms of the maze were comparable between the BER50 group and the FLX group.
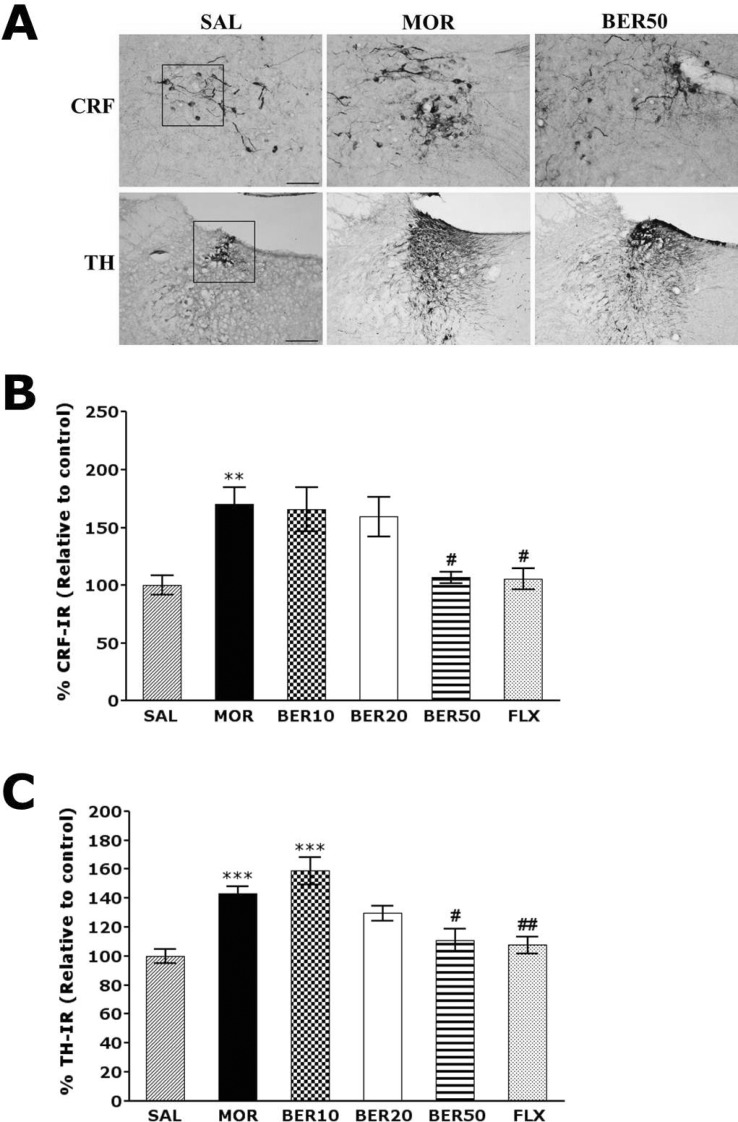
Effect of BER on morphine-induced CRF- and TH-like immunoreactivity
Following withdrawal from repeated morphine injection, CRF-like immunoreactivity was primarily detected in cell bodies of hypothalamic regions, including the paraventricular nucleus (PVN; Fig. 4). In the brains of the MOR group, the number of CRF immunoreactive neurons in the PVN was increased by 170.04%. Analysis of the number of CRF-immunoreactive neurons revealed a significant increase in CRF expression in the MOR group compared with those in the SAL group (p<0.01). The number of CRF-immunoreactive neurons was significantly decreased in hypothalamic PVN regions in the BER50 group (p<0.05) compared with those in the MOR group. TH-like immunoreactivity was also analyzed in the cell bodies of major noradrenergic regions, including the LC (Fig. 4). In the brains of the MOR group, the number of TH immunoreactive fibers in the LC was increased by 142.98%. Analysis of the number of TH-immunoreactive neurons revealed that rats in the MOR group had a significant increase in TH expression compared with those in the SAL group (p<0.001). The numbers of TH-immunoreactive neurons significantly decreased in central adrenergic regions of the BER50 group (p<0.05) compared with those in the MOR group. This indicated that the repeated morphine administration withdrawal-induced increase in CRF- and TH-immunoreactivity was significantly restored by BER administration, and the number of CRF- and TH-immunopositive neurons in the BER50 group was similar to that in the FLX group.
Fig. 4.
Representative photomicrographs showing CRF expression in the paraventricular nucleus (PVN) of the hypothalamus and TH expression in the locus coeruleus (LC) (A). Effect of BER on the expression of CRF (B) and TH (C) after withdrawal from repeated saline or morphine administration. Cells of the hypothalamus area were counted as the CRF reactive cells, and cells of the LC area were counted as the TH reactive cells within actual square box of defined size (100×100 µm2). The scale bar represents 50 µm. **p<0.01, ***p<0.001 vs. SAL; #p<0.05, ##p<0.01 vs. MOR group.
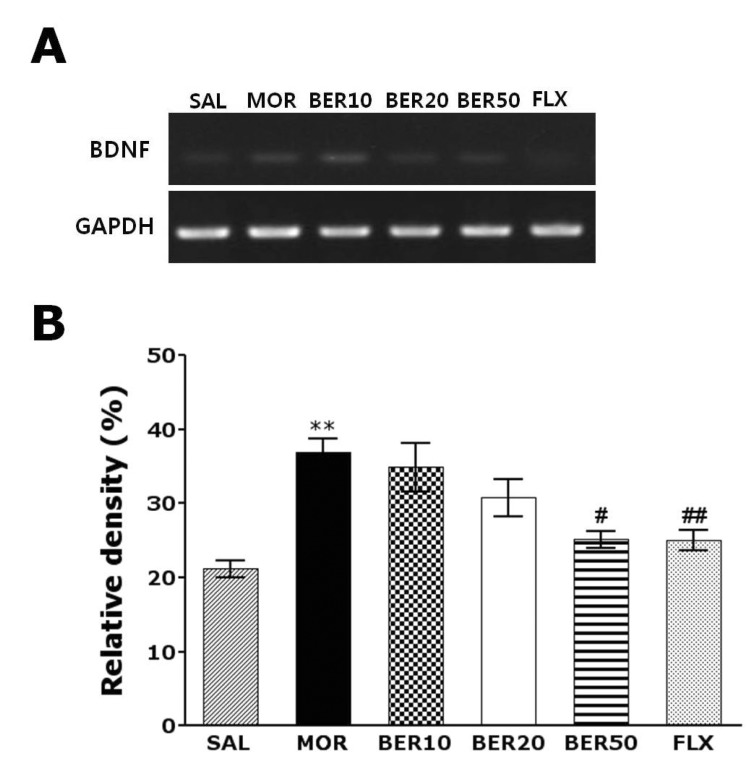
Effect of BER on morphine-induced BDNF mRNA expression in the hippocampus
The effect of BER administration on BDNF mRNA expression was investigated in the rat hippocampus following withdrawal from repeated morphine injection using RT-PCR analysis (Fig. 5). BDNF mRNA expression was normalized against glyceraldehydes-3-phophate dehydrogenase (GAPDH) mRNA, a housekeeping gene used as an internal control. BDNF mRNA expression in the MOR group hippocampus significantly increased compared with that in the SAL group (p<0.01). The increased BDNF mRNA expression in the MOR groups was significantly restored in the BER50 group (p<0.05). The recovery of the expression levels of BNDF mRNA in the BER50 group was comparable to that in the FLX group.
Fig. 5.
RT-PCR bands (A) and their relative intensities (B) of brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus after withdrawal from repeated saline or morphine administration. **p<0.01 vs. SAL group; #p<0.05, ##p<0.01 vs. MOR group.
DISCUSSION
BER has multiple pharmacological activities and has been used for treating various psychosomatic and neurodegenerative diseases, such as oxidative stress and ischemia [15]. Although BER is known to be clinically effective in alleviating depression-related symptoms, the underlying mechanisms of BER's effects on depression- and anxiety-like behavior during morphine withdrawal have not been fully characterized. Recently, several studies have shown that BER exerts antidepressant-like effects in various behavioral paradigms of despair and increases brain biogenic amines, such as norepinephrine, serotonin, and dopamine [16], and alleviates β-amyloid (Aβ)-induced spatial memory impairment, and inhibits pro-inflammatory cytokines expression, such as interleukin (IL)-1β [17]. BER can penetrate the blood-brain barrier and reach the striatum, cortex, and hippocampus, and thus BER might act directly in brain nuclei to produce pharmacological effects [9].
Our results clearly demonstrate that BER pretreatment prior to every morphine treatment significantly decreased the duration of immobility in the FST and increased open-arm exploration in the EPM test following withdrawal from repeated morphine administration by modulating hypothalamic CRF and the noradrenergic system in the CNS. Thus, the results of our study support the possibility that BER has antidepressant and anxiolytic effects. Interestingly, we examined dose-dependent activity of BER (10, 20, or 50 mg/kg) and found that 50 mg/kg was most effective in inhibiting harmful effects induced by repeated morphine administration, such as depression- and anxiety-like behaviors, using the FST and EPM test. The optimal dose and usage determined in this study was also referred to a previous study [17,18]. Additionally, on 3rd morphine treatment day, a significant increase in the immobility in the FST and decrease in open-arm exploration time in the EPM test were observed compared with saline-treated controls. However, only small differences in FST and EPM behavioral tests were observed on days 1 and 2 after ending repeated morphine administration. The third day (day 3) of repeated morphine administration was thus found to be the optimal time to observe changes in depression- and anxiety-like behaviors. Based on these results, we concluded that BER pretreatment prior to every morphine treatment might inhibited development of morphine dependence, and has antidepressant and anxiolytic effects, and BER can be a powerful regulator of psychiatric side-effects and a potential therapeutic agent against disease.
The FST is a valuable and reliable behavioral research model of depression in rodents and an important tool to study neurobiological mechanisms involved in antidepressant responses [18]. The observed immobility behavior in the FST is similar to a state of lowered mood or helplessness and depression in humans [19]. Although the FST provides information about mood (i.e., depression and anxiety) in rodents, it is important to use caution when extrapolating the data to humans. Our results are consistent with previous findings showing that withdrawal-related behavior from repeated morphine administration increased immobility during the FST [20]. Also, BER administration significantly decreased immobility and increased climbing behavior in the FST. However, there was no effect on swimming time in the FST, which confirmed that the antidepressant-like activity was not caused by a motor function deficit [18]. Some studies have reported that immobility and climbing behavior in the FST are associated with the central noradrenergic system [21]. Also, many studies have suggested that morphine dependence and withdrawal show increases the number of TH positive neurons in the LC [22,23]. Activation of the LC produces intense anxiety, hypervigilance and inhibition of exploratory behavior [24,25]. It has been proposed that clinical anxiety or depression may be the result of alterations in the activity of the LC in central noradrenergic system [26]. Thus, our results suggest that the central adrenergic system is involved in the antidepressant effect of BER on despair-like behavior that persisted for 3 days in rats following withdrawal from repeated morphine administration. Anxiety is another complex feature of depression, and thus anxiety-like symptoms in chronically stressed animals are not surprising. Many studies have suggested that rats receiving repeated morphine administration show decreases in the proportion of time spent in and the number of entries into the open arms of the EPM compared with saline-treated controls [27]. Although the EPM is based on conflict and subsequent movement of an animal between an open and an illuminated environment and an aversive environment, the test includes two additional anxiety-provoking environmental parameters, height and an open area [28]. In the present study, BER administration prior to repeated morphine injections significantly reduced anxiety-like behaviors in the EPM test, as indicated by an increase in the percentage of time spent in and the number of entries into an open arm [29]. Accordingly, these results suggest that chronic BER co-treatment with morphine on withdrawal-related behaviors in response to development of morphine dependence has anxiolytic activity.
Previous studies have shown that increased CRF systems activity in the brain is involved in behavioral and physiological manifestations of drug withdrawal and in relapses to drug-taking behaviors in both animals and human clinical populations [30]. CRF contributes to the anxiogenesis and aversive symptoms of withdrawal from exposure to several drugs of abuse, including morphine [31]. Our data suggest that the CRF circuits in the hypothalamus were potently activated by morphine administration, and this activation might be responsible for inducing depression and anxiety related to morphine withdrawal [32]. Furthermore, our results showed that alterations in hypothalamic CRF underlie the antidepressant and anxiolytic activities of BER following withdrawal from repeated morphine administration in rats. Many studies have shown that morphine withdrawal induces hyperactivity of noradrenergic pathways and an increase in TH modulation in the LC [33]. TH is an enzyme involved in response to morphine withdrawal-related psychopathological conditions, such as depression and anxiety [34]. Accordingly, TH expression in the LC is elevated following repeated morphine exposure and subsequent withdrawal, possibly due to long-term drug seeking or relapse in anticipation of drug discontinuation [35]. Therefore, our results are consistent with previous reports indicating that depression- and anxiety-like behaviors induced by morphine withdrawal are the result of alterations in the central noradrenergic system [36]. Thus, we demonstrated that BER is capable of attenuating complex behaviors involved in depression and anxiety via the modulation of the central noradrenergic system. Furthermore, some findings suggested that increased BDNF expression in the ventral tegmental area (VTA) produces long-lasting enhancement of cocaine seeking and locomotor stimulation during repeated cocaine administration [37,38]. BDNF may drive synaptic changes underlying drug craving and drug-seeking behavior [39]. In the present study, BER pretreatment prior to every morphine treatment restored the decreased expression level of BDNF mRNA in the hippocampus and ameliorated depression- and anxiety-like behaviors in rats experiencing withdrawal from morphine administration. These findings indicate that BER might be effective in preventing patients with drug addiction from relapsing into drug-seeking while trying to quit, by relieving some of the discomfort of morphine withdrawal symptoms, such as anxiety and depression.
In summary, our results demonstrated that BER pretreatment prior to every morphine treatment reduced depression- and anxiety-like symptoms strongly associated with morphine discontinuation, probably by modulating hypothalamic CRF and the noradrenergic system in the CNS. Therefore, BER may be a useful compound in the development of alternative medicines for treating morphine withdrawal-related symptoms, such as depression and anxiety.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Kyung Hee University in 2011 (KHU-20110087) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2005-0049404).
ABBREVIATIONS
- BER
berberine
- CRF
corticotrophin-releasing factor
- TH
tyrosine hydroxylase
- LC
locus coeruleus
- BDNF
brain-derived neurotrophic factor
- SSRIs
serotonin reuptake inhibitors
- TCAs
tricyclic antidepressants
- CNS
central nervous system
- FST
forced swimming test
- TST
tail-suspension test
- EPM
elevated plus maze
- FLX
fluoxetine
- PBS
phosphate-buffered saline
- ABC
avidin-biotin-peroxidase complex
- DAB
diaminobenzidine
- RT-PCR
reverse transcription-polymerase chain reaction
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- VTA
ventral tegmental area
References
- 1.Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, Kalivas PW. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology (Berl) 2009;203:677–684. doi: 10.1007/s00213-008-1414-2. [DOI] [PubMed] [Google Scholar]
- 2.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157:217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- 4.Maj M, Turchan J, Smiałowska M, Przewłocka B. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003;37:105–110. doi: 10.1016/s0143-4179(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 5.Lee B, Kim H, Shim I, Lee H, Hahm DH. Wild ginseng attenuates anxiety- and depression-like behaviors during morphine withdrawal. J Microbiol Biotechnol. 2011;21:1088–1096. doi: 10.4014/jmb.1106.06027. [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki D, Tsujita R, Yamada S, Kawasaki K, Otsuka Y, Hashimoto S, Hattori T, Kitamura Y, Miki N. Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacology (Berl) 2002;162:323–332. doi: 10.1007/s00213-002-1111-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Zhang HT, Bootzin E, Millan MJ, O'Donnell JM. Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs. Neuropsychopharmacology. 2009;34:1467–1481. doi: 10.1038/npp.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui HS, Matsumoto K, Murakami Y, Hori H, Zhao Q, Obi R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: involvement of B-cell lymphoma 2 phosphorylation suppression. Biol Pharm Bull. 2009;32:79–85. doi: 10.1248/bpb.32.79. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Yang JQ, He BC, Zhou QX, Yu HR, Tang Y, Liu BZ. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med J. 2009;30:760–766. [PubMed] [Google Scholar]
- 10.Peng WH, Lo KL, Lee YH, Hung TH, Lin YC. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007;81:933–938. doi: 10.1016/j.lfs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Peng WH, Wu CR, Chen CS, Chen CF, Leu ZC, Hsieh MT. Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models: interaction with drugs acting at 5-HT receptors. Life Sci. 2004;75:2451–2462. doi: 10.1016/j.lfs.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Erdtmann-Vourliotis M, Mayer P, Linke R, Riechert U, Höllt V. Long-lasting sensitization towards morphine in motoric and limbic areas as determined by c-fos expression in rat brain. Brain Res Mol Brain Res. 1999;72:1–16. doi: 10.1016/s0169-328x(99)00184-9. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 14.Vieira C, De Lima TC, Carobrez Ade P, Lino-de-Oliveira C. Frequency of climbing behavior as a predictor of altered motor activity in rat forced swimming test. Neurosci Lett. 2008;445:170–173. doi: 10.1016/j.neulet.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, Umathe S, Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220:30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008;589:163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 17.Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006;7:78. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaker EY, Sninsky CA. Effect of berberine on myoelectric activity and transit of the small intestine in rats. Gastroenterology. 1989;96:1506–1513. doi: 10.1016/0016-5085(89)90519-2. [DOI] [PubMed] [Google Scholar]
- 19.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinmardi N, Fathollahi Y, Naghdi N, Javan M. Theta pulse stimulation: a natural stimulus pattern can trigger long-term depression but fails to reverse long-term potentiation in morphine withdrawn hippocampus area CA1. Brain Res. 2009;1296:1–14. doi: 10.1016/j.brainres.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Consoni FT, Vital MA, Andreatini R. Dual monoamine modulation for the antidepressant-like effect of lamotrigine in the modified forced swimming test. Eur Neuropsychopharmacol. 2006;16:451–458. doi: 10.1016/j.euroneuro.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Carmona JA, Almela P, Baroja-Mazo A, Milanes MV, Laorden ML. Restricted role of CRF1 receptor for the activity of brainstem catecholaminergic neurons in the negative state of morphine withdrawal. Psychopharmacology (Berl) 2012;220:379–393. doi: 10.1007/s00213-011-2478-y. [DOI] [PubMed] [Google Scholar]
- 23.Núñez C, Földes A, Pérez-Flores D, García-Borrín JC, Laorden ML, Kovács KJ, Milanés MV. Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase mRNA expression, phosphorylation, and enzyme activity in the nucleus of the solitary tract during morphine withdrawal. Endocrinology. 2009;150:3118–3127. doi: 10.1210/en.2008-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redmond DE, Jr, Huang YH. The primate locus coeruleus and effects of clonidine on opiate withdrawal. J Clin Psychiatry. 1982;43:25–29. [PubMed] [Google Scholar]
- 25.Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 26.Park HJ, Shim HS, Kim H, Kim KS, Lee H, Hahm DH, Shim I. Effects of glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Korean J Physiol Pharmacol. 2010;14:371–376. doi: 10.4196/kjpp.2010.14.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y. Anxiety profile in morphine-dependent and withdrawn rats: effect of voluntary exercise. Physiol Behav. 2012;105:195–202. doi: 10.1016/j.physbeh.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Rezayof A, Hosseini SS, Zarrindast MR. Effects of morphine on rat behaviour in the elevated plus maze: the role of central amygdala dopamine receptors. Behav Brain Res. 2009;202:171–178. doi: 10.1016/j.bbr.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Aguiar DC, Terzian AL, Guimarães FS, Moreira FA. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl) 2009;205:217–225. doi: 10.1007/s00213-009-1532-5. [DOI] [PubMed] [Google Scholar]
- 30.Shi J, Li SX, Zhang XL, Wang X, Le Foll B, Zhang XY, Kosten TR, Lu L. Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse. 2009;35:267–272. doi: 10.1080/00952990902933878. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Zaragoza J, Núñez C, Laorden ML, Milanés MV. Effects of corticotropin-releasing factor receptor-1 antagonists on the brain stress system responses to morphine withdrawal. Mol Pharmacol. 2010;77:864–873. doi: 10.1124/mol.109.062463. [DOI] [PubMed] [Google Scholar]
- 32.Papaleo F, Kitchener P, Contarino A. Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron. 2007;53:577–589. doi: 10.1016/j.neuron.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Núñez C, Féldes A, Pérez-Flores D, García-Borrín JC, Laorden ML, Kovács KJ, Milanés MV. Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase mRNA expression, phosphorylation, and enzyme activity in the nucleus of the solitary tract during morphine withdrawal. Endocrinology. 2009;150:3118–3127. doi: 10.1210/en.2008-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl) 2003;170:80–88. doi: 10.1007/s00213-003-1504-0. [DOI] [PubMed] [Google Scholar]
- 36.Cameron OG. Anxious-depressive comorbidity: effects on HPA axis and CNS noradrenergic functions. Essent Psychopharmacol. 2006;7:24–34. [PubMed] [Google Scholar]
- 37.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 39.Chu NN, Zuo YF, Meng L, Lee DY, Han JS, Cui CL. Peripheral electrical stimulation reversed the cell size reduction and increased BDNF level in the ventral tegmental area in chronic morphine-treated rats. Brain Res. 2007;1182:90–98. doi: 10.1016/j.brainres.2007.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]