Abstract
In this study, we examined the antinociceptive effect of Cyperi rhizoma (CR) and Corydalis tuber (CT) extracts using a chronic constriction injury-induced neuropathic pain rat model. After the ligation of sciatic nerve, neuropathic pain behavior such as mechanical allodynia and thermal hyperalgesia were rapidly induced and maintained for 1 month. Repeated treatment of CR or CT (per oral, 10 or 30 mg/kg, twice a day) was performed either in induction (day 0~5) or maintenance (day 14~19) period of neuropathic pain state. Treatment of CR or CT at doses of 30 mg/kg in the induction and maintenance periods significantly decreased the nerve injury-induced mechanical allodynia. In addition, CR and CT at doses of 10 or 30 mg/kg alleviated thermal heat hyperalgesia when they were treated in the maintenance period. Finally, CR or CT (30 mg/kg) treated during the induction period remarkably reduced the nerve injury-induced phosphorylation of NMDA receptor NR1 subunit (pNR1) in the spinal dorsal horn. Results of this study suggest that extracts from CR and CT may be useful to alleviate neuropathic pain.
Keywords: Chronic constriction injury, Corydalis tuber, Cyperi rhizoma, Neuropathic pain, NMDA receptor
INTRODUCTION
Traditionally, both of Cyperi rhizoma (CR, a root of Cyperus rotundus L.) and Corydalis tuber (CT, a root of Corydalis yanhusuo W.T. Wang) is well known as medicinal herbs [1] and recent pharmacological studies have revealed pharmacological actions of these substances [2-7]. CR has used to treat spasm, bowel and stomach disorder, candidiasis, malarial fever, psychotic disorders and several inflammatory diseases [3-5,8,9] and CT has used to treat pain, inflammation and gastrointestinal dysfunctions [2,6,7,10]. However more scientific approach is needed to reveal their pharmacological effect.
Peripheral neuropathic pain can be induced by a traumatic and/or metabolic nerve injury and neuropathic pain patients commonly suffer from the spontaneous burning pain (causalgia), allodynia (pain sensation after innocuous stimuli) and hyperalgesia (enhanced pain sensation by noxious stimuli) [11,12]. Existing treatments by using opioids, neurotransmitter reuptake inhibitors, and calcium channel blockers elicit an insufficient pain relief and side effects including sedation, dependence, tolerance, and other neurological disorders have limited the use of these drugs [13]. Numerous studies have revealed that the neuropathic pain is evoked by a variety of pathological changes including central and peripheral sensitization [14,15]. At the spinal cord level, N-methyl-D-aspartate (NMDA) receptor plays a major role in the induction of central sensitization [16]. The NMDA receptor is an ionotropic receptor for a major excitatory neurotransmitter, glutamate and composed of three related families of subunits NR1, NR2, and NR3 [17]. Among these subunits, NR1 is known to be essential for the functional NMDA receptor and phosphorylation of NR1 subunit (pNR1) of spinal dorsal horn may be critical to produce pathological chronic pain [18,19].
We designed this study to determine whether the repeated treatment of CR or CT is able to inhibit neuropathic pain (i.e. mechanical allodynia and thermal hyperalgesia) using chronic constriction injury (CCI) model in rats [20]. Repeated treatment of CR or CT (per oral, 10 or 30 mg/kg, twice a day) was performed either in induction (day 0~5) or maintenance (day 14~19) period after sciatic nerve ligation. In addition, pNR1 expression of the spinal dorsal horn was analyzed by using immunohistochemistry and computer-based image analysis technique to determine the antinociceptive mechanism of CR and CT on CCI-induced neuropathic pain in rats.
METHODS
Animals
Male Sprague-Dawley rats (Samtako, Osan, Korea) weighing 200 to 250 g were used in this experiment. Food and water were supplied ad libitum and animals were kept in a 12-hour light-dark cycle. All surgical and experimental procedures were approved by the Animal Care and Use Committee at Chungnam National University and conform to NIH guidelines (NIH publication no. 86-23, revised 1985).
Induction of chronic constriction injury (CCI)
A CCI of common sciatic nerve was performed according to the method as previously described [20]. Briefly rats were anesthetized with 3% isoflurane in a mixture of N2O/O2 gas. The right sciatic nerve was exposed at the mid-thigh level, and 4 loose ligatures of 4~0 chromic gut were placed around the nerve with a 1.0 to 1.5 mm interval between each ligature. Sham rats received only surgical opening without nerve ligation. After surgery, animals were housed in clear plastic cages with a thick layer of sawdust bedding.
Drugs
Extracts from both CR and CT were purchased from the Plant Diversity Research Center at the Korea Research Institute of Bioscience & Biotechnology (Daejeon, Korea). CR or CT was immersed in ethanol and sonicated at 50℃ for 7 days. Extracts were pressure-filtered by using non-fluorescence cotton, concentrated with a rotary evaporator and lyophilized for 24 h. Vehicle was prepared by the combination of dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA, 20%), polyethylene glycol 400 (PEG, Sigma, 50%), and sterile saline solution (30%). CR or CT at a dose of 10 or 30 mg/kg was administered orally with a volume of 0.3 ml/100 g body weight. As a positive control drug, pregabalin (Pfizer, Seoul, Korea) was used. Dose of pregabalin (30 mg/kg) was selected by previous reports [21,22]. Oral treatment of CR, CT and pregabalin was performed twice a day, either on postoperative day (POD) 0~5 (induction period) or on POD 14~19 (maintenance period) in a separated set of experiment and control animals received vehicle solution.
Behavioral assessments
To obtain normal baseline values of withdrawal response to mechanical and heat stimuli, behavioral tests were performed 1 day before CCI induction on all animals. Animals were randomly assigned to each drug-treatment group, and all subsequent behavioral testing was performed blindly. The number of paw withdrawal responses to normally innocuous mechanical stimuli was measured by using a von Frey filament with a force of 2.0 g (North Coast Medical, Morgan Hill, CA, USA). Rats were placed on a metal mesh grid under a plastic chamber, and the von Frey filament was applied from underneath the metal mesh flooring to each hind paw. The von Frey filament was applied 10 times to each hind paw, and the number of paw withdrawal responses out of 10 was then counted. The results of mechanical behavioral testing in each experimental animal were expressed as a percent withdrawal response frequency (PWF), which represented the percentage of paw withdrawals out of the maximum of 10 as previously described [23,24]. To assess nociceptive responses to heat stimuli, we measured paw withdrawal response latency (PWL) by using the plantar paw-flick latency test as previously described [25]. Briefly rats were placed in a plastic chamber with a glass floor and were allowed to acclimatize for 10 min before testing. A radiant heat source was positioned under the glass floor beneath the hind paw to be tested, and withdrawal latency was measured using a plantar analgesia meter (IITC Life Science Inc., Woodland Hills, CA, USA). The test was duplicated in the ipsilateral hind paw of each animal, and the mean withdrawal latency was calculated. Cutoff time in the absence of a response was set at 20 sec to prevent possible tissue damage.
Immunohistochemistry and imaging analysis for pNR1 (ser897)
Rats were euthanized at the endpoint of each drug treatment (either 5 or 19 days after CCI surgery), and the L4~L6 segments of the spinal cord were removed for pNR1 (ser897) immunohistochemistry at 5 or 19 days after CCI induction. Animals were deeply anesthetized with 5% isoflurane and then perfused transcardially with calcium-free Tyrode solution, followed by a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 6.9). The spinal cord was removed immediately, postfixed at 4℃ overnight in the same fixative, and then cryoprotected in 30% sucrose in phosphate-buffered saline (pH 7.4) for 48 h. Frozen serial sections (40 µm) were cut through the lumbar L4~L6 spinal cord using a cryostat (Microm, Walldorf, Germany). After elimination of endogenous peroxidase activity with 0.3% hydrogen peroxide and preblocking with 3% normal goat serum in phosphate-buffered saline, the free-floating sections were incubated in rabbit anti-pNR1 antibody (1:1,000, cat# 06~641, Upstate Biotechnology, NY, USA) at 4℃ for 48 h. To determine immunohistochemical method specificity, some sections were processed in an identical fashion, but without primary antibodies. The sections were subsequently processed using the avidin-biotin-peroxidase procedure previously described [26]. Finally visualization was performed using 3,3'-diaminobenzidine (Sigma) with 0.2% nickel chloride intensification. The sections were examined under a bright-field microscope (Zeiss Axioscope; Hallbergmoos, Germany) at ×100 to localize pNR1-immunoreactive (ir) neurons. For quantitative analysis of pNR1-ir neurons, five spinal cord sections from the L4~L6 lumbar spinal cord segments were randomly selected from each animal and subsequently scanned. Individual sections were digitized with 4,096 gray levels using a cooled charge-coupled device camera (Micromax Kodak 1317; Princeton Instruments, Tucson, AZ, USA) connected to a computer-assisted image analysis system (Metamorph®, Molecular devices, USA). To maintain a constant threshold for each image and to compensate for subtle variability of the immunostaining, we only counted neurons that were at least 70% darker than the average gray level of each image after background subtraction and shading correction. The microscope illumination and data acquisition settings were fixed throughout the entire analysis procedure. The average number of pNR1-ir neurons per section from each animal was obtained, and these values were averaged across each group and presented as group data. The expression of pNR1 was quantified in the following three dorsal horn regions: (1) the superficial dorsal horn (laminae I and II), (2) the nucleus proprius (laminae III and IV), and (3) the neck region (laminae V and VI). These regions were identified based on cytoarchitectonic criteria as defined by previous report [27]. All pNR1 quantitation procedures described above were performed blindly with regard to the experimental condition of each animal.
Rota-rod test
The rota-rod test is a commonly used screening procedure to detect motor incoordination and/or ataxia in rodents [28]. Using a rota-rod tester (SciTech Korea Inc., Seoul, Korea), rats were placed on a cylindrical platform (12 cm wide; 6 cm diameter) suspended 33 cm above the bottom of the apparatus. Escape to either side was prevented by Plexiglas walls, and falls were cushioned by wood shaving bedding. After 3 days of acclimation period, rota-rod test has done before and at every 0, 30, 60 and 90 min after per oral treatment of test drugs. Spent time on a rotating rod (constant speed of 3 revolutions per minute) was measured at each time point and cut-off time for this test was 2 minutes. As a positive control, combination of Zoletil (5 mg/kg) and xylazine (0.75 mg/kg) was intraperitoneally treated.
Statistical analysis
Data values were expressed as mean±SEM. The level of statistical significance was determined by unpaired Student's t-test for comparisons between two means, and by analysis of variance (ANOVA) followed by Dunnett's test for multiple comparisons. Graphpad Prism (version 4.0, San Diego, CA, USA) was used to perform the statistical analysis and a value of p<0.05 was considered statistically significant.
RESULTS
Antinociceptive effect of Cyperi rhizoma (CR) on neuropathic pain
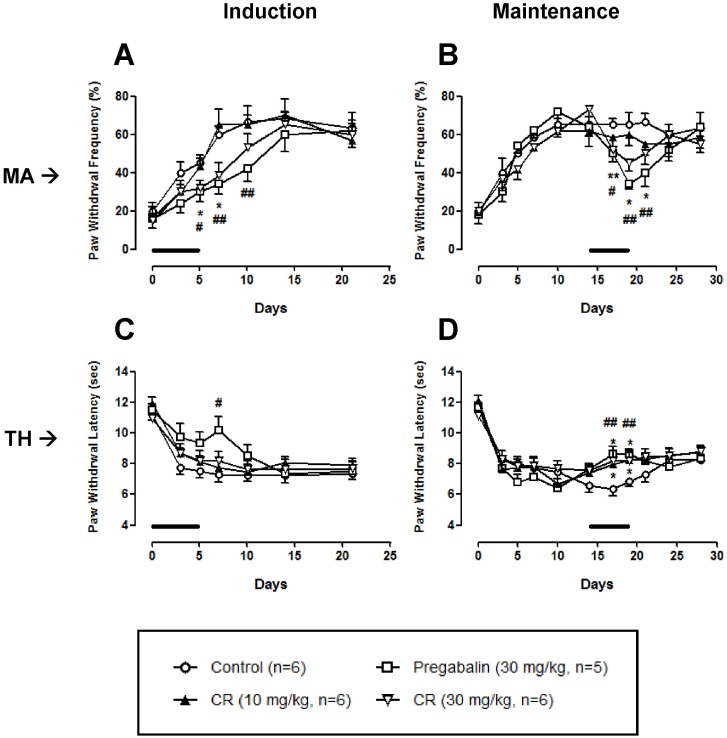
Chronic constriction injury significantly produced a typical neuropathic pain behavior such as mechanical allodynia and thermal hyperalgesia (Control in Fig. 1). These pain behaviors are rapidly induced just after the surgery and sustained for 1 month. As shown in Fig. 1A, 1B, high dose (30 mg/kg) of CR, but not low dose (10 mg/kg), significantly reduced mechanical allodynia both in induction and maintenance phases. In the thermal hyperalgesia test, CR did not produce antinociceptive effect when it was treated in the induction phase (Fig. 1C). However, CR (10 and 30 mg/kg) treated in the maintenance phase significantly alleviated nerve injury-associated thermal hyperalgesia (Fig. 1D). Antinociceptive effect of CR on neuropathic pain was abolished after the discontinuation of treatment.
Fig. 1.
Effect of Cyperi rhizoma (CR) on chronic constriction injury (CCI)-induced mechanical allodynia (MA) and thermal hyperalgesia (TH). CR at a dose of 30 mg/kg significantly reduced mechanical allodynia (A, B) when it was treated in the induction and maintenance phases. In a thermal hyperalgesia test, CR at doses of 10 and 30 mg/kg increased the paw withdrawal latency in the maintenance phase (D), but not induction phase (C). Pregabalin (30 mg/kg) was used as a positive control drug. Lines in each panel indicate the treatment period of CR or pregabalin. *p<0.05 and **p<0.01 control vs CR. #p<0.05 and ##p<0.01 control vs pregabalin.
Antinociceptive effect of Corydalis tuber (CT) on neuropathic pain
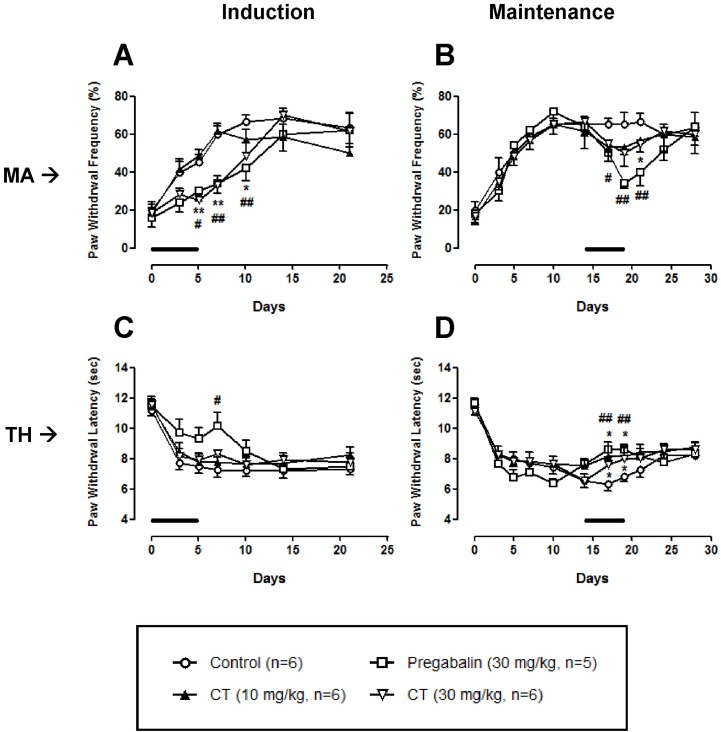
As shown in Fig. 2A and B, only high dose (30 mg/kg) of CT significantly decreased nerve injury-induced mechanical allodynia both in the induction and maintenance phases. In the thermal hyperalgesia test, CT (10 and 30 mg/kg) treated in the maintenance phase, but not induction phase, produced a significant antinociception (Fig. 2C and D). These CT-induced analgesic effects were abolished after the discontinuation of treatment.
Fig. 2.
Effect of Corydalis tuber (CT) on chronic constriction injury (CCI)-induced mechanical allodynia (MA) and thermal hyperalgesia (TH). CT at a dose of 30 mg/kg significantly reduced mechanical allodynia when it was treated in the induction and maintenance phases (A, B). In a thermal hyperalgesia test, CT at doses of 10 and 30 mg/kg increased the paw withdrawal latency in the maintenance phase (D), but not induction phase (C). Pregabalin (30 mg/kg) was used as a positive control drug. Lines in each panel indicate the treatment period of CT or pregabalin. *p<0.05 and **p<0.01 control vs CR. #p<0.05 and ##p<0.01 control vs pregabalin.
Inhibitory effect of Cyperi rhizoma (CR) and Corydalis tuber (CT) on chronic constriction injury-induced phosphorylation of NMDA receptor NR1 subunit (pNR1)
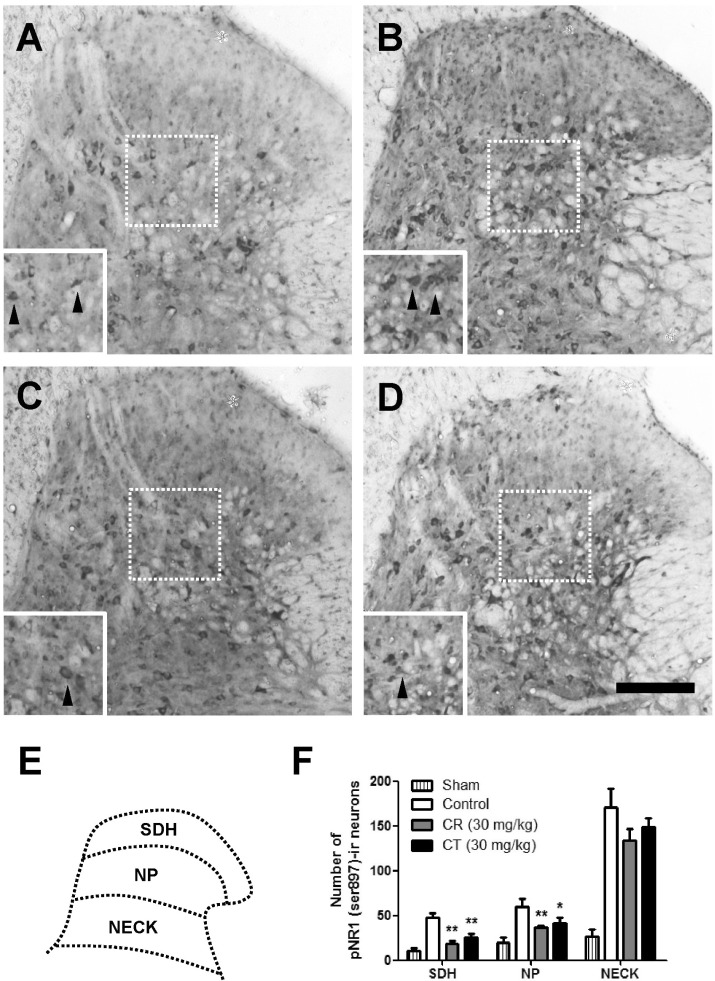
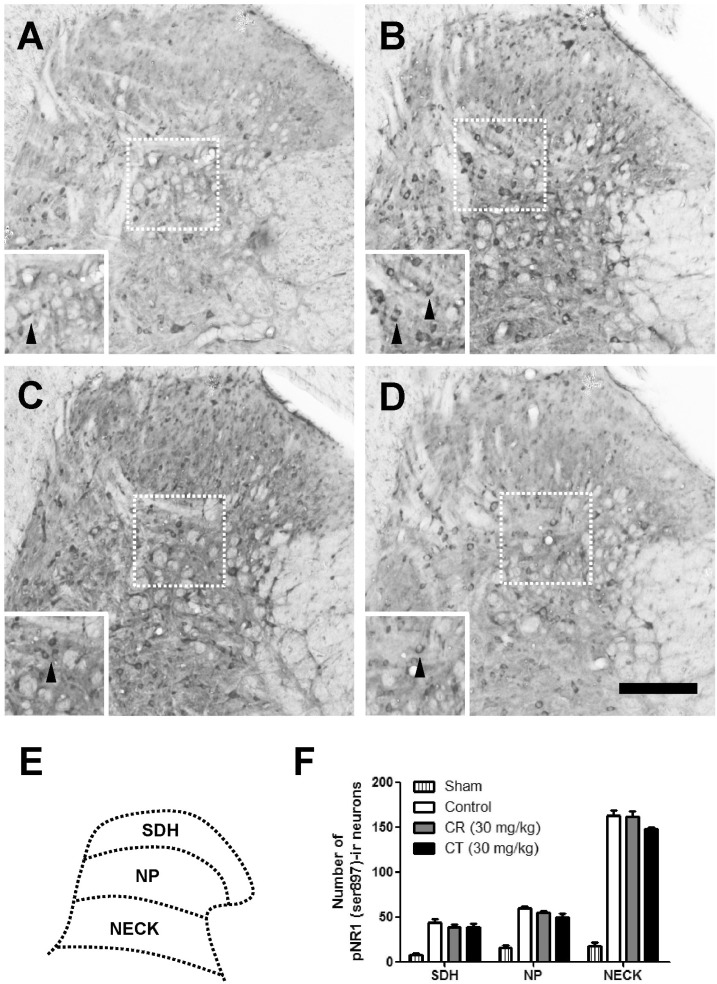
As compared with sham rats, CCI remarkably increased the number of pNR1-positive neurons as shown in Fig. 3 and 4. When CR and CT (30 mg/kg) were treated in the induction phase (POD 0-5), the number of pNR1 immunoreactive neurons was significantly decreased in the superficial dorsal horn (SDH) and nucleus proprius (NP) as shown in Fig. 3. However, both CR and CT did not affect the chronic constriction injury-induced pNR1 in the neck of dorsal horn (NECK). This result indicates that CR and CT of induction period might have an antinociceptive effect on chronic constriction injury-induced neuropathic pain via the mediation of NMDA receptor NR1 subunit activity of spinal dorsal horn (i.e. SDH and NP). When CR or CT (30 mg/kg) was treated in the maintenance phase (POD 14-19) of chronic constriction injury-induced neuropathic pain, they did not reduce the pNR1 expression in SDH, NP and NECK area as compared with that of control (Fig. 4).
Fig. 3.
Effect of Cyperi rhizoma (CR) and Corydalis tuber (CT) on chronic constriction injury (CCI)-induced phosphorylation of NMDA receptor NR1 subunit (pNR1) in induction phase. The number of pNR-1 positive neurons was significantly increased by CCI surgery (B) as compared with Sham group (A). Treatment of CR or CT (30 mg/kg) in the induction phase significantly reduced the number of pNR1-immunoreactive neurons both in superficial dorsal horn (SDH) and nucleus proprius (NP) as compared with that of control group (B). However, there is no statistically significant change in neck of dorsal horn (NECK) area. The pNR1 immunoreactivity was observed in Sham (A), control (B), CR (C) and CT (D)-treated groups. Panel (E) shows a diagram depicting the location of the different spinal cord regions analyzed in this study. Results of each group are represented in panel (F). Inserted panels show a magnified image of rectangles in panel A, B, C, and D, respectively. The black arrow heads indicate representative pNR1 neurons. The number of animals is 3 in each group. Scale bar=200 µm. *p<0.05 and **p<0.01 compared to control.
Fig. 4.
Effect of Cyperi rhizoma (CR) and Corydalis tuber (CT) on chronic constriction injury (CCI)-induced phosphorylation of NMDA receptor NR1 subunit (pNR1) in maintenance phase. The number of pNR-1 positive neurons was significantly increased by CCI surgery (B) as compared with Sham group (A). Treatment of CR or CT (30 mg/kg) in the maintenance phase did not affect the chronic constriction injury-induced pNR1 in the superficial dorsal horn (SDH), nucleus proprius (NP) and neck of dorsal horn (NECK). The pNR1 immunoreactivity was observed in Sham (A), control (B), CR (C) and CT (D)-treated groups. Panel (E) shows a diagram depicting the location of the different spinal cord regions analyzed in this study. Results of each group are represented in panel (F). Inserted panels show a magnified image of rectangles in panel A, B, C, and D, respectively. The black arrow heads indicate representative pNR1 neurons. The number of animals is 3 in each group. Scale bar=200 µm.
Rota-rod test
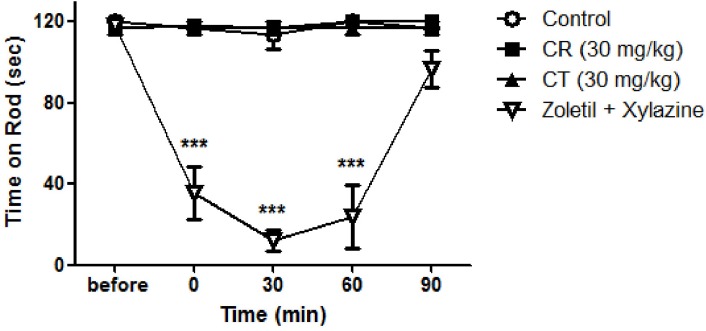
Per oral treatment of CR (30 mg/kg) or CT (30 mg/kg) did not affect normal motor function of rats as shown in Fig. 5. This result indicated that antinociceptive effect of CR and CT are not induced by possible sedative action. Intraperitoneal injection of anesthetic (Zoletil and xylazine) significantly suppressed normal motor function.
Fig. 5.
Effect of Cyperi rhizoma (CR) and Corydalis tuber (CT) on motor function. Rota-rod test revealed that orally treated CR or CT did not affect normal motor function of rats. Positive control drug (Zoletil; 5 mg/kg and xylazine; 0.75 mg/kg, i.p.) significantly suppressed normal motor function. ***p<0.001 compared to control. The number of animals is 3 in each group.
DISCUSSION
In this study, we determined the antinociceptive effect of extracts from CR or CT using a CCI-induced neuropathic pain model in rats. When CR or CT was treated in the induction period (POD 0-5) of neuropathic pain, high dose (30 mg/kg, oral, twice a day) of CR or CT significantly alleviated the mechanical allodynia. In addition, they remarkably suppressed the chronic constriction injury-induced pNR1 expression of the spinal dorsal horn including SDH (laminae I and II) and NP (laminae III and IV). These results suggested that the antinociceptive effect of CR and CT against the nerve injury-induced pathological pain might be produced by the mediation of spinal NMDA receptor NR1 subunit activity. Since the phosphorylation of NMDA receptor have been accepted to play a critical role in the central sensitization which is one of the most important phenomena for neuropathic pain [11,18,19], results of this study indicated that the CR and CT might be good candidates as a new analgesic for the treatment of initial phase of neuropathic pain since a mechanical allodynia is the most disturbing symptom and decreases the quality of life of neuropathic pain patient. The antinociceptive effect of CR and CT at doses of 30 mg/kg on nerve injury-induced mechanical allodynia was also reproduced when they were treated in the maintenance period (POD 14-19). However, CR and CT treated in the maintenance period did not reduce the nerve injury-induced pNR1 expression of spinal dorsal horn as compared with that of control group. It is thought that higher dose than 30 mg/kg of CR and CT might be needed to suppress nerve injury-induced pNR1 expression or other neuronal mechanisms were responsible for the antinociceptive action of CR and CT in this period.
In addition to mechanical allodynia, thermal heat hyperalgesia is another typical pain symptom of nerve injury-induced neuropathic pain [29,30]. In neuropathic pain state, innocuous (i.e. non-noxious) touch stimulation can cause mechanical allodynia and thermal hyperalgesia is induced by a noxious heat stimulus. These two typical pain behaviors seemed to be induced by a different neuronal mechanism. Activity of heat transducer receptors such as transient receptor potential vanilloid 1 (TRPV1) and TRPV2 is known to be critical to induce thermal heat hyperalgesia [11,31]. Results of this study showed that the thermal hyperalgesia was rapidly induced just after the surgery and sustained for 1 month. When CR and CT were treated in the induction period of neuropathic pain, they did not produce any noticeable antinociceptive effect on thermal hyperalgesia. However, CR and CT at doses of 10 and 30 mg/kg significantly alleviated thermal hyperalgesia during the maintenance phase, suggesting that CR and CT may be valuable to cure the thermal heat hyperalgesia after the development of neuropathic pain.
Both CR and CT have been traditionally used to treat several diseases as a medicinal herb because they had a significant pharmacological effect. CR has used to treat spasm, bowel and stomach disorder, candidiasis, malarial fever, psychotic disorders and inflammatory diseases [3-5,8,9]. Recent reports have revealed that the water extract of CR produces a protective effect on 6-hydroxydopamine-induced neuronal toxicity [3] and modulates the immune function by the enhancing of Th1 and suppressing of Th2 lineage development [4]. Moreover, animal study has suggested that the CR may recover the mood disorder such as a depression [5]. CT has used to treat pain, inflammation and gastrointestinal dysfunctions [2,6,7,10]. Traditionally, CT has been used to treat pain and recent report indicates that the CT modulates glycine-induced ion current of periaqueductal gray neuron which is important to generate or mediate the descending pain modulatory system, suggesting the main active site of CT may be a higher brain center [6]. In this study, all experimental animals showed a normal motor behavior, suggesting that CR and CT may not produce unwanted side effect such as sedation.
In conclusion, we have demonstrated that the antinociceptive effect of CR and CT using a rat model of chronic constriction injury-induced neuropathic pain. Repeated treatment of CR or CT in the induction (POD 0-5) and maintenance (POD 14-19) periods significantly decreased the nerve injury-induced mechanical allodynia. In addition, CR or CT alleviated thermal heat hyperalgesia when they were treated in the maintenance period. Finally, CR or CT treated during the induction period remarkably reduced the nerve injury-induced pNR1 of spinal dorsal horn, suggesting that the antinociceptive effect of CR and CT might be produced by the mediation of NMDA receptor NR1 subunit activity. Results of this study suggest that the CR and CT may be useful to treat nerve injury-induced neuropathic pain.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A084541).
ABBREVIATIONS
- CCI
chronic constriction injury
- CR
Cyperi rhizoma
- CT
Corydalis tuber
- ir
immunoreactive
- MA
mechanical allodynia
- pNR1
phosphorylation of NMDA receptor NR1 subunit
- POD
postoperative day
- TH
thermal hyperalgesia
References
- 1.Ding GS. Important Chinese herbal remedies. Clin Ther. 1987;9:345–357. [PubMed] [Google Scholar]
- 2.Lee TH, Son M, Kim SY. Effects of corydaline from Corydalis tuber on gastric motor function in an animal model. Biol Pharm Bull. 2010;33:958–962. doi: 10.1248/bpb.33.958. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Hwang DS, Kim HG, Oh H, Park H, Cho JH, Lee JM, Jang JB, Lee KS, Oh MS. Protective effect of Cyperi rhizoma against 6-hydroxydopamine-induced neuronal damage. J Med Food. 2010;13:564–571. doi: 10.1089/jmf.2009.1252. [DOI] [PubMed] [Google Scholar]
- 4.Bae H, Park N, Kim Y, Hong M, Shin M, Kim SH, Kim J. The modulative effect of Cyperi Rhizoma on Th1/Th2 lineage development. Cytokine. 2010;51:259–265. doi: 10.1016/j.cyto.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Han J, Seog DH, Chung JY, Kim N, Hong Park Y, Lee SK. Antidepressant effect of Chaihu-Shugan-San extract and its constituents in rat models of depression. Life Sci. 2005;76:1297–1306. doi: 10.1016/j.lfs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Cheong BS, Choi DY, Cho NH, Lee JD, Chang HK, Shin MC, Shin MS, Kim CJ. Modulation of Corydalis tuber on glycine-induced ion current in acutely dissociated rat periaqueductal gray neurons. Biol Pharm Bull. 2004;27:1207–1211. doi: 10.1248/bpb.27.1207. [DOI] [PubMed] [Google Scholar]
- 7.Kubo M, Matsuda H, Tokuoka K, Ma S, Shiomoto H. Anti-inflammatory activities of methanolic extract and alkaloidal components from Corydalis tuber. Biol Pharm Bull. 1994;17:262–265. doi: 10.1248/bpb.17.262. [DOI] [PubMed] [Google Scholar]
- 8.Weenen H, Nkunya MH, Bray DH, Mwasumbi LB, Kinabo LS, Kilimali VA, Wijnberg JB. Antimalarial compounds containing an alpha,beta-unsaturated carbonyl moiety from Tanzanian medicinal plants. Planta Med. 1990;56:371–373. doi: 10.1055/s-2006-960985. [DOI] [PubMed] [Google Scholar]
- 9.Weenen H, Nkunya MH, Bray DH, Mwasumbi LB, Kinabo LS, Kilimali VA. Antimalarial activity of Tanzanian medicinal plants. Planta Med. 1990;56:368–370. doi: 10.1055/s-2006-960984. [DOI] [PubMed] [Google Scholar]
- 10.Ma WG, Fukushi Y, Tahara S, Osawa T. Fungitoxic alkaloids from Hokkaido Papaveraceae. Fitoterapia. 2000;71:527–534. doi: 10.1016/s0367-326x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 11.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 13.Rice AS, Hill RG. New treatments for neuropathic pain. Annu Rev Med. 2006;57:535–551. doi: 10.1146/annurev.med.57.121304.131324. [DOI] [PubMed] [Google Scholar]
- 14.Son JS, Kwon YB. Sigma-1 receptor antagonist BD1047 reduces allodynia and spinal ERK phosphorylation following chronic compression of dorsal root ganglion in rats. Korean J Physiol Pharmacol. 2010;14:359–364. doi: 10.4196/kjpp.2010.14.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 16.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 18.Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki-Yatsugi S, Nagakura Y, Ogino S, Sekizawa T, Kiso T, Takahashi M, Ishikawa G, Ito H, Shimizu Y. Automated measurement of spontaneous pain-associated limb movement and drug efficacy evaluation in a rat model of neuropathic pain. Eur J Pain. 2012;16:1426–1436. doi: 10.1002/j.1532-2149.2012.00142.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar N, Laferriere A, Yu JS, Leavitt A, Coderre TJ. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2010;113:552–561. doi: 10.1111/j.1471-4159.2010.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-D-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008;12:552–563. doi: 10.1016/j.ejpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Roh DH, Kwon YB, Kim HW, Ham TW, Yoon SY, Kang SY, Han HJ, Lee HJ, Beitz AJ, Lee JH. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: involvement of spinal alpha 2-adrenoceptors. J Pain. 2004;5:297–303. doi: 10.1016/j.jpain.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YB, Jeong YC, Kwon JK, Son JS, Kim KW. The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats. Korean J Physiol Pharmacol. 2009;13:425–429. doi: 10.4196/kjpp.2009.13.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbadie C, Besson JM. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. Pain. 1994;57:45–54. doi: 10.1016/0304-3959(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 28.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 29.Vranken JH. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2009;9:71–78. doi: 10.2174/187152409787601932. [DOI] [PubMed] [Google Scholar]
- 30.Bennett GJ, Chung JM, Honore M, Seltzer Z. Models of neuropathic pain in the rat. Curr Protoc Neurosci. 2003;Chapter 9:Unit 9.14. doi: 10.1002/0471142301.ns0914s22. [DOI] [PubMed] [Google Scholar]
- 31.Baron R. Neuropathic pain: a clinical perspective. Handb Exp Pharmacol. 2009;(194):3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]