Abstract
The spontaneous axon regeneration of damaged neurons is limited after spinal cord injury (SCI). Recently, mesenchymal stem cell (MSC) transplantation was proposed as a potential approach for enhancing nerve regeneration that avoids the ethical issues associated with embryonic stem cell transplantation. As SCI is a complex pathological entity, the treatment of SCI requires a multipronged approach. The purpose of the present study was to investigate the functional recovery and therapeutic potential of human MSCs (hMSCs) and polymer in a spinal cord hemisection injury model. Rats were subjected to hemisection injuries and then divided into three groups. Two groups of rats underwent partial thoracic hemisection injury followed by implantation of either polymer only or polymer with hMSCs. Another hemisection-only group was used as a control. Behavioral, electrophysiological and immunohistochemical studies were performed on all rats. The functional recovery was significantly improved in the polymer with hMSC-transplanted group as compared with control at five weeks after transplantation. The results of electrophysiologic study demonstrated that the latency of somatosensory-evoked potentials (SSEPs) in the polymer with hMSC-transplanted group was significantly shorter than in the hemisection-only control group. In the results of immunohistochemical study, β-gal-positive cells were observed in the injured and adjacent sites after hMSC transplantation. Surviving hMSCs differentiated into various cell types such as neurons, astrocytes and oligodendrocytes. These data suggest that hMSC transplantation with polymer may play an important role in functional recovery and axonal regeneration after SCI, and may be a potential therapeutic strategy for SCI.
Keywords: Electrophysiology, Mesenchymal stem cells, Polymer, Spinal cord injury
INTRODUCTION
After spinal cord injury (SCI), failure of regeneration is attributed to the nonpermissive environment of the damaged spinal cord. Injured spinal cord is characterized by astrocyte-derived inhibitory molecules in the scar tissue, demyelination of axons, lack of tropic support for axotomized neurons, and intrinsic neuronal changes such as cell atrophy and death [1,2]. Therefore, effective repair strategies for SCI require the creation of a permissive environment within the injured spinal cord in order to protect damaged neurons from the effects of secondary injury and also to facilitate axonal regeneration. The repair of the injured human spinal cord in many cases requires neuronal survival, axonal growth and remyelination [3,4], as well as reconnection across the trauma cavity by means of bridging grafts.
Clinically, the limited access to autologous donor materials and the immunological problems associated with allograft rejection have prompted a search for artificial biomaterials that may be implanted as bridges in injured spinal cords. Investigators have attempted to overcome some of these barriers by using implantable scaffolds made of a variety of materials, including biodegradable polymers and other, nondegradable materials [5,6]. Recent preclinical studies include the implantation of tubes made from polymeric materials [7,8], polymer hydrogels [9-11] and biodegradable polylactide implants [12]. Although hydrogels, water-swollen insoluble polymers that can be made degradable, have recently been used as scaffolds for axon regeneration [13], their use in delivering molecules to promote regenerative growth in injured central nervous system has not been explored. Therefore, we expect that implantation of the polymer with hMSC may be more efficient in improving functional recovery than implantation of the polymer alone.
A variety of experimental strategies including stem cell transplantation are emerging to promote regeneration of injured spinal cords. Mesenchymal stem cells (MSCs) constitute an alternative source of pluripotent stem cells. They have the capacity to differentiate into cells of mesodermal lineage, and also have a much broader differentiation potential [14-16]. After MSC transplantation into host CNS tissue, MSCs show the capacity to differentiate into neurons [17-20] and astrocytes [21,22]. Recent studies have reported that MSCs promote partial functional recovery after grafting to sites of SCI [23-25], although mechanisms underlying this recovery have not been defined. Grafted MSCs survive in spinal cord tissue, forming cell bridges within the traumatic centromedullary cavity. In spinal cord tissue, cells expressing neuronal and astroglial markers have been observed, together with a marked ependymal proliferation and nestin-positivity, after MSC transplantation [26]. Moreover, MSC transplantation in a variety of animal models of SCI has been shown to produce remyelination and improved functional recovery [27-30]. Our previous reports have also shown that transplantation of human mesenchymal stem cells (hMSCs) into the spinal cord after contusion injury promotes a functional outcome [31]. Thus, the present study was conducted to investigate the functional recovery and the therapeutic potential of human MSCs (hMSCs) and polymer in a spinal cord hemisection injury model using behavioral, electrophysiological and immunohistochemical evaluations.
METHODS
Preparation of human mesenchymal stem cells
MSCs were cultured from the iliac crests of six normal patients between the ages of 10 and 15 years who had undergone bone marrow harvests for future allogeneic transplantation at the Ajou University Medical Center. Permission to use human MSCs was granted by the Institutional Review Board of Ajou University Medical Center, and all experiments were conducted with the understanding and written consent of each subject. Approximately 5 ml of bone marrow aspirates were centrifuged through a density gradient and plated in culture media [31]. LacZ-expressing hMSCs were cultured in a 100-mm tissue culture Petri dish (Falcon, Franklin Lakes, NJ, USA) in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated standard fetal bovine serum (FBS; HyClone, Logan, UT, USA), penicillin-streptomycin (Gibco), and 10 ng/ml bFGF (Sigma, St. Louis, MO, USA). The culture medium was changed every two days. After reaching confluency, the cells were harvested.
Spinal cord hemisection injury and transplantation
Male Sprague-Dawley rats (300~350 g) were anesthetized with pentobarbital (50 mg/kg, i.p.). Under microscopic observation, a laminectomy was conducted at vertebral levels T10-11. After a longitudinal opening of the dura mater, a spinal cord hemisection was made at T11 spinal cord level, followed by the removal of 3 mm hemicord segment along the midline using micro-scissors (hemisection only group). Polymer (BD™ PuraMatrix™ Peptide Hydrogel, San Jose, CA, USA; polymer only group), polymer with hMSCs (4×105; polymer with hMSC group) was implanted into the cavity. The muscle and fascia were sutured and the skin was closed. Cyclosporine A (1 mg/100 g, i.p.) was injected daily starting two days before transplantation. Prophylactic gentamycin sulfate (1 mg/kg, i.m.) was regularly administered for a week. All animal experiments were approved by the Institutional Animal Care and Use Committee of Yonsei University Health System. The number of animals used was kept to a minimum, and procedures were performed in a manner that minimized suffering.
Behavioral studies
Motor function was tested using the BBB open field testing procedure described by Basso et al. [32]. The BBB test on the injured leg side was performed 1 d, 4 d, and once a week from 1 to 8 weeks after the hemisection surgery. Sensory function was tested using mechanical thresholds and the up-and-down method [33]. The 50% paw withdrawal threshold (PWT) to von Frey filament stimuli applied to the paw was measured and used as an indicator of mechanical sensitivity of the affected paw.
Electrophysiological studies
Eight weeks after transplantation, the animals were reanesthetized with urethane (1.25 g/kg, i.p.). Each animal was placed on a stereotaxic device (Narishige Scientific Instrument Laboratory, Tokyo, Japan) and artificially ventilated using a small animal respirator (Model 683, Rodent Ventilator, Harvard, Holliston, MA, USA). Levels of CO2 in expired air were maintained within the physiological range using a capnometer (Model 2200, Traverse Medical Monitors, Saline, MI, USA). Somatosensory evoked potentials (SSEPs) were recorded to measure the conduction recovery of the sensory system. A special electrode (NE-120, Rhodes Medical Instruments, Inc., Woodland Hills, CA, USA) was used for SSEP recording. For the SSEP recording, the recording electrode was placed in the sensorimotor cortex (bregma: -2 mm, lateral: 2 mm; recording side). A bipolar platinum wire electrode placed in the contralateral sciatic nerve (stimulating side) was used as a stimulating electrode.
Motor evoked potentials (MEPs) were recorded to measure the conduction recovery of the motor system. Special electrodes that were identical to the SSEP recording electrodes wereused to stimulate the motor cortex (stimulating side). Laminectomy was performed at the L1 spinal cord for the placement of the recording electrodes. Following the laminectomy, the same special electrode was inserted into the contralateral gray matter of the L1 spinal cord (recording side) to record MEPs.
A single square pulse (0.1 ms duration) of electrical stimulus was delivered by a stimulus isolator (A365D, World Precision Instruments, Inc., New Haven, CT, USA) that was driven by a pulse generator (Pulsemaster A300, World Precision Instruments). The analog signals of the evoked potentials were amplified (×10,000), filtered (bandpass 300~1,000 Hz), and fed to an IBM-compatible PC through an AD/DA converter (CED 1401, Cambridge, UK) to be averaged using Spike 2 software. Each SSEP or MEP consisted of an average of 100~300 single sweep epochs. The effect of the stimulation intensity on SSEPs or MEPs was analyzed in the wave forms by latencies and amplitudes.
X-gal-positive cell counts
Eight weeks after transplantation, the rats were sacrificed and perfused with normal saline and 4% paraformaldehyde. The spinal cords were removed and post-fixed for 24 h followed by incubation in 30% sucrose overnight. The spinal cord was longitudinally sectioned by cryostat (10 µm thickness: Microm/HM500V, Walldorf, Germany) and the specimens were stored at -20℃. For the detection of β-galactosidase activity in the spinal cord tissue, fresh frozen sections stained with X-gal reagent (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside in dimethylformamide) were stored in a 37℃ thermostat container overnight to produce a blue color in β-galactosidase-expressing cells. Five slides from each of four animals were used for counting X-gal-positive cells. The number of X-gal-positive cells was counted in a 9-mm long section of tissue, within 3 mm around the injury region site.
Immunohistochemical studies
Immunohistochemistry was performed to evaluate the morphological features of transplanted cells in vivo. The sections were fixed in 4% paraformaldehyde and rinsed with PBS. Blocking solution (PBS containing 0.3% Triton X-100, 5.0% bovine serum albumin, 3.0% normal donkey serum) was used to treat the sections. The sections were incubated with primary antibodies at 4℃ overnight. The following primary antibodies were used: anti β-galactosidase (1:100, Biogenesis, Kingston, NH, USA), anti-GFAP (glial fibrillary acidic protein, astrocytes, 1:100, BD Biosciences, San Jose, CA, USA), anti-Tau (neurons, 1:50, Abcam, Cambridge, UK), or anti-APC (mature oligodendrocytes, 1:50, Chemicon, Temecula, CA, USA). Secondary antibodies, Cy3 (β-galactosidase, 1:500, Jackson, West Grove, PA, USA) and FITC (GFAP, Tau, APC, 1:250, Jackson, West Grove, PA, USA) were applied to the sections for three hours. The sections were mounted with fluorescent mounting medium (Vector, Burlingame, CA, USA) and observed by confocal microscope (LSM 510, Zeiss, Gottingen, Germany).
Statistical analyses
Data are expressed as mean±SEM. One-way ANOVA followed by Dunnett's post-hoc multiple comparison test was used to evaluate the behavioral, electrophysiological and immunohistochemistry results to compare the hemisection-only group with the polymer group or the polymer with hMSC-transplanted group.
RESULTS
Behavioral Improvement after Cell Transplantation
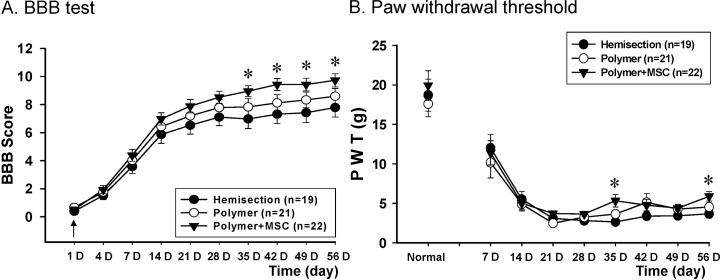
Hindlimb performance was tested in all rats using BBB open field scaling [32]. The hemisection-only group (n=19) scored 0 at 1 day after post injury and the score gradually increased to a final score of 7.8±0.7 on the injured leg side eight weeks after injury (Fig. 1A). The polymer-only and polymer with hMSC-transplanted groups showed improved hindlimb performance at five weeks after transplantation compared to the hemisection-only group. The mean BBB score of the polymer-only group (n=21) was 8.6±0.5. The polymer with hMSC-transplanted group (n=22) was 9.7±0.5 and showed a significantly improved hindlimb performance at four weeks after transplantation compared to the hemisection-only group. Even though the polymer with hMSC-transplanted group showed significantly improved hindlimb recovery over time, there were no differences when compared with the polymer-only group.
Fig. 1.
Behavioral effects of hMSC transplantation. The behavior in hemisected rats without transplantation (n=19), with polymer transplantation (n=21), and with polymer combined with hMSC transplantation (n=22) was tested before hemisection surgery and after 1 d, 4 d, and once a week from one to eight weeks after hemisection surgery. (A) The polymer and hMSC-transplanted group significantly improved hindlimb performance in injured (left) legs at five weeks after transplantation. (B) In injured (left) hindlimb, the PWT was significantly different at five and eight weeks after transplantation (↓ or ↑: hemisection or transplantation time, *p<0.05).
The time course of changes in PWT of hemisection-only, polymer transplantation and polymer with hMSC transplantation group is shown in Fig. 1B. After hemisection of the left spinal cord at T11, the PWT values to von Frey filament stimulation showed a remarkable decrease on the injury side of the hind paw as compared with the pre-hemisection value in all groups. The PWT of the polymer with hMSC-transplanted group was increased compared with the hemisection-only group starting at three weeks after transplantation. And on injured leg side, the PWT was significantly different at five and eight weeks after transplantation.
Recovery of neural conduction
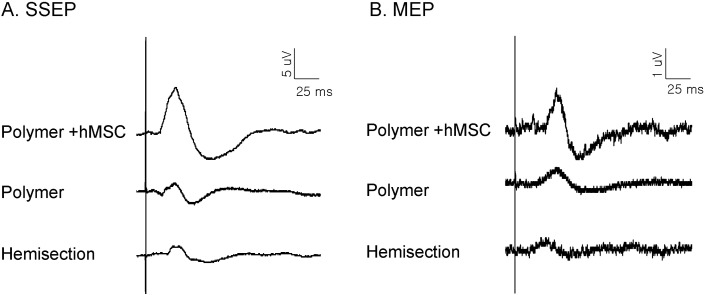
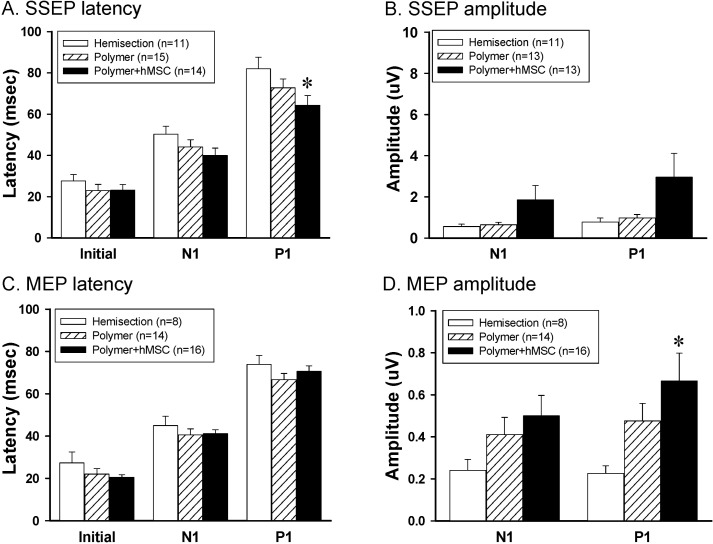
Electrophysiological measurements of SSEPs and MEPs activity were used to determine if axons conducting sensory and/or motor information crossed the damage site during the recovery period (Figs. 2 and 3). The SSEPs were recorded in the sensorimotor cortex following stimulation of the sciatic nerve. Fig. 2A shows representative wave forms of SSEPs by 6 mA stimulation in different experimental groups. When the sciatic nerve was stimulated, a negative-positive-negative deflection with a short latency was observed at the sensorimotor cortex. The latencies of the SSEPs were classified as initial, N1- and P1-peak latencies. The latency of the polymer-only group and polymer with hMSC group on the injured side was shorter than that of the hemisection-only group. Especially, the P1-peak latency of polymer with the hMSC-transplanted group was significantly shorter than that of the hemisection-only group (Fig. 3A). The amplitudes of polymer with the hMSC group tended to increase as compared with those of the hemisection-only group, but there was no significant difference (Fig. 3B).
Fig. 2.
Representative wave forms of somatosensory evoked potentials (A), and motor evoked potentials (B) at different intensity stimulations.
Fig. 3.
In vivo electrophysiological effects of hMSCs transplantation. (A, B) Latencies and amplitudes of SSEPs. The P1-peak latencies in polymer with hMSC group were shorter than that with the hemisection group on the injured side (A). (C, D) Latencies and amplitudes of MEPs. P1-peak amplitudes in polymer with hMSC were significantly higher than in hemisection group on the injured side (C). Asterisks indicate statistically significant differences compared to the hemisection-only group, based on Dunnett's post-hoc multiple comparison test (*p<0.05).
The MEPs were recorded using a bipolar disk electrode in the L1 spinal cord after stimulation of the hindlimb area of the sensorimotor cortex. Fig. 2B shows representative wave forms of the MEPs recorded in the hemisection, polymer and polymer with hMSC transplanted animals. The wave forms were very similar to SSEPs as negative-positive-negative deflection. After spinal hemisection injury, the animals showed lengthened MEP latencies and reduced amplitudes. In the MEPs, the latency of polymer with the hMSC-transplanted group tended to be short compared to that with the hemisection-only group, but there was no significant difference (Fig. 3C). In amplitudes, the P1-peak amplitudes of the polymer with hMSC transplanted group were significantly increased compared to those of the hemisection-only group (Dunnett's post-hoc multiple comparison test, p<0.05) (Fig. 3D). However, there was no difference between the polymer only- and polymer with hMSC-transplanted groups. Therefore, the improvement in SSEPs and MEPs may indicate that the damaged axons had recovered to conduct the action potentials following polymer with hMSC-transplantation.
Immunohistochemical evidence to support survival and differentiation patterns of transplanted cells
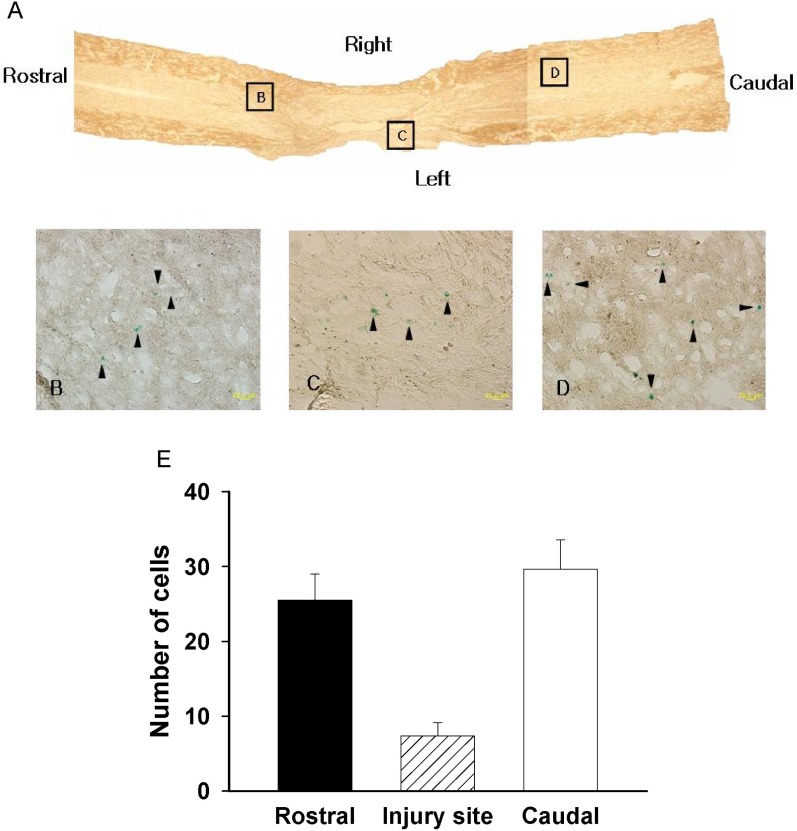
We determined the exogenous hMSC cell survival and amounts of settled cells by X-gal staining (Fig. 4). The X-gal-positive cells appeared in the injury site and in adjacent sites to the spinal cord (Fig. 4A-D). The number of positive cells is summarized in Fig. 4E. The number of X-gal-positive cells in the rostral site, injured site and caudal site were 25.5±3.48, 7.3±1.78 and 29.6±3.9, respectively, along the whole section (Fig. 4E). Therefore, these data indicate that the polymer with hMSC transplanted cells migrated to rostral and caudal sites, even though the cells were transplanted in the hemisected area.
Fig. 4.
Staining with X-gal. (A) X-gal-positive cells were observed around the injury site and in adjacent sites, indicating that transplanted cells had migrated. (B~D) High magnification of box B, C, D in A (arrowhead: X-gal positive cells). (E) The number of X-gal-positive cells along the whole section (rostral, injured, and caudal sites).
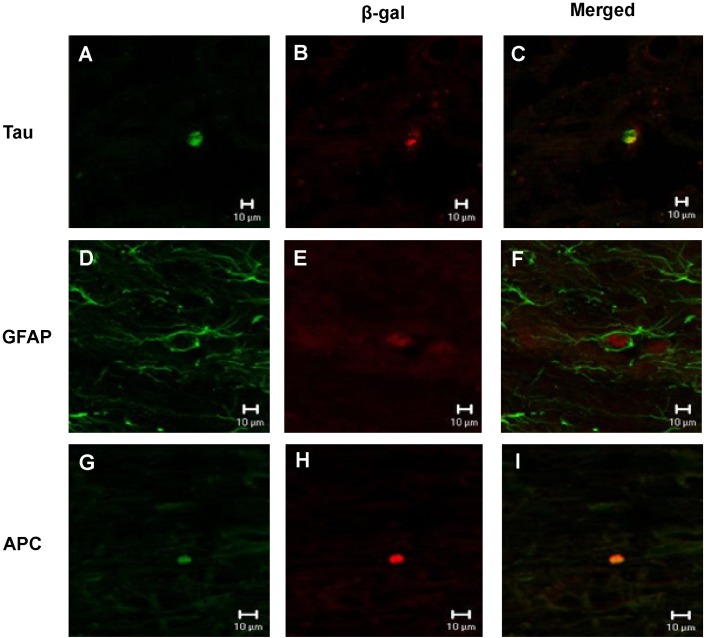
Immunoreactivity of β-galactosidase (β-gal) was observed by confocal microscopy in the injured site at eight weeks after transplantation, which indicates that transplanted hMSCs have successfully been grafted into the spinal cord. The double staining of β-gal and Tau, β-gal and GFAP, or β-gal and APC-positive cells was present in the transplanted site and in adjacent sites, indicating that some of the transplanted cells had settled and differentiated into neurons, astrocytes or oligodendrocytes (Fig. 5), although the number of differentiated cells was very low.
Fig. 5.
Double staining of β-gal and Tau, GFAP or APC analyzed by confocal microscopy. (A, D, G) β-gal-positive cells. (B, E, H) Tau, GFAP, APC-positive cells. (C, F, I) merged.
DISCUSSION
In this study, we observed that the transplantation of polymer with hMSCs into the spinal cord after a hemisection injury results in improved functional outcomes as determined by behavioral tests, electrophysiology and immunostaining analyses. Previous reports suggest that MSCs can promote functional recovery when grafted into contusion injury models [23,24,28,31], which is in agreement with the results of our study. The results of hindlimb performance assessments showed significantly improved functional outcomes at five weeks after injury in subjects that received transplantations of polymer with hMSC. Although there were no significant differences, the function of injured hindlimbs of the polymer-transplanted group tended to show improvement compared to the hemisection-only group. Therefore the polymer may have the capacity to provide an axonal guiding bridge and improve functional recovery in a hemisection model, even without transplantation of MSCs.
The hemisection model used in this study produced somewhat severe injury compared to the other models, by eliminating 3 mm thick of the spinal cord. So it might have low BBB scores than the other models given light hemisection injury among those studies reported previously. Similar BBB scores can be found in a study using similar hemisection model [34]. SCI induces the loss of sensory and motor function and the development of chronic pain in the majority of patients. SCI-induced pain is characterized as spontaneous burning pain, allodynia and hyperalgesia, all of which occur at or below the level of the lesion [35,36]. Hindpaw withdrawal thresholds to von Frey stimulation decrease after spinal contusion, indicating that mechanical allodynia develops below the level of the injury [37]. In agreement with the results of a study by Yoon et al. [37], our results also showed that spinal hemisection injury decreases the PWT to mechanical allodynia. The PWT values of polymer with hMSCs-transplanted rats increased at five weeks after transplantation and were significantly increased in comparison to PWT values in the hemisection-only group. This indicates that the transplantation of polymer with hMSC is more effective than the transplantation of polymer only for functional recovery, and this difference may be due to the ability of the polymer to form a bridge across the trauma cavity and provide a supportive environment for survival and differentiation of transplanted hMSC cells. While there have been several reports demonstrating behavioral improvement, few have shown evidence of conduction recovery. Normal rats show a reflexive withdrawal when they are stimulated with 20 g of von Frey as the result of this study. About 20 g of stimulation is needed to produce a reflex for von Frey filament. Therefore, the threshold of the stimulation is low owing to the pain but not a reflex reaction. Thus, we might guess lowered paw withdrawal threshold is for pain.
Our electrophysiological results demonstrate that subjects that received transplantations of polymer with hMSC had a shorter SSEP latency, which is consistent with our behavioral results and with our previous study in which hMSC transplantation reduced the pain and recovered the conduction in contusion SCI [31]. Functional recovery includes sensory as well as motor components. Behavioral tests in our study showed that somatosensory as well as locomotor performance was improved after polymer with hMSC transplantation. The SSEPs and MEPs may have improved because the damaged neurons and axons had recovered to conduct the action potentials after polymer with hMSC transplantation. In the injured spinal cord, there is neuron demyelination as well as neuronal cell death. Many studies have reported axonal regeneration and remyelination following transplantation of various cells. When directly injected into demyelinated rat spinal cord, marrow cells derived from the mononuclear layer can remyelinate these axons [38]. In animal models of SCI, grafts of MSC have been shown to promote remyelination [39] as well as partial recovery of function [23,24,40]. Consequently, reconnection and remyelination of axons in the injured spinal cord might be easier after the transplantation of the polymer with hMSC.
We used β-gal to observe survival and differentiation patterns of transplanted cells. The extent of cells that have settled and survived after the transplantation was determined by observing β-gal positive in longitudinal sections of the spinal cord including the hemisected injury site at eight weeks after transplantation. The hMSCs transplanted into the hemisection cavity had also migrated from the injured site to rostral and caudal sites. This result indicates that the transplantation of polymer with hMSCs results in successful implantation, with the polymer playing an important role in connection within the cavity site. Lu and colleagues [41] mentioned that MSCs also migrate for short distances from the lesion site into the host tissue.
In previous studies, MSCs have been shown to differentiate into cells with neural characteristics in vivo [22,23,42]. MSCs can differentiate into astrocytes when transplanted into rodent brain and neurons in vitro, under the appropriate cell culture conditions [43]. Our immunohistochemistry data also supports these results from previous studies. After double staining, we observed that the transplanted hMSCs were coated with β-gal and Tau, GFAP or APC in the transplanted and the adjacent sites, indicating that the transplanted cells had settled and differentiated into neurons, astrocytes and oligodendrocytes. Tau and APC seem equal to β-gal (red) in transplanted cells as it is stained in internal part of the cell cytoplasm due to the nature of staining. However β-gal shows round shape (red) as exists in internal part of cytoplasm infected with viruses into the transplanted cells while GFAP-positive staining (green) seems different as it stained external part of membrane surrounding cytoplasm. Therefore, it is shown by staining of external (GFAP-positive fibers) and internal (cytoplasm) parts on the basis of cell body. Therefore, our data demonstrate that the transplanted polymer with hMSCs results in differentiation of the hMSCs into a variety of cell-types and successful functional recovery and bridge generation for axonal recovery, resulting in improved behavioral performance and neural conduction.
Neurotrophins released from grafts or provided exogenously have a neuroprotective effect due to the decrease in retrograde degenerative changes in axotomized neurons [44-46]. Therefore, we hypothesize that the specific combinatorial treatment of polymer delivery with the secreted factors produced by MSCs may improve recovery in a hemisection model. There is growing evidence that MSCs produce a variety of neurotrophic factors as well as chemokines and cytokines in vitro and in vivo [47]. In the present study, we did not study the secretion produced by hMSC. However, recovery of damaged neurons and axons, and remyelination may be due to secretion from hMSCs as well as to the direct effect of polymer combined with hMSC. Thus, our results suggest that polymer with hMSCs may be useful as a combinatorial repair approach to treating SCI.
ACKNOWLEDGEMENTS
The research was supported by grants from the Stem Cell Research Center of the 21st Century Frontier Research Program (SC4140) and from Basic Science Research Program (No. 2005-0049404, 20090076605) through the National Research Foundation funded by the Ministry of Education, Science and Technology.
ABBREVIATIONS
- DMEM
Dulbecco's Modified Eagle's Medium
- GFAP
glial fibrillary acidic protein
- hMSC
human mesenchymal stem cell
- MEP
motor evoked potential
- MSC
mesenchymal stem cell
- PWT
paw withdrawal threshold
- SCI
spinal cord injury
- SSEP
somatosensory evoked potential
References
- 1.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 2.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 3.Liu WG, Wang ZY, Huang ZS. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011;33:686–693. doi: 10.1179/1743132810Y.0000000031. [DOI] [PubMed] [Google Scholar]
- 4.Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 5.Kang CE, Poon PC, Tator CH, Shoichet MS. A new paradigm for local and sustained release of therapeutic molecules to the injured spinal cord for neuroprotection and tissue repair. Tissue Eng Part A. 2009;15:595–604. doi: 10.1089/ten.tea.2007.0349. [DOI] [PubMed] [Google Scholar]
- 6.Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201:359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Houle JD, Ziegler MK. Bridging a complete transection lesion of adult rat spinal cord with growth factor-treated nitrocellulose implants. J Neural Transplant Plast. 1994;5:115–124. doi: 10.1155/NP.1994.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, Bunge MB. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22:1125–1136. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 9.Jendelová P, Herynek V, Urdzíková L, Glogarová K, Kroupová J, Andersson B, Bryja V, Burian M, Hájek M, Syková E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 10.Lesný P, De Croos J, Prádný M, Vacík J, Michálek J, Woerly S, Syková E. Polymer hydrogels usable for nervous tissue repair. J Chem Neuroanat. 2002;23:243–247. doi: 10.1016/s0891-0618(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 11.Woerly S, Doan VD, Evans-Martin F, Paramore CG, Peduzzi JD. Spinal cord reconstruction using NeuroGel implants and functional recovery after chronic injury. J Neurosci Res. 2001;66:1187–1197. doi: 10.1002/jnr.1255. [DOI] [PubMed] [Google Scholar]
- 12.Maquet V, Martin D, Scholtes F, Franzen R, Schoenen J, Moonen G, Jér me R. Poly(D,L-lactide) foams modified by poly (ethylene oxide)-block-poly(D,L-lactide) copolymers and a-FGF: in vitro and in vivo evaluation for spinal cord regeneration. Biomaterials. 2001;22:1137–1146. doi: 10.1016/s0142-9612(00)00357-4. [DOI] [PubMed] [Google Scholar]
- 13.Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 2004;21:789–804. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 15.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 18.Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. [PubMed] [Google Scholar]
- 21.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 24.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazdani SO, Pedram M, Hafizi M, Kabiri M, Soleimani M, Dehghan MM, Jahanzad I, Gheisari Y, Hashemi SM. A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat. Tissue Cell. 2012;44:205–213. doi: 10.1016/j.tice.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004;15:1105–1108. doi: 10.1097/00001756-200405190-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 28.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 29.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromalcells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 30.Zurita M, Vaquero J. Bone marrow stromal cells can achieve cure of chronic paraplegic rats: functional and morphological outcome one year after transplantation. Neurosci Lett. 2006;402:51–56. doi: 10.1016/j.neulet.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 31.Lee KH, Suh-Kim H, Choi JS, Jeun SS, Kim EJ, Kim SS, Yoon do H, Lee BH. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars) 2007;67:13–22. doi: 10.55782/ane-2007-1628. [DOI] [PubMed] [Google Scholar]
- 32.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 33.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Hu YR, Wan H, Xia L, Li JH, Yang F, Qu X, Wang SG, Wang ZC. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells and Schwann cells. Chin Med J (Engl) 2010;123:2424–2431. [PubMed] [Google Scholar]
- 35.Quertainmont R, Cantinieaux D, Botman O, Sid S, Schoenen J, Franzen R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012;7:e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddall PJ, Yezierski RP, Loeser JD. Pain following spinal cord injury: clinical features, prevalence, and taxonomy. IASP Newsletter. 2000;3:3–7. [Google Scholar]
- 37.Yoon YW, Dong H, Arends JJ, Jacquin MF. Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens Mot Res. 2004;21:25–31. doi: 10.1080/0899022042000201272. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, Suzuki Y, Ejiri Y, Noda T, Bai H, Kitada M, Kataoka K, Ohta M, Chou H, Ide C. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res. 2003;72:343–351. doi: 10.1002/jnr.10587. [DOI] [PubMed] [Google Scholar]
- 41.Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Zhang YQ, He LM, Xing B, Zeng X, Zeng CG, Zhang W, Quan DP, Zeng YS. Neurotrophin-3 gene-modified Schwann cells promote TrkC gene-modified mesenchymal stem cells to differentiate into neuron-like cells in poly(lactic-acid-co-glycolic acid) multiple-channel conduit. Cells Tissues Organs. 2012;195:313–322. doi: 10.1159/000327724. [DOI] [PubMed] [Google Scholar]
- 43.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 44.Himes BT, Goldberger ME, Tessler A. Grafts of fetal central nervous system tissue rescue axotomized Clarke's nucleus neurons in adult and neonatal operates. J Comp Neurol. 1994;339:117–131. doi: 10.1002/cne.903390111. [DOI] [PubMed] [Google Scholar]
- 45.Himes BT, Liu Y, Solowska JM, Snyder EY, Fischer I, Tessler A. Transplants of cells genetically modified to express neurotrophin-3 rescue axotomized Clarke's nucleus neurons after spinal cord hemisection in adult rats. J Neurosci Res. 2001;65:549–564. doi: 10.1002/jnr.1185. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Himes BT, Murray M, Tessler A, Fischer I. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002;178:150–164. doi: 10.1006/exnr.2002.7977. [DOI] [PubMed] [Google Scholar]
- 47.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]