Abstract
The purpose of this study is to investigated the mechanism of pharmacological action of local anesthetic and provide the basic information about the development of new effective local anesthetics. Fluorescent probe techniques were used to evaluate the effect of lidocaine·HCl on the physical properties (transbilayer asymmetric lateral and rotational mobility, annular lipid fluidity and protein distribution) of synaptosomal plasma membrane vesicles (SPMV) isolated from bovine cerebral cortex, and liposomes of total lipids (SPMVTL) and phospholipids (SPMVPL) extracted from the SPMV. An experimental procedure was used based on selective quenching of 1,3-di(1-pyrenyl)propane (Py-3-Py) and 1,6-diphenyl-1,3,5-hexatriene (DPH) by trinitrophenyl groups, and radiationless energy transfer from the tryptophans of membrane proteins to Py-3-Py. Lidocaine·HCl increased the bulk lateral and rotational mobility of neuronal and model membrane lipid bilayes, and had a greater fluidizing effect on the inner monolayer than the outer monolayer. Lidocaine·HCl increased annular lipid fluidity in SPMV lipid bilayers. It also caused membrane proteins to cluster. The most important finding of this study is that there is far greater increase in annular lipid fluidity than that in lateral and rotational mobilities by lidocaine·HCl. Lidocaine·HCl alters the stereo or dynamics of the proteins in the lipid bilayers by combining with lipids, especially with the annular lipids. In conclusion, the present data suggest that lidocaine, in addition to its direct interaction with proteins, concurrently interacts with membrane lipids, fluidizing the membrane, and thus inducing conformational changes of proteins known to be intimately associated with membrane lipid.
Keywords: Annular lipid fluidity, Lidocaine·HCl, Membrane protein clustering, Neuronal and model membranes, Transbilayer lateral and rotational mobility
INTRODUCTION
Two general theories have been proposed to explain the action of local anesthetics on sodium channel: one considers a direct binding of local anesthetic molecules to specific receptors on sodium channels [1-4] and the other proposes the general perturbation of the bulk membrane structure by anesthetics and its consequences on channel function [3-9]. There is a large amount of evidence in support of the specific receptor hypothesis [10]. General membrane perturbation may also contribute to an explanation of anesthetic actions [10]. However, the precise location of molecular mechanism of action of local anesthetics has continued to be a subject of controversy to the present day.
Effects of local anesthetics on motion, order and phase transitions of bulk bilayer systems of native or model membranes have received considerable attention in past decades. This is due to the interest in biological membranes as well as the unique information on intermolecular interactions that can be derived from the investigation of volume changes. It is known that the potency of an anesthetic increases roughly in proportion with its lipid/water partition coefficient, strongly suggesting an amphiphilic site for anesthetic molecules [3,4,8,11-13]. Yun et al. [8] reported that local anesthetics decreased microviscosity of synaptosomal plasma membrane vesicles isolated from the bovine cerebral cortex (SPMV). In addition, differential scanning thermograms of dimyristoylphosphatidylcholine multilamellar liposomes showed that local anesthetics significantly lowered the phase transition temperature, broadened the thermogram peaks, and reduced the size of the cooperative unit.
If local anesthetics cause expansion of neuronal and model membranes, this expansion is probably due to the increased fluidity in neuronal and model membrane lipid bilayers induced by local anesthetics. Our questions were what the role of local anesthetics (which is believed to have more interaction with protein than other lipids) was and to what degree the neuronal and model membrane lipid bilayers was expanded by local anesthetics.
More specifically, our questions were; first, how much of an increase do local anesthetics bring to rotational and lateral mobilities of the neuronal and model membrane lipid bilayers; second, whether such increasing effects were shown evenly on both lipid bilayers or differently between inner and outer monolayers; third, it the degree of increase is different between the inner and outer monolayers, then which monolayer has been mostly affected; fourth, whether annular lipid fluidity of the neuronal membrane lipid bilayers is increased or decreased by local anesthetics, and whether the degree of such increase or decrease is approximately the same as or much greater than the degree of changes in rotational and lateral mobilities; and fifth, to what degree the neuronal and model membrane is expanded by local anesthetics.
We are here to present the results of our study on how we solved the aforementioned questions by employing fluorescence technique, including the fluorescence quenching technique which Prof. Yun first developed specifically for the study to measure the rate and range of asymmetrical lateral mobilities between inner and outer monolayers of the lipid bilayers of neuronal and model membranes.
METHODS
Materials
The fluorescent probes, 1,3-di(1-pyrenyl)propane (Py-3-Py), 1-annilinonaphthalene-8-sulfonic acid (ANS) and 1,6-diphenyl-1,3,5-hexatriene (DPH) were purchased from Molecular Probes, Inc. (Junction City, OR, USA). Lidocaine·HCl, N-2-hydroxyethyl-piperazine-N'-2-ethanesulfonic acid (Hepes) and bovine serum albumin (BSA) were purchased from Sigma Chemical (St. Louis, MO, USA). 2,4,6-Trinitrobenzenesulfonic acid (TNBS) was obtained from Fluka (Switzerland). All other reagents were purchased commercially and were of the highest quality available. Water was deionized.
Preparation of synaptosomes
Membrane isolation
SPMV were isolated according to the procedure reported from earlier studies [3,4,12,14-18]. Their protein concentration was determined by the method of Lowry et al. [19] with BSA as a standard.
Liposomes preparation and TNBS labeling
Total lipids were extracted from the SPMV as previously described [12]. Cholesterol content of the extracted total lipids was determined according to the Liebermann-Buchard reaction [20]. Phospholipids were quantitated by measuring the amounts of inorganic phosphate [21] after hydrolysis of the phospholipids at 180℃ in 70% HClO4 [22]. The SPMV had a high lipid to protein ratio (0.942 mg total lipids/1 mg protein) and a low cholesterol to phospholipid molar ratio (0.593±0.011: cholesterol 0.208±0.010, phospholipids 0.702±0.025). An average molecular weight of 775 for phospholipids is assumed and the molecular weight of cholesterol is 387 for the calculation. Phospholipids were composed (mol%) of phosphatidylcholine (41.55±0.91), phosphatidylethanolamine (36.83±0.48), phosphatidylserine (13.60±0.26), sphingomyelin (4.15±0.16), phosphatidylinositol (2.90±0.09) and lysophosphatidylcholine (0.97±0.03).
Stock solutions of total lipids and phospholipids were made in chloroform. The concentration of the phospholipid stock solutions was 0.2 mg/ml. Giant unilamellar vesicles (GUVs: SPMVTL and SPMVPL) with a mean diameter of 45 µm were prepared by the method developed by Angelova and Dimitrov [23,24] and Angelova et al. [25]. To grow the GUVs, a special temperature-controlled chamber, which was previously described [26,27], was used. The experiments were carried out in the same chamber after the vesicle formation, using an inverted microscope (Axiovert35: Zeiss, Thornwood, NY). The following steps were used to prepare the GUVs: 1) ~3 µl of the lipid and phospholipid stock solution was spread on each Pt wire under a stream on N2. To remove the residues of organic solvent we put the chamber in a liophilizer for ~2 h. 2) To add the aqueous solvent inside the chamber (Millipore water 17.5 MΩ/cm), the bottom part of the chamber was sealed with a coverslip. The Millipore water was previously heated to the desired temperature (80℃), and then sufficient water was added to cover the Pt wires. Just after this step, the Pt wires were connected to a function generator (Hewlett-Packard, Santa Clara, CA), and a low-frequency AC field (sinusoidal wave function with a frequency of 10 Hz and an amplitude of 3 V) was applied for 90 min. After the vesicle formation, the AC field was turned off.
To determine the fluorescence parameters of probe molecules in each of the membrane monolayers, TNBS labeling reactions were performed as described [12,13,15-17,28,29] with a few modifications. The synaptosomal pellet was gently resuspended in 50 ml of 4 mM TNBS in buffer A for 80 min (in the case of asymmetric lateral mobility) or 50 ml of 2 mM TNBS in buffer A for 40 min (in the case of asymmetric rotational mobility) or in buffer A alone. Model membranes were gently resuspended in 50 ml of 0.5 mM TNBS in buffer A for 20 min buffer A alone. CO2 was bubbled through the solution. Buffer A composed of 30 mM NaCl, 120 mM NaHCO3, 11 mM glucose and 1% bovine serum albumin (BSA), and its pH was adjusted to 8.5 with NaOH. To assure complete exposure of all synaptosomal and model membrane outer monolayers to TNBS, the pellet was passed slowly through an Eberbach tissue grinder (3 up and down strokes). Unless otherwise specified, treatment was carried out at 4℃. The TNBS labeling reaction was terminated by adding an equal volume of 1% BSA in phosphate buffered saline (PBS; 8 g/l NaCl, 0.2 g/l KCl, 0.2 g/l KH2PO4, 1.15 g/l Na2HPO4·7H2O, 0.48 g/l Hepes, pH 7.4).
Fluorescence measurements
The fluorescence measurements were taken using a modified method of earlier studies [13,15-17,29].
All fluorescence measurements were obtained with a Multi Frequency Cross-Correlation Phase and Modulation Fluorometer (Model; ISS K2-003). Cuvette temperature was maintained at 37.0±0.1℃ in a circulating water bath (pH 7.4). Bandpass slits were 10 nm on excitation and 5 nm on emission. Blanks, prepared under identical conditions without fluorescent probes, served as controls.
Py-3-Py was incorporated by adding aliquots of a 5×10-5 M stock solution absolute ethanol to the SPMV, SPMVTL and SPMVPL, such that the final probe concentration was less than 5×10-7 M [13,15-17,29]. Mixtures were initially vigorously vortexed for 10 s at room temperature and then incubated at 4℃ for 18 h with gentle stirring [13,15-17,29].
DPH was dissolved in tetrahydrofuran, and 0.5 µl tetrahydrofuran per ml of PBS was added directly to the membrane suspension to a concentration of 0.01 µg/50 µg membrane protein or membrane lipid (fluorescent probe DPH 2: membrane protein or lipid 10,000) as described previously [13,15-17,28,29]. After probe incorporation the membrane suspension was placed in cuvettes, and control fluorescence was determined. Concentrated solutions of lidocaine·HCl were prepared in 10 mM Tris-HCl (pH 7.4) and added to the labeled membrane suspension (or untreated SPMV, SPMVTL and SPMVPL suspension) to give the desired concentration of lidocaine·HCl (in this case, for 30 min incubation).
Excitation wavelengths were 286 nm for tryptophan and 330 nm for Py-3-Py. Emission wavelengths were 335 nm for tryptophan, 379 nm for Py-3-Py monomer and 480 nm for Py-3-Py excimer. For Py-3-Py excimer emission, a GG-455 cut-off filter was used. The excimer to monomer fluorescence intensity ratio, I'/I, was calculated from the 480 nm to 379 nm signal ratio. The excitation wavelength for DPH was 362 nm and emission wavelength was 424 nm.
Effect of lidocaine·HCl on the structure of the individual monolayers of SPMV, SPMVTL and SPMVPL: selective quenching of Py-3-Py
The method used is based on the assumption that the system is composed of fluorescing compartments that are differentially accessed by TNBS. The excimer to monomer fluorescence intensity ratios, I'/I, of Py-3-Py in bulk (inner plus outer), and in the inner and outer monolayers were calculated from the following equations:
| (I'/I)t=I't / It | equation 1 |
| (I'/I)i=I'i / Ii | equation 2 |
| (I'/I)o=(I't-I'i) / (It-Ii) | equation 3 |
where (I'/I)t, (I'/I)i and (I'/I)o are the excimer to monomer fluorescence intensity ratios of Py-3-Py (I'/I) in bulk, and in the inner and outer monolayers, respectively. The values of I't (excimer fluorescence intensity for inner plus outer monolayers) and I'i (excimer fluorescence intensity for the inner monolayer) were determined for Py-3-Py from SPMV, SPMVTL and SPMVPL incubated with buffer A and buffer A plus TNBS, respectively, at 4℃ (pH 8.5) (non-penetrating conditions).
Determination of annular lipid fluidity in SPMV
The experimental determination of the annular lipid fluidity in SPMV is based on a method previously established for synaptic plasma membrane [30,31] and SPMV [15-17].
Incorporated Py-3-Py in the SPMV was excited by radiationless energy transfer (RET) from tryptophan (excitation at 286 nm) and the excimer to monomer fluorescence intensity ratio (I'/I) of Py-3-Py was calculated from the ratio 480 nm to 379 nm signal. Taking into account that the Förster radius (the RET-limiting distance) for the tryptophan-Py-3-Py donor-acceptor pair is 3 nm [15-17,32], only Py-3-Py located in annular lipids (close to proteins) was excited, and the fluidity of annular lipids was considered proportional to I'/I [13,15-17,26,27].
Determination of protein distribution in the SPMV llipid bilayers
This was based on a method previously established for membranes [15-17,30,31]. The fluorescence intensity of endogenous tryptophan in SPMV was determined. Thereafter the Py-3-Py probe was incorporated at a concentration of 5×10-7 M (in absolute ethanol), and after 10 min, tryptophan emission fluorescence intensity was measured again. The efficiency of RET from tryptophan to Py-3-Py was calculated from the equation:
| RET=(Id-Ida) / Id | equation 4 |
where Id and Ida represent the fluorescence intensities of donor (in this case, endogenous tryptophan) in the absence and presence, respectively, of acceptor (in this case, Py-3-Py). The wavelengths of excitation and emission of tryptophan were 286 and 335 nm, respectively.
Effect of lidocaine·HCl on the rotational mobility of bulk SPMV, SPMVTL and SPMVPL
The intensities of the components of fluorescence parallel (I∥) and perpendicular (I┴) to the direction of the vertically polarized excitation light were determined by measuring the light emitted through polarizers oriented vertically and horizontally. The polarization (P) was obtained from the intensity measurements using P=(I∥-GI┴) / (I∥+GI┴) where G is a grating correction factor for the transmission efficiency of the monochromator for vertically and horizontally polarized light. It is given by the ratio of the fluorescence intensities of the vertical to horizontal components when the exciting light is polarized in the horizontal direction. The polarization was expressed as the anisotropy [r=2P / (3-P)] (equation 5).
Effect of lidocaine·HCl on the separate monolayers of SPMV, SPMVTL and SPMVPL: selective quenching of DPH
The experimental determination of the separate monolayer structure in SPMV, SPMVTL and SPMVPL is based on a method by the method developed for tumor cell plasma membranes by Schroeder [33]. And a method previously established for LM fibroblast plasma membrane [34], for synaptic plasma membrane [35-38] and for synaptosomal plasma membrane vesicles [3,4,12,15-18] for the plasma membrane vesicles of Chinese hamster ovary cells, for Mar 18.5 hybridoma cells [29] and for the myeloma cell line Sp2/0-Ag14 [13]. It does not simply provide a theoretically calculated or average value: instead it is based on the assumption that the system is composed of fluorescing compartments of different accessibility to TNBS. If fluorescence intensity, F, and anisotropy, r, are measured simultaneously, then
| r=ΣFjrj | equation 6 |
where Fj is the fraction of fluorescence intensity in compartment j. For a binary system composed of the outer and inner monolayers of the SPMV, SPMVTL and SPMVPL, this leads to
| equation 7 |
where F and Fi are the DPH fluorescence obtained for SPMV incubated with buffer A and buffer A plus 2 mM TNBS for 40 min at 4℃ (pH 8.5) (non-penetrating conditions), (in the case of model membranes, incubated with buffer A and buffer A plus 0.5 mM TNBS for 20 min) respectively. The values of fluorophore concentration-independent parameter anisotropies, r (anisotropy for both monolayers) and ri (inner monolayer anisotropy), were also determined for DPH in SPMV, SPMVTL and SPMVPL incubated with buffer A and buffer A plus TNBS at 4℃, respectively. The equation was then solved for ro (outer monolayer anisotropy).
RESULTS
In order to determine the effect of the lidocaine·HCl on the bulk and asymmetric rotational and lateral mobilities of the SPMV, SPMVTL and SPMVPL monolayers, it is, first, necessary to demonstrate that this local anesthetic does not interact directly with Py-3-Py and DPH and thereby quench its fluorescence. No quenching of absorbance-corrected fluorescence intensity of both Py-3-Py and DPH by the lidocaine·HCl was observed at all tested concentrations.
In our judgment, it would be rational to use liposome that is made up of total lipids extracted from neuronal membranes and liposome that is made up of phospholipids extracted from neuronal and model membranes as samples in the study of the structure-activity relationship of local anesthetics.
The purity of SPMV
We assessed the purity of SPMV by enzymatic and morphological criteria. The specific activities of Na+, K+-ATPase, acetylcholinesterase and 5'-nucleotidase were enriched about 4-, 2.5- and 3-fold, respectively, in the plasma membrane fraction with respect to crude homogenates. The transmission electron microscopic examination of the SPMV indicated very high purity. The vesicles, which were separated according to size demonstrated homogeneous distribution and no longer showed the presence of intracellular organelles or leakage. The protein concentration was determined by the method of Lowry et al. [19] using BSA as a standard.
Effect of lidocaine·HCl on the rate and range of lateral mobility in SPMV, SPMVTL and SPMVPL bulk bilayer
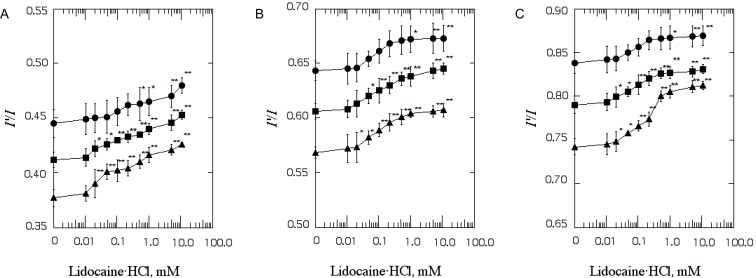
The I'/I value in intact SPMV, SPMVTL and SPMVPL (lidocaine·HCl-untreated) was 0.412±0.007, 0.606±0.007, 0.790±0.010, respectively (at 37℃, pH 7.4). Incubation with lidocaine·HCl increased the range and rate of lateral mobility of bulk (inner+outer monolayer) SPMV, SPMVTL and SPMVPL lipid bilayers at concentrations as low as 0.02 mM (n=5, p<0.05), respectively, and above as demonstrated in Fig. 1.
Fig. 1.
The effect of lidocaine·HCl on excimer to monomer fluorescence intensity ratio (I'/I) of Py-3-Py in SPMV (A), SPMVTL (B) and SPMVPL (C). The excitation wavelength of Py-3-Py was 330 nm and the I'/I values were calculated from the 480 nm to 379 nm signal ratio. SPMV was treated±4mM TNBS, pH 8.5, at 4℃ for 80 min. SPMVTL and SPMVPL were treated±0.5 mM TNBS, pH 8.5, at 4℃ for 20 min. Py-3-Py was incorporated into SPMV, SPMVTL and SPMVPL and fluorescence measurements were performed at 37℃ (pH 7.4). Untreated (inner and outer monolayers, ▪); TNBS treated (inner monolayer, ▴); calculated for outer monolayer (•) by eq. 3 as described in Materials and Methods. Each point represents the mean±SEM of 5 determinations. An asterisk and double asterisks signify p<0.05 and p<0.01, respectively, compared to control by Student's t-test.
The I'/I value of Py-3-Py in bulk SPMV, SPMVTL and SPMVPL incubated with 1 mM lidocaine·HCl was 0.441±0.004, 0.639±0.009 and 0.828±0.007 (n=5, p<0.01), respectively, and the change in I'/I value before and after adding the 1 mM lidocaine·HCl was 0.028, 0.032 and 0.037. The I'/I values of Py-3-Py in the SPMV, SPMVTL and SPMVPL bilayer were 0.356±0.006, 0.524±0.005 and 0.715±0.007 (n=5) at 25℃ (pH 7.4), respectively. Hence the effect of 1 mM lidocaine·HCl was equivalent to that produced by a temperature increase of approximate 6.0, 4.7 and 5.9℃, respectively.
Effect of lidocaine·HCl on the rate and range of transbilayer asymmetric lateral mobility of SPMV, SPMVTL and SPMVPL monolayers
The effect of increasing concentrations of lidocaine·HCl on the I'/I values in the individual SPMV, SPMVTL and SPMVPL monolayers is shown in Fig. 1. Lidocaine·HCl increased the rate and range of lateral mobility of the SPMV, SPMVTL, SPMVPL inner monolayer to a significant extent (0.390±0.013, 0.582±0.006 and 0.748±0.011, p<0.01, p<0.05 and p<0.05, n=5) at 0.02, 0.05 and 0.02 mM lidocaine·HCl, respectively (Fig. 1). It had a greater increasing effect on the lateral mobility of the inner monolayer (Fig. 1, filled triangles) than that of the outer monolayer (Fig. 1, filled circles). Since the changes in I'/I values is due primarily from the effect on the inner monolayer, we studied the selective effect of lidocaine·HCl on the rate and range of mobility of the probe. To the best of our knowledge, the results for asymmetric lateral mobility presented here are the first to demonstrate that the Sheetz-Singer hypothesis [39] is valid in neuronal membranes.
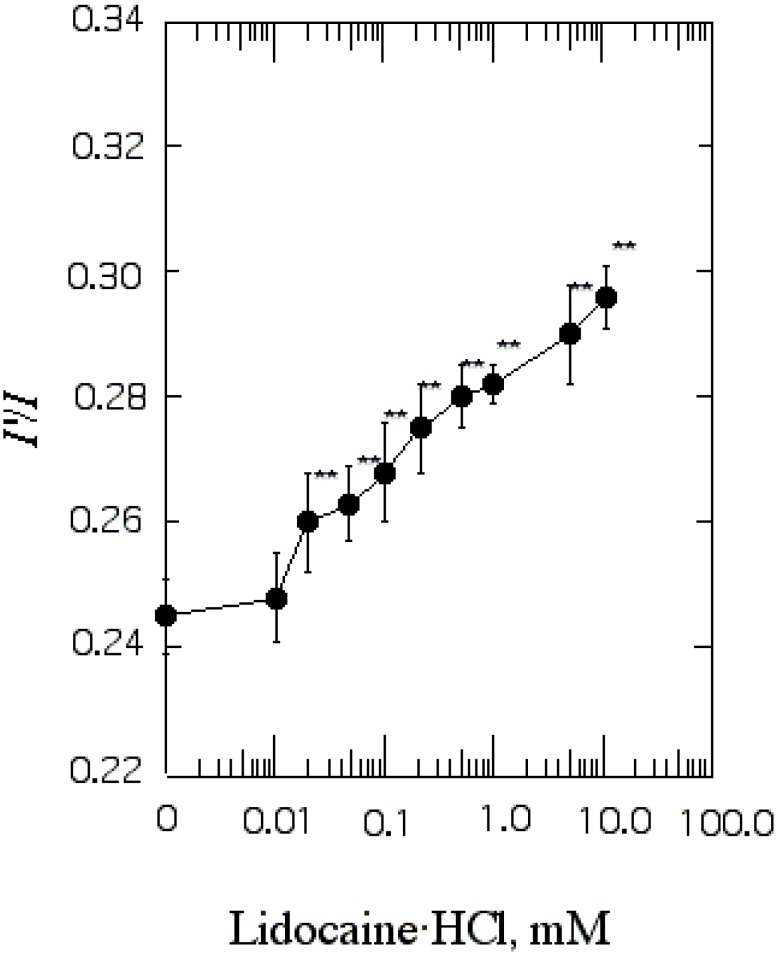
Effect of lidocaine·HCl on annular lipid fluidity in the SPMV lipid bilayers
I'/I measurements showed that the annular lipid fluidity of SPMV (intact membrane) was 0.245±0.006 (37℃, pH 7.4), and in this increased in response to concentration of 0.02 mM lidocaine·HCl and above (Fig. 2).
Fig. 2.
The effect of lidocaine·HCl on annular lipid fluidity in SPMV. Py-3-Py was excited through RET from tryptophan (excitation wavelength, 286 nm) and the excimer to monomer fluorescence intensity ratio (I'/I) was calculated from the 480 nm to 379 nm signal ratio. Fluorescence measurements were performed at 37℃ (pH 7.4). Each point represents the mean±SEM of 5 determinations. An asterisk and double asterisks signify p<0.05 and p<0.01, respectively, compared to control by Student's t-test.
The I'/I values of Py-3-Py in the bilayer are 0.245±0.006 (n=5) and 0.199±0.002 (n=5) at 37℃ and 25℃, respectively. Thus the effect by 1 mM lidocine·HCl was the same as that produced by a temperature increase of approximate 9.6℃. The important finding is that there was much greater increase in annular lipid fluidity in the lateral and rotational mobilities.
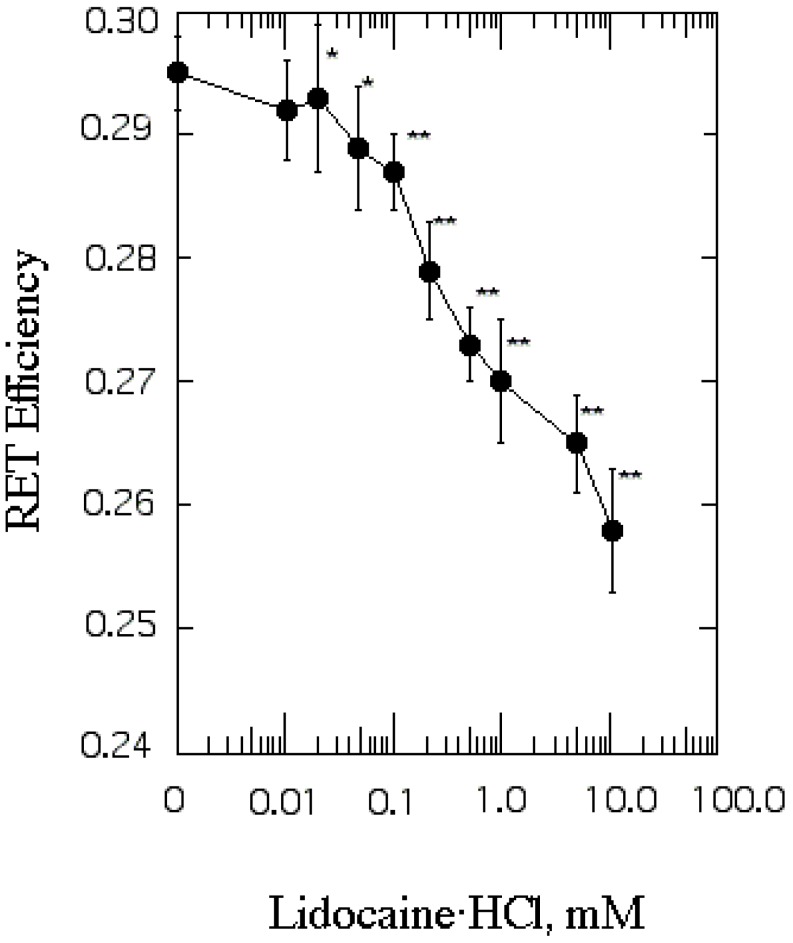
Effect of lidocaine·HCl on protein distribution in SPMV
We evaluated protein distribution by RET from tryptophan to Py-3-Py. The RET value of untreated SPMV was 0.295±0.003 (37℃, pH 7.4) and it was lowered by concentrations of lidocaine·HCl of 0.02 mM or more (Fig. 3). Protein clustering is probably caused by interaction among phospholipids, especially annular lipids, whose movement is increased by lidocaine·HCl and proteins around them.
Fig. 3.
The effect of lidocaine·HCl on protein distribution in SPMV. Efficiency of RET from tryptophan to Py-3-Py was taken as a measure of protein clustering and calculated by eq. 4. Fluorescence measurements were performed at 37℃ (pH 7.4). Each point represents the mean±SEM of 5 determinations. An asterisk and double asterisks signify p<0.05 and p<0.01, respectively, compared to control by Student's t-test.
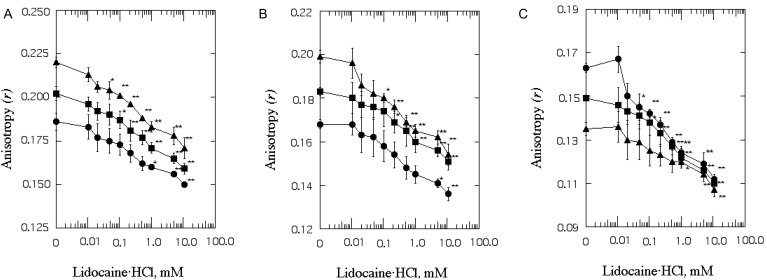
Effect of lidocaine·HCl on the range of rotational mobility of SPMV, SPMVTL and SPMVPL bulk bilayer
The anisotropy (r) of DPH in the intact SPMV, SPMVTL and SPMVPL was 0.202±0.004, 0.183±0.002 and 0.149±0.001 (at 37℃, pH 7.4), respectively (Fig. 4). Lidocaine·HCl inceased rotational mobility, with a significant decrease in anisotropy (r) values in SPMV, SPMVTL and SPMVTL even at 0.1, 0.2 and 0.1 mM, respectively (Fig. 4). The difference in anisotropy of the bulk SPMV, SPMVTL and SPMVPL lipid bilayers before and after adding 1 mM lidocaine·HCl was 0.031, 0.023 and 0.027, respectively. This can be evaluated by comparison with the effect of temperature on this parameter. The anisotropy of DPH in the SPMV, SPMVTL and SPMVPL bilayer was 0.257±0.002, 0.242±0.003 and 0.201±0.001 (n=5) at 25℃ (pH 7.4), respectively. The effect of 1 mM lidocaine·HCl was thus the same as that of a temperature increase of approximate 6.8, 4.7 and 6.2℃, respectively.
Fig. 4.
The effect of lidocaine·HCl on the anisotropy (r) of DPH in SPMV (A), SPMVTL (B) and SPMVPL (C). The excitation wavelength for DPH was 362 nm and fluorescence emission was read at 424 nm. SPMV was treated±2 mM TNBS, pH 8.5, at 4℃ for 40 min. SPMVTL and SPMVPL were treated±0.5 mM TNBS, pH 8.5, at 4℃ for 20 min. DPH was incorporated into SPMV, SPMVTL and SPMVPL and fluorescence measurements were performed at 37℃ (pH 7.4). Untreated (inner and outer monolayers, ▪); TNBS treated (inner monolayer, ▴); calculated for outer monolayer (•) by eq. 7 as described in Materials and Methods. Each point represents the mean±SEM of 5 determinations. An asterisk and double asterisks signify p<0.05 and p<0.01, respectively, compared to control by Student's t-test.
Effect of lidocaine·HCl on the range of transbilayer asymmetric rotational mobility of SPMV monolayers
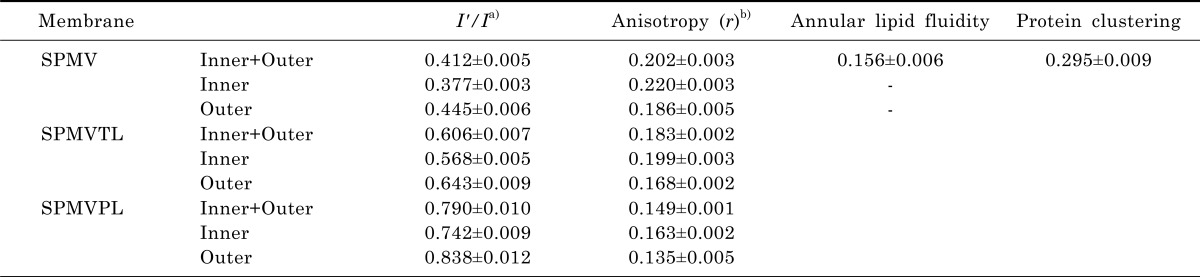
The structures of the intact SPMV, SPMVTL and SPMVPL (inner plus outer monolayers), and the outer (extracellular) and inner (intracellular) monolayers separately, were evaluated with DPH as a fluorescent reporter and trinitrophenyl groups as quenching agents. Trinitrophenylation of the intact synaptosome at 4℃ (non-penetrating conditions) results in covalent attachment of trinitrophenyl quenching agents to the outer monolayers. Approximately half of the DPH fluorescence was quenched in the treated SPMV outer monolayer. When TNBS labeling was conducted in penetrating conditions (37℃), greater than 80% of the fluorescence of the DPH was quenched. Values of fluorescence parameters in intact SPMV, SPMVTL and SPMVPL (both monolayers) and in TNBS-treated SPMV, SPMVTL and SPMVPL (inner monolayer) are listed in Table 1. The anisotropy of DPH in SPMV, SPMVTL and SPMVPL of the inner monolayer was 0.034, 0.031, 0.028 significantly greater than that calculated for the outer monolayer, as demonstrated in Table 1.
Table 1.
Structural parameters of intact SPMV, SPMVTL and SPMVPL
a)SPMV was treated±4 mM TNBS, pH 8.5, at 4℃ for 80 min (I'/I). SPMVTL and SPMVPL were treated±0.5 mM TNBS, pH 8.5, at 4℃ for 20 min (I'/I). Py-3-Py was incorporated and fluorescence measurements were performed at 37℃ (pH 7.4); b)SPMV was treated±2 mM TNBS, pH 8.5, at 4℃ for 40 min (anisotropy). SPMVTL and SPMVPL were treated±0.5 mM TNBS, pH 8.5, at 4℃ for 20 min (anisotropy). DPH was incorporated and fluorescence measurements were performed at 37℃ (pH 7.4).
Values from untreated membranes represent inner+outer monolayer; Values from TNBS treated membranes represent the inner monolayer; Values for the outer monolayer were calculated as described in Materials and Methods. Values are represented as the mean±SEM of 5 determinations.
Fig. 4 shows that the anisotropy (r) of DPH in the TNBS untreated membrane (inner plus outer monolayers) decreased gradually (fluidization) with increasing lidocaine·HCl concentrations (Fig. 4, filled squares). There was a similar, but more gradual, decrease in the calculated anisotropy of the inner monolayer at any lidocaine·HCl concentration (Fig. 4, filled triangles). However, there was no statistically significant decrease in the anisotropy (the rotational mobility range) of the outer monolayer of SPMV, SPMVPL at 0.01~0.5 mM (in case of SPMVTL 0.01~1 mM) of the lidocaine·HCl concentrations used. These results suggest that the fluidizing effect (range of rotational mobility) of lidocaine·HCl is selective.
DISCUSSION
We have reported through our earlier study developed by prof. Yun in our laboratory the facile method to measure transbilayer asymmetric lateral mobility of native membrane lipid bilayers [40]. In this study, we report here developed by prof. Yun in the lavoratory the facile method to measure transbilayer asymmetric lateral and rotational mobilities of model membrane lipid bilayers.
We used Py-3-Py, a pyrene derivative that has been used to quantify lateral mobility within native and model membranes [13,28,29,41,42], to determine the rate and range of lateral mobility in the SPMV, SPMVTL and SPMVPL. With this probe one monitors emission of both the monomer (I) and the excimer (I') components in such a way that a ratio can be derived and used as a measure of lateral mobility [13,28,29,41,42]. As the probe mobility increases emission from the excimer predominates, since formation of the intramolecular excimer is dependent upon lateral movement of its two components. Therefore, an increase in the I'/I ratio indicates increased lateral mobility of the probe within the membranes. The excimer fluorescence technique using Py-3-Py has the advantage over its counterpart based on intermolecular excimerization that very low probe concentrations can be used (<10-7 M) and perturbation of the SPMV, SPMVTL and SPMVPL by the probe molecule is minimized.
The covalently linked trinitrophenyl group has a broad absorbance range with a maximum near 420 nm. This peak has a large overlap with the fluorescence emission of Py-3-Py. This overlap is responsible in part for the high transfer (quenching) efficiency of the probe. Approximately half of the Py-3-Py fluorescence was quenched in the trinitrophenylated SPMV, SPMVTL and SPMVPL. When TNBS labeling was conducted under penetrating conditions (37℃), nearly 80% of the fluorescence of the Py-3-Py was quenched. Values of the excimer to monomer fluorescence intensity ratio (I'/I) of Py-3-Py in intact SPMV, SPMVTL and SPMVPL (both monolayers) and in TNBS-treated SPMV, SPMVTL and SPMVPL (inner monolayer) are listed in Table 1. The I'/I of Py-3-Py in the outer monolayer of SPMV, SPMVTL and SPMVPL were 0.068, 0.075 and 0.096 respectively, greater than that calculated for the inner monolayer. This means that the rate and range of lateral mobility of the outer monolayer is greater than that of the inner monolayer.
Although many researchers have reported that the inner and outer monolayers of native and model membranes differ in fluidity, all previous studies of asymmetric bilayer fluidity have examined the rotational range but not the rate and range of lateral mobility. In this study, using the selective quenching of Py-3-Py and DPH fluorescence by trinitrophenyl groups, we examined transbilayer asymmetric fluidity.
The TNBS labeling reaction must be carefully monitored in order to ensure that the reagent does not penetrate into the synaptosomes and label both sides of the plasma membrane. For this purpose, three control procedures are routinely used. First, as an "internal control", mitochondria and microsomes are isolated from the synaptosomes from which the trinitrophenylated plasma membranes are isolated. If any significant degree of penetration of TNBS into the synaptosome occurs, these intracellular organelles also become trinitrophenylated. Only 1.8±0.2% and 2.1±0.4% of microsomal and mitochondrial phosphatidylethanolamine were trinitrophenylated by our treatment [12]. In contrast, when the TNBS treatment is performed under penetrating conditions (37℃), 60~80% of the phosphatidylethanolamine in microsomes or mitochondria is trinitrophenylated [12]. Second, approximately half of the Py-3-Py fluorescence was quenched in the trinitrophenylated SPMV, SPMVTL and SPMVPL. Third, the trinitrophenylation of the synaptosomes may alter membrane enzyme activities. Unlike the results obtained under penetrating conditions (37℃), the activity of neither Na+, K+-ATPase nor 5'-nucleotidase was significantly altered by the TNBS reaction under non-penetrating conditions [12].
It is important to note that the term "membrane fluidity" is often misused. It arose from a combination of spectroscopic studies, the realization that membranes can be regarded as two-dimensional fluids, and the desire to obtain a simple single physical parameter that would describe their properties. The difficulty with the membrane fluidity concept is that any physical parameter chosen will be a function of the spectroscopic method employed, specifically its particular time window, and the properties of the probe (shape, charge, location etc) [43]. The membrane fluidity concept also depends on the assumption that the hydrophobic region of cell membranes is structurally and dynamically homogeneous, an assumption that is now under serious challenge. Thus while it may be true to say that the bulk or average spectroscopic properties of cell membranes may not be useful in building a hypothesis for the pharmacological action(s) of drug(s), local properties pertaining to domains or the immediate environment of a membrane protein may be very relevant.
As already pointed out, membrane bilayer mobility is one of the important factors controlling membrane microviscosity or fluidity. Membrane bilayer mobility includes lateral mobility, rotational mobility and flip-flop and it is well known that the most important of these is lateral mobility. We are pleased to have been able to develop and describe, for the first time, a fluorescence quenching technique that can measure membrane transbilayer lateral mobility. We therefore believe that this study will make a contribution to the study of drug-membrane interactions.
The clear mechanism of action of the drug on the increasing effects of annular lipid fluidity of the SPMV is unknown. However, the mechanism through which lidocaine·HCl increases the annular lipid fluidity in the SPMV lipid bilayers can be assumed as follows.
Annular lipids are known to surround proteins with or without being physically associated with them. Lidocaine·HCl may alter the stereo structure or dynamics of these proteins by combining with the lipids, especially with the annular lipids, increasing their mobility and indirectly affecting the dynamic behavior of the proteins. Because biological membranes are of highly complex composition, it has not been feasible to monitor changes in the local lipid environment and at the same time to determine their effect on membrane protein function. Nevertheless is likely that the observed effects are not only due to the influence of lidocaine·HCl on lipids, but are magnified by the interactions between lipids and proteins.
Lidocaine·HCl thus affects the lateral and rotational mobility of SPMV, SPMVTL and SPMVPL mainly via an effect on the inner monolayer of the SPMV, SPMVTL and SPMVPL. This is the first demonstration that lidocaine·HCl has a differential effect on the transbilayer lateral and rotational mobility of the inner and outer monolayers of neuronal and model membranes. It seems that native and model membranes, specifically inner monolayer, are much more sensitive to the fluidizing effects of lidocaine·HCl, and this finding can be extended to the transbilayer asymmetric fluidity of neuronal and model membranes.
It was confirmed that while the local anesthetic lidocaine·HCl used in this study did not generate significant increasing effect of lateral mobility on the outer monolayer of the SPMV, SPMVPL as well as the bulk bilayer of the SPMV, SPMPL at a concentration of 0.02 mM, it did generate significant increasing effect of the mobility on the inner monolayer at the concentration of 0.02 mM. It was confirmed that while the local anesthetic lidocaine·HCl used in this study did not generate significant increasing effect of lateral mobility on the outer monolayer of the SPMVTL as well as the bulk bilayer of the SPMVTL at a concentration of 0.05 mM, it did generate significant increasing effect of the mobility on the inner monolayer at the concentration of 0.05 mM.
It was confirmed that while the local anesthetic lidocaine·HCl used in this study did not generate significant increasing effect of rotational mobility on the outer monolayer of the SPMV, SPMVPL as well as the bulk bilayer of the SPMV, SPMPL at a concentration of 0.05 mM, it did generate significant increasing effect of the mobility on the inner monolayer at the concentration of 0.05 mM. It was confirmed that while the local anesthetic lidocaine·HCl used in this study did not generate significant increasing effect of rotational mobility on the outer monolayer of the SPMVTL as well as the bulk bilayer of the SPMVTL at a concentration of 0.1 mM, it did generate significant increasing effect of the mobility on the inner monolayer at the concentration of 0.1 mM. Thus lidocaine·HCl has a selective fluidizing effect within the transbilayer domains of the SPMV, SPMVTL and SPMVPL.
From the results of our study, it is without a doubt that lidocaine·HCl increased lateral and rotational mobility of the neuronal and model membrane lipid bilayers. Lidocaine·HCl which increase rotational and lateral mobility of the neuronal and model lipid bilayers mostly increased the mobility of inner monolayer, thereby expanding membranes. These effects are not solely due to the influence of lidocaine·HCl on lipids, but they are magnified by the interaction between lipids and proteins. This conclusion can be drawn because we confirmed that the magnititude of increasing effect on annular lipid fluidity in SPMV lipid bilayers induced by lidocaine·HCl was significantly far greater than the magnitude of increasing effect of the drug on the lateral and rotational of bulk SPMV lipid bilayers.
Opinions have been divided as to whether local anesthetics interfered with membrane protein function by directly binding to the proteins, or whether the main modes of action occurred indirectly through a change in the physicochemical properties of the lipid membranes into which the local anesthetics readily diffused. Because biological membranes are of highly complex composition, it has not been feasible to monitor changes in the local lipid environment and to determine its effect on the membrane protein function at the same time. It is possible to explain the multiplication effects citing the increased mobility of protein triggered by lipids, but the reverse case of protein triggering change in lipids is more likely. It is certain that local anesthetics increase the mobility of the neuronal lipid bilayers but the direct effects of local anesthetics on protein appear to have magnified such effects on the lipid. That is to say, before or during or even after the interaction of the local anesthetics with sodium channels, the fluidization of membrane lipids may provide an ideal microenvironment for optimum local anesthetic effects. In conclusion, the present data suggest that local anesthetics, in addition to its direct interaction with sodium channels, concurrently interact with membrane lipids, affecting fluidity of the neuronal membrane lipid bilayers. The most important finding of this study is that there is far greater increase in annular lipid fluidity by lidocaine·HCl than that than in lateral and rotatonal mobilities by the local anesthetics. Lidocaine·HCl alters the stereo or dynamics of the proteins in the lipid bilayers by combining with lipids, especially with the annular lipids. In conclusion, the present data suggest that lidocaine, in addition to its direct interaction with proteins, concurrently interacts with membrane lipids, fluidizing the membrane, and thus inducing conformational changes of proteins known to be intimately associated with membrane lipid.
The side effects of the lidocaine·HCl are summarized as follow. Although local anesthetics including lidocaine·HCl are remarkably safe in therapeutic usage, the importance of their systemic toxicity cannot be ignored [44]. Although blood levels of lidocaine of 4 mg/ml are considered safe, serial central nerves system reactions occur at blood levels higher than 6 mg/ml. The unusual patient may show toxicity with blood levels as low as 3 mg/ml [44]. Local anesthetics convulsions are transient and are not in themselves lethal. Systemic reactons usually occur rapidly but may be delayed for as much as 30 minutes after the drug is injected [45]. Allergic reactions to local anesthetics are uncommon. However, mild dermatologic reaction (urticarial or rashes), delayed reactions (serum sickness), or immediate reactions (anaphylaxis) may occur after the use of any local anesthetic. A carefully obtained medical history should disclose known sensitizations, but it is no assurance that an allergy dose not exist. A true allergy to the drug means that the patient is more likely to be allergic to drugs with a similar chemical structure. The esters have a greater allergic potential than do the amides. In fact, wheather a true allergic reaction (antigen-antibody) can occur with the amide is still being debated [44]. As a rule, use of the amides is safe in patients who are allergic to the ester type of drugs and vise versa. Cross-allergenicity dose not appear to exist between the amides lidocaine and mepivacaine. Consequently, they may usually be substituted for each other. However, some individuals are allergic to several anesthetics, and no subsititute is an absolute quarantee that an allergic reaction [45].
We have tried to solve the correlation between the results of this study and the side effect of lidocaine·HCl. However we have found that there is no significant correlation between them.
ABBREVIATIONS
- BSA
bovine serum albumin
- DPH
1,6-Diphenyl-1,3,5-hexatriene
- PBS
phosphate-buffered saline
- Py-3-Py
1,3-Di (1-pyrenyl) propane
- RET
radiationless energy transfer
- SPMV
synaptosomal plasma membrane vesicles isolated from bovine cerebral cortex
- SPMVTL
liposome of total lipids extracted from SPMV
- SPMVPL
liposome of phospholipids extracted from SPMV
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
References
- 1.Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973;62:37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterworth JF, 4th, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Lee YH, Park NS, Kwon JD, Park JS, Shin GB, Lee CS, Jung TS, Choi NJ, Yoon JH, Ok JS, Yoon UC, Bae MK, Jang HO, Yun I. Amphiphilic effects of dibucaine HCl on rotational mobility of n-(9-anthroyloxy)stearic acid in neuronal and model membranes. Chem Phys Lipids. 2007;146:33–42. doi: 10.1016/j.chemphyslip.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Kim DI, Mun H, Lee SK, Park JS, Kim JH, Lee JH, Park YH, Jeon YC, Yoon UC, Bae MK, Jang HO, Wood WG, Yun I. The effect of propoxycaine.HCl on the physical properties of neuronal membranes. Chem Phys Lipids. 2008;154:19–25. doi: 10.1016/j.chemphyslip.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972;24:583–655. [PubMed] [Google Scholar]
- 6.Lee AG. Model for action of local anaesthetics. Nature. 1976;262:545–548. doi: 10.1038/262545a0. [DOI] [PubMed] [Google Scholar]
- 7.Singer MA. Interaction of dibucaine and propranolol with phospholipid bilayer membranes-effect of alterations in fatty acyl composition. Biochem Pharmacol. 1977;26:51–57. doi: 10.1016/0006-2952(77)90129-0. [DOI] [PubMed] [Google Scholar]
- 8.Yun I, Han SK, Baek SW, Kim NH, Kang JS, Chung IK, Lee EJ. Effects of local anesthetics on the fluidity of synaptosomal plasma membrane vesicles isolated from bovine brain. Korean J Pharmacol. 1987;24:43–52. [Google Scholar]
- 9.Smith IC, Auger M, Jarrell HC. Molecular details of anesthetic-lipid interaction. Ann N Y Acad Sci. 1991;625:668–684. doi: 10.1111/j.1749-6632.1991.tb33901.x. [DOI] [PubMed] [Google Scholar]
- 10.Strichartz GR, Richie JM. The action of local anesthetics on ion channels of excitable tissues. In: Strichartz GR, editor. Local anesthetics. New York: Springer-Verlag; 1987. pp. 21–52. [Google Scholar]
- 11.Miller KW, Braswell LM, Firestone LL, Dodson BA, Forman SA. In: Molecular and Cellular Mechanisms of Anesthetics. Roth H, editor. New York: Plenum; 1986. pp. 157–171. [Google Scholar]
- 12.Yun I, Kang JS. The general lipid composition and aminophospholipid asymmetry of synaptosomal plasma membrane vesicles isolated from bovine cerebral cortex. Mol Cells. 1990;1:15–20. [Google Scholar]
- 13.Kang JS, Choi CM, Yun I. Effects of ethanol on lateral and rotational mobility of plasma membrane vesicles isolated from cultured mouse myeloma cell line Sp2/0-Ag14. Biochim Biophys Acta. 1996;1281:157–163. doi: 10.1016/0005-2736(95)00301-0. [DOI] [PubMed] [Google Scholar]
- 14.Yun I, Kim YS, Yu SH, Chung IK, Kim IS, Baik SW, Cho GJ, Chung YZ, Kim SH, Kang JS. Comparision of several procedures for the preparation of synaptosomal plasma membrane vesicles. Arch Pharm Res. 1990;13:325–329. [Google Scholar]
- 15.Jang HO, Shin HG, Yun I. Effects of dimyristoylphosphatidylethanol on the structural parameters of neuronal membranes. Mol Cells. 2004;17:485–491. [PubMed] [Google Scholar]
- 16.Jang HO, Jeong DK, Ahn SH, Yoon CD, Jeong SC, Jin SD, Yun I. Effects of chlorpromazine HCl on the structural parameters of bovine brain membranes. J Biochem Mol Biol. 2004;37:603–611. doi: 10.5483/bmbrep.2004.37.5.603. [DOI] [PubMed] [Google Scholar]
- 17.Bae MK, Jeong DK, Park NS, Lee CH, Cho BH, Jang HO, Yun I. The effect of ethanol on the physical properties of neuronal membranes. Mol Cells. 2005;19:356–364. [PubMed] [Google Scholar]
- 18.Yun I, Cho ES, Jang HO, Kim UK, Choi CH, Chung IK, Kim IS, Wood WG. Amphiphilic effects of local anesthetics on rotational mobility in neuronal and model membranes. Biochim Biophys Acta. 2002;1564:123–132. doi: 10.1016/s0005-2736(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Huang TC, Chen CP, Wefler V, Raftery A. A stable reagent for the Liebermann-Burchard reaction. Anal Chem. 1961;33:1405–1407. [Google Scholar]
- 21.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 22.Madeira VMC, Antunes-Madeira MC. Lipid composition of biomembranes: a complete analysis of sarcoplasmic reticulum phospholipids. Cienc Biol (Coimbra) 1976;2:265–291. [Google Scholar]
- 23.Angelova ML, Dimitrov DS. Liposome electroformation. Faraday Discuss Chem Soc. 1986;81:303–311. [Google Scholar]
- 24.Dimitrov DS, Angelova MI. Lipid swelling and liposome formation on solid surfaces in external electric fields. Progr Colloid Polym Sci. 1987;73:48–56. [Google Scholar]
- 25.Angelova ML, Soléau S, Meléard PH, Faucon JF, Bothorel P. Preparation of giant vesicles by external AC fields, kinetics and application. Prog Colloid Polym Sci. 1992;89:127–131. [Google Scholar]
- 26.Bagatolli LA, Gratton E. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys J. 1999;77:2090–2101. doi: 10.1016/S0006-3495(99)77050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagatolli LA, Gratton E. Two photon fluorescence microscopy of coexisting lipid domainsin giant unilamellar vesicles of binary phospholipid mixtures. Biophys J. 2000;78:290–305. doi: 10.1016/S0006-3495(00)76592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun I, Yang MS, Kim IS, Kang JS. Bulk vs. transbilayer effects of ethanol on the fluidity of the plasma membrane vesicles of cultured Chinese hamster ovary cells. Asia Pacific J Pharmacol. 1993;8:9–16. [Google Scholar]
- 29.Yun I, Lee SH, Kang JS. The effect of ethanol on lateral and rotational mobility of plasma membrane vesicles isolated from cultured Mar 18.5 hybridoma cells. J Membr Biol. 1994;138:221–227. doi: 10.1007/BF00232794. [DOI] [PubMed] [Google Scholar]
- 30.Avdulov NA, Wood WG, Harris RA. Effects of ethanol on structural parameters of rat brain membranes: relationship to genetic differences in ethanol sensitivity. Alcohol Clin Exp Res. 1994;18:53–59. doi: 10.1111/j.1530-0277.1994.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 31.Avdulov NA, Chochina SV, Draski LJ, Deitrich RA, Wood WG. Chronic ethanol consumption alters effects of ethanol in vitro on brain membrane structure of high alcohol sensitivity and low alcohol sensitivity rats. Alcohol Clin Exp Res. 1995;19:886–891. doi: 10.1111/j.1530-0277.1995.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 32.Dobretsov GE, Spirin MM, Chekrygin OV, Karmansky IM, Dmitriev VM, Vladimirov YuA. A fluorescence study of apolipoprotein localization in relation to lipids in serum low density lipoproteins. Biochim Biophys Acta. 1982;710:172–180. doi: 10.1016/0005-2760(82)90147-3. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder F. Differences in fluidity between bilayer halves of tumour cell plasma membranes. Nature. 1978;276:528–530. doi: 10.1038/276528a0. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder F, Kier AB, Sweet WD. Role of polyunsaturated fatty acids and lipid peroxidation in LM fibroblast plasma membrane transbilayer structure. Arch Biochem Biophys. 1990;276:55–64. doi: 10.1016/0003-9861(90)90009-n. [DOI] [PubMed] [Google Scholar]
- 35.Wood WG, Gorka C, Schroeder F. Acute and chronic effects of ethanol on transbilayer membrane domains. J Neurochem. 1989;52:1925–1930. doi: 10.1111/j.1471-4159.1989.tb07278.x. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder F, Morrison WJ, Gorka C, Wood WG. Transbilayer effects of ethanolon fluidity of brain membrane leaflets. Biochim Biophys Acta. 1988;946:85–94. doi: 10.1016/0005-2736(88)90460-9. [DOI] [PubMed] [Google Scholar]
- 37.Wood WG, Schroeder F. Membrane effects of ethanol: bulk lipid versus lipid domains. Life Sci. 1988;43:467–475. doi: 10.1016/0024-3205(88)90147-6. [DOI] [PubMed] [Google Scholar]
- 38.Sweet WD, Wood WG, Schroeder F. Charged anesthetics selectively alter plasma membrane order. Biochemistry. 1987;26:2828–2835. doi: 10.1021/bi00384a026. [DOI] [PubMed] [Google Scholar]
- 39.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joo HJ, Ahn SH, Lee HR, Jung SW, Choi CW, Kim MS, Bae MK, Chung IK, Bae SK, Jang HO, Yun I. The effect of methanol on the structural parameters of neuronal membrane lipid bilayers. Korean J Physiol Pharmacol. 2012;16:255–264. doi: 10.4196/kjpp.2012.16.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schachter D. Fluidity and function of hepatocyte plasma membranes. Hepatology. 1984;4:140–151. doi: 10.1002/hep.1840040124. [DOI] [PubMed] [Google Scholar]
- 42.Zachariasse KA, Vaz WL, Sotomayor C, Kühnle W. Investigation of human erythrocyte ghost membranes with intramolecular excimer probes. Biochim Biophys Acta. 1982;688:323–332. doi: 10.1016/0005-2736(82)90343-1. [DOI] [PubMed] [Google Scholar]
- 43.Stubbs CD, Williams BW. Fluorescence in membranes. In: Lakowicz JR, editor. Fluorescence Spectroscopy in Biochemistry. Vol III. New York: Plenum; 1992. pp. 231–263. [Google Scholar]
- 44.Swinyard EA. Local anesthetics. In: Gennaro AR, editor. Remington's pharmaceutical Sciences. 17th ed. Faston, Pennsylvania: Mack Publishing Company; 1985. pp. 1048–1058. [Google Scholar]
- 45.Requa-Clark B, Halroyd SV. Local anesthetic. In: Halroyd SV, Wynn RL, Requa-Clark B, editors. Clinical pharmacology in ental practice. 4th ed. Washington, DC, Tokyo: C.V. Mosby Company. St Louis; 1988. [Google Scholar]