Abstract
TLR6 forms a heterodimer with TLR2 and TLR4. While proinflammatory roles of TLR2 and TLR4 are well documented, the role of TLR6 in inflammation is poorly understood. In order to understand mechanisms of action of TLR6 in inflammatory responses, we investigated the effects of FSL-1, the TLR6 ligand, on expression of chemokine CCL2 and cytokine IL-1β and determined cellular factors involved in FSL-1-mediated expression of CCL2 and IL-1β in mononuclear cells. Exposure of human monocytic leukemia THP-1 cells to FSL-1 resulted not only in enhanced secretion of CCL2 and IL-1β, but also profound induction of their gene transcripts. Expression of CCL2 was abrogated by treatment with OxPAPC, a TLR-2/4 inhibitor, while treatment with OxPAPC resulted in partially inhibited expression of IL-1β. Treatment with FSL-1 resulted in enhanced phosphorylation of Akt and mitogen-activated protein kinases and activation of protein kinase C. Treatment with pharmacological inhibitors, including SB202190, SP6001250, U0126, Akt inhibitor IV, LY294002, GF109203X, and RO318220 resulted in significantly attenuated FSL-1-mediated upregulation of CCL2 and IL-1β. Our results indicate that activation of TLR6 will trigger inflammatory responses by upregulating expression of CCL2 and IL-1β via TLR-2/4, protein kinase C, PI3K-Akt, and mitogen-activated protein kinases.
Keywords: Chemokine, FSL-1, Monocytes/macrophages, TLR6
INTRODUCTION
In addition to oxidized forms of cholesterol, multiple infectious agents including Chlamydia pneumoniae, Mycoplasma pneumoniae, Porphymonas gingivalis, Staphylococcus species, and Streptococcus species are found in the atherosclerotic plaque [1,2]. The pathogens comprise pathogen-associated molecular patterns (PAMPs) that are recognized by the toll-like receptors (TLRs). TLRs are type 1 transmembrane proteins with an ectodomain consisting of leucine rich repeats required for recognition of PAMPs, a transmembrane domain that determines cellular localization, and an intracellular toll interleukin 1 receptor (TIR) domain needed for downstream signaling [3]. Expression and function of TLRs are associated with atherosclerosis, which is caused by inflammation. For instance, mice deficient in TLR4 and TLR2 showed a 55% decrease in atherosclerotic lesions, while a 65% decrease in macrophage infiltration was observed in ApoE-/- mice deficient in TLR4 [4,5]. TLR6 forms a heterodimer with TLR2 and TLR4 [6,7]. Coexpression of TLR2 and TLR6 at the cell surface is crucial for recognition of diacylated lipopeptide from mycoplasma and peptidoglycan and subsequent cellular activation [8]. TLR6 activation induces production of tumor necrosis factor (TNF)-α in macrophages [9]. However, signaling molecules associated with TLR6 are clarified.
Chemokines and cytokines play important roles in atherosclerosis by promoting recruitment and migration of inflammatory cells into the atherosclerotic lesion and by inducing activation of endothelial cells and leukocyte subsets. The activated cells, in turn, release more cytokines and chemokines, leading to enhanced inflammatory responses in atherosclerotic lesions [10-12]. Chemokine (C-C motif) ligand 2 (CCL2), monocyte chemotactic protein-1 (MCP-1), is critical for recruitment of monocytes to atherosclerotic plaques and its expression has been linked to atherosclerosis [13]. Deletion of the CCL2 gene or the gene for its receptor resulted in reduced development of atherosclerotic plaques [14]. The decrease in lesion size in the absence of TLRs was associated with a decrease in peripheral levels of CCL2 [4]. In addition to CCL2, expression of interleukin-1 (IL-1), a prototypic proinflammatory cytokine that stimulates both local and systemic inflammatory responses to multiple infectious agents, is also elevated in macrophages of human atherosclerotic plaques [15]. Genetic deficiency and inhibition of IL-1β and its receptor IL-1R1 resulted in a decrease in atherosclerotic plaque formation in animal models of atherosclerosis [16-18]. IL-1 protein is considered to induce production of cytokines and chemokines and increases expression of adhesion molecules on endothelial cells, thus enhancing the recruitment of inflammatory cells [16-18]. Therefore, due to their close association with atherosclerosis, understanding the regulation of CCL2 and IL-1 expression is important.
Although TLR6 is believed to participate in inflammatory responses through forming heterodimers with TLR2 and TLR4 [6,7], the proinflammatory property of TLR6 itself is poorly characterized. To test the hypothesis that TLR6 is able to activate signaling pathways contributing to or leading to inflammation, we evaluated the ability of the TLR6 agonist FSL-1 (Pam2CGDPKHPKSF), a synthetic lipoprotein derived from Mycoplasma salivarium, to induce CCL2 and IL-1β expression in macrophages. In addition, we determined signaling molecules downstream of TLR6 that were involved in FSL-1-mediated upregulation of CCL2 and IL-1β.
METHODS
Cells
THP-1 cells were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere of 5% CO2 in the presence of penicillin (50 units/ml) and streptomycin (50 µg/ml). THP-1 cells were passaged every 2~3 days in order to maintain between 1,000 to 1,000,000 cells per ml in the culture medium. THP-1 cells in passages between 7 and 10 were used for experiments.
Reagents
FSL-1 and oxidized 1-palmitoyl-2-arachidonosyl-sn-phosphatidylcholine (OxPAPC) were purchased from Invivogen (San Diego, CA, USA). LY294002, RO318220, GF109203X, and SP600125 were purchased from Sigma-Aldrich (St. Louis, MO, USA). U0126, SB202190, and Akt inhibitor IV (Akti IV) were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-phospho-ERK antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-p38 MAPK antibody was purchased from R&D systems (Minneapolis, MN, USA). Anti-phospho-Akt antibody and anti phospho-JNK antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-α-tubulin antibody was purchased from Calbiochem.
Enzyme-linked immunosorbent assay (ELISA)
The amount of CCL2 and IL-1β released from THP-1 cells into the culture medium was determined using commercially available ELISA kits according to the manufacturer's instructions (R&D Systems, Minneapolis). Recombinant standards of CCL2 or IL-1β provided in the kit and the isolated culture medium were added to a plate pre-coated with a monoclonal antibody against the chemokine. After incubation for 2 h, the plate was washed and incubated with an enzyme-linked polyclonal antibody specific for CCL2 or IL-1β. After several washes, the substrate solution was added, and the color intensity was measured. A standard curve was used for determination of the amount of CCL2 or IL-1β present in the samples. Data are expressed as average±standard deviation of triplicates experiments.
Reverse transcription (RT)-polymerase chain reaction (PCR)
RT-PCR and real-time PCR were performed as previously described [19]. In brief, total RNAs isolated were reverse-transcribed for one hour at 42℃ with Moloney Murine Leukemia Virus reverse transcriptase, followed by PCR analysis using primers. The cDNA was denatured at 90℃ for 5 min followed by 25 cycles of PCR (95℃ for 30 sec, 55℃ for 30 sec, 72℃ for 30 sec). The primers for CCL2 were 5-TCTGTGCCTGCTGCTCATAG-3 (forward) and 5-CAGATCTCCTTGGCCACAAT-3 (reverse), and the primers for IL-1β were 5-GGACAAGGGAGGAAGATGC-3 (forward) and 5-TCTTTCAACACGCAGGACAG-3 (reverse). Transcripts of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were amplified as an internal control. Primers for GAPDH were 5-GAGTCAACGGATTTGGTCCT-3 (forward) and 5-TGTGGTCATGAGTCCTTCCA-3 (reverse). The intensities of CCL2 and IL-1β bands were normalized relatively to that of GAPDH which was not changed by each treatment. Error bars represents the standard deviation of triplicates experiments.
Western blot analysis
THP-1 cells were lysed with lysis buffer (1% SDS, 1 mM NaVO3, 10 mM Tris-HCl, pH 7.4) containing protease inhibitors, and supernatants were isolated after centrifugation (15,000×g, for 5 min, at 4℃). Cell lysates containing an equal amount of protein were separated by 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes. After blocking for one hour in 5% skim milk in 0.1% Tween 20/TBS, the membranes were incubated at 4℃ with appropriate secondary antibody diluted in blocking solution overnight. After washing three times with 0.1% Tween 20/TBS for 10 min each, the membranes were incubated for one hour at room temperature with horseradish peroxidase-conjugated secondary antibodies diluted in blocking solution (1:5,000). After washing three times with washing buffer for 10 min each, bands were detected with chemiluminescent reagents.
RESULTS
Upregulation of CCL2 and IL-1β in response to FSL-1 at the messenger and protein levels
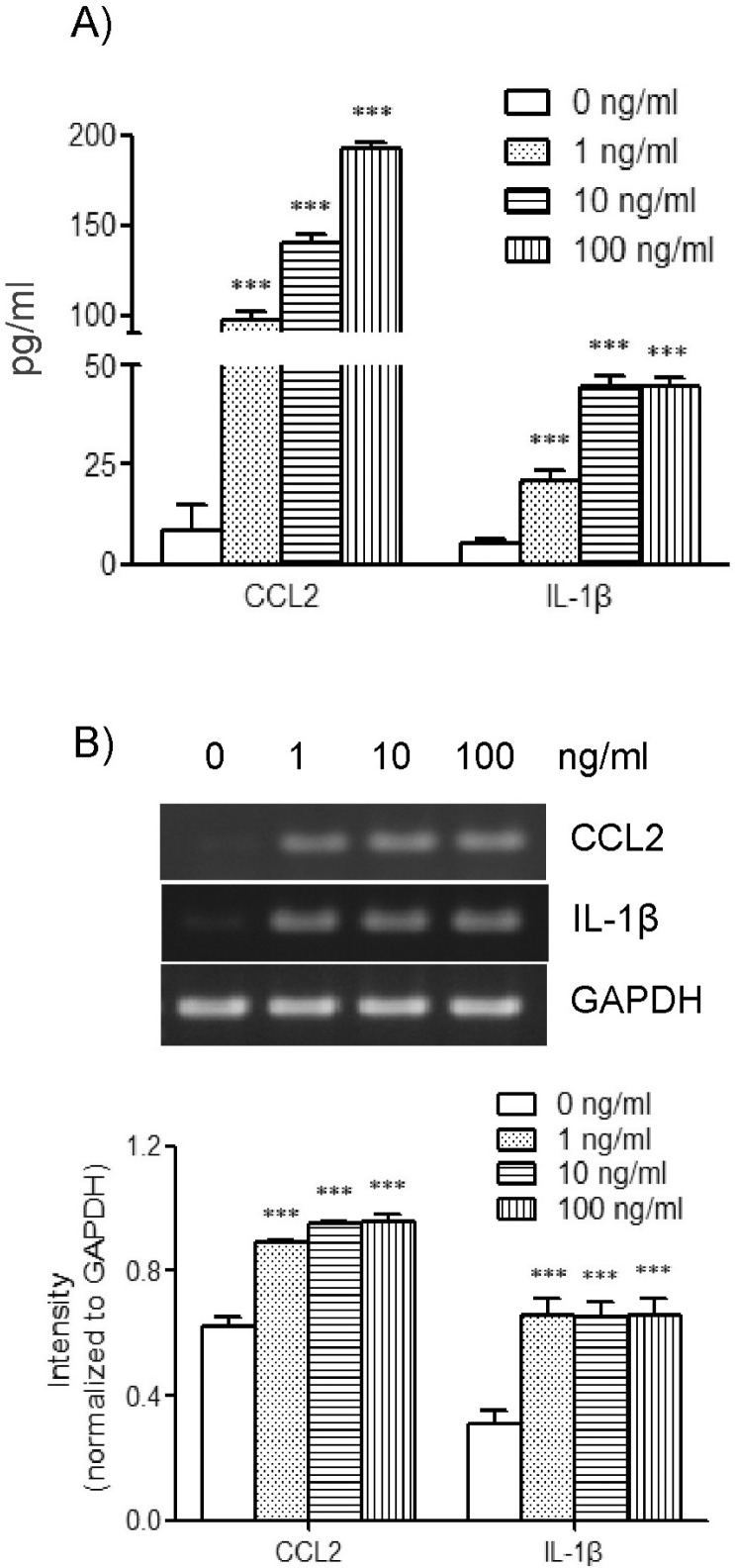
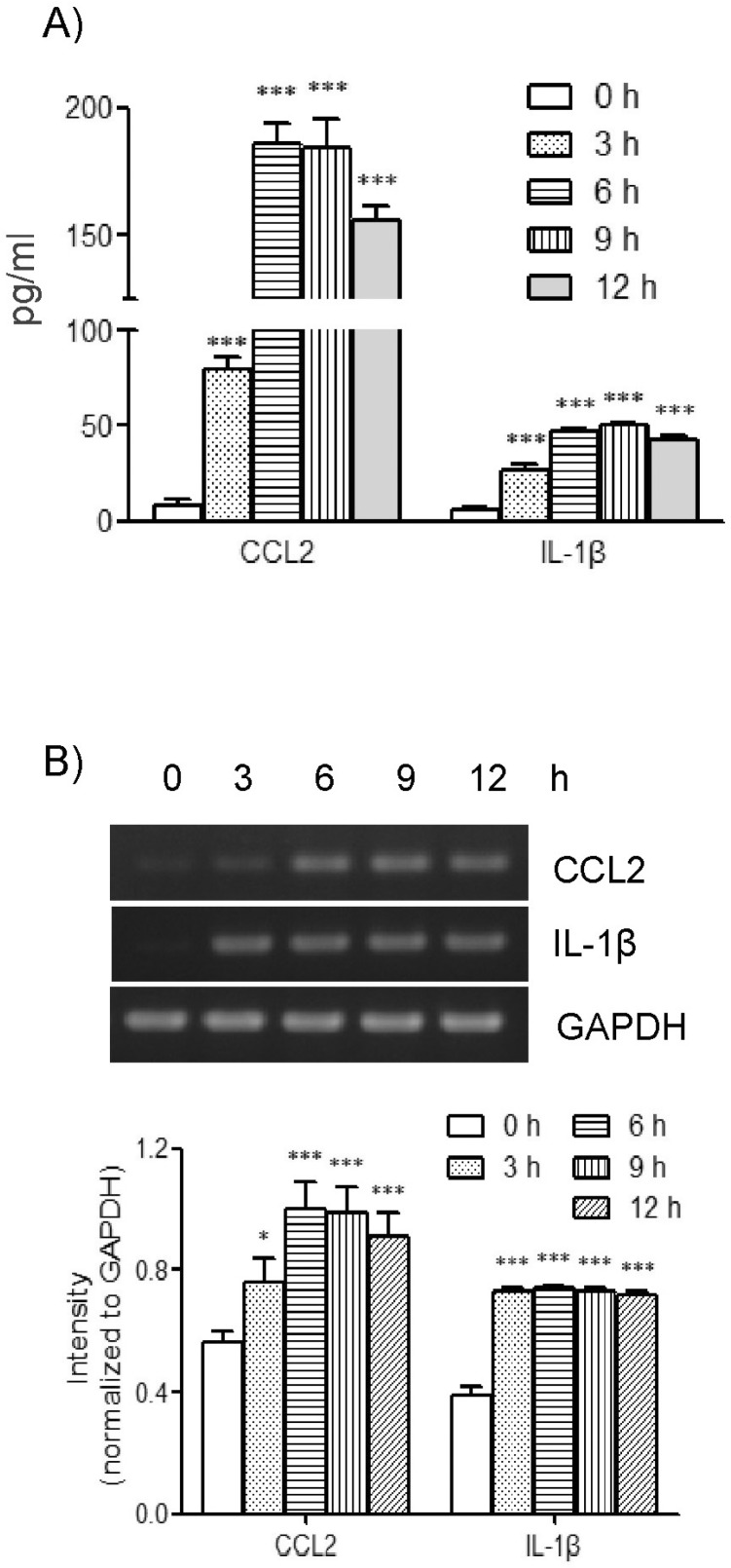
In order to examine the effects of TLR-6 activation on expression of CCL2 and IL-1β in THP-1 cells, we performed concentration and time course experiments using FSL-1. Results of ELISA analyses revealed that THP-1 cells significant increase in the presence of FSL-1. The amount of CCL2 secreted increased by 7.2-, 10.3-, and 14.3-fold in the presence of 1, 10 and 100 ng/ml of FSL-1, respectively, in comparison with control cells cultured in the absence of FSL-1 (Fig 1A). The amount of IL-1β secreted was increased by 3.9-, 8.3-, and 8.2-fold in the presence of 1, 10, and 100 ng/ml of FSL-1, respectively, compared with control cells. We also performed RT-PCR in order to determine whether FSL-1 had an influence on expression of CCL2 and IL-1β at the mRNA level. CCL2 and IL-1β gene transcripts were barely detected in THP-1 cells in the absence of FSL-1, whereas their transcripts were induced in the presence of 1, 10, and 100 ng/ml of FSL-1 (Fig. 1B). FSL-1-enhanced secretion of CCL2 and IL-1β was observed as early as 3 h post-treatment, and appeared to reach a plateau at 6 h post-treatment, and was sustained up to 12 h post-treatment with FSL-1 (Fig. 2A). FSL-1-induced transcription of CCL2 was observed as early as 3 h post-treatment transcripts, and the induction became more evident 6 h post-treatment and thereafter. FSL-1 induced transcription of IL-1β as early as 3 h post-treatment transcript and persisted up to 12 h post-treatment with FSL-1 (Fig. 2B).
Fig. 1.
Effects of FSL-1 concentrations on secretion and gene transcription of CCL2 and IL-1β. (A) THP-1 cells (1×106 cells/ml) were incubated for 9 h in the absence or presence of the indicated concentrations of FSL-1. The amounts of CCL2 and IL-1β released into the medium were measured by ELISA. ***p<0.001 vs. 0 ng/ml. (B) Transcripts of CCL2 and IL-1β genes were amplified by RT-PCR after the treatment. ***p<0.001 vs. 0 ng/ml.
Fig. 2.
Effects of FSL-1 treatment periods on secretion and gene transcription of CCL2 and IL-1β. (A) THP-1 cells (1×106 cells/ml) were incubated for the indicated time periods in the absence or presence of FSL-1 (100 ng/ml). The amounts of CCL2 and IL-1β released into the medium were measured by ELISA. ***p<0.001 vs. 0 h. (B) Transcripts of CCL2 and IL-1β genes were amplified by RT-PCR after the treatment. *p<0.05 vs. 0 h. ***p<0.001 vs. 0 h.
Participation of TLR2/4 in FSL-1-induced expression of CCL2 and IL-1β
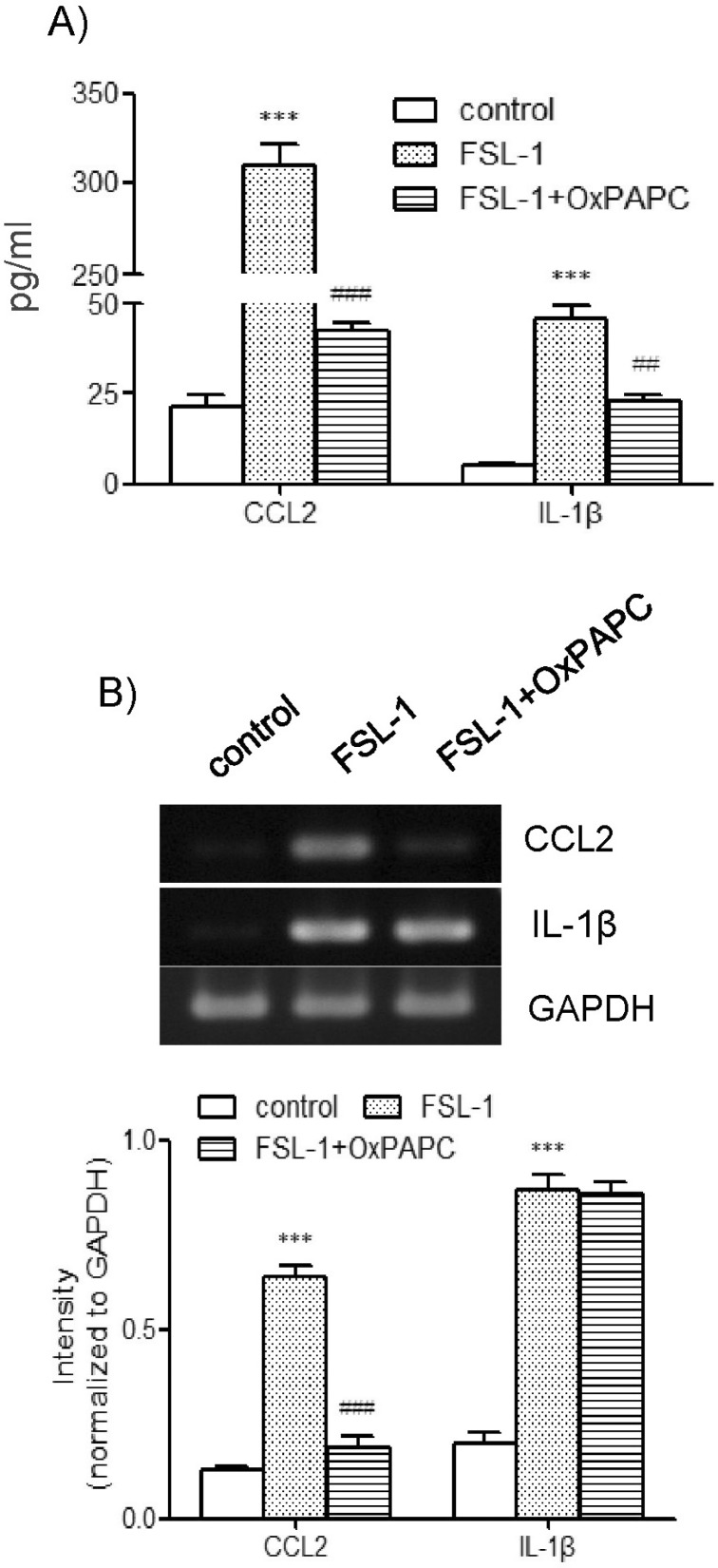
Since TLR6 co-operates with TLR2/4 via heterodimerization to induce expression of proinflammatory genes [6-8], we investigated the question of whether TLR2/4 played a role in FSL-1-mediated expression of CCL2 and IL-1β using OxPAPC, the TLR2/4 inhibitor. OxPAPC had a profound influence on secretion of CCL2 and IL-1β (Fig. 3A). Secretion of CCL2, which increased by 10.6-fold in the presence of FSL-1, was reduced to 1.9-fold and secretion of IL-1β, which increased by 8.9-fold in response to FSL-1, was attenuated to 4.5-fold in the presence of OxPAPC in comparison with control cells. The TLR2/4 inhibitor affected transcription of CCL2 but not of IL-1β. In particular, upregulation of CC2 transcripts induced by FSL-1 was abrogated in the presence of the inhibitor (Fig. 3B).
Fig. 3.
Effects of OxPAPC on secretion and gene transcription of CCL2 and IL-1β. (A) THP-1 cells were stimulated for 9 h with or without FSL-1 (100 ng/ml) after treatment for 1 h with OxPAPC (30 µg/ml). The amounts of CCL2 and IL-1β released into the medium were measured by ELISA. ***p<0.001 vs. control. ##p <0.01 vs. FSL-1. ###p<0.001 vs. FSL-1. (B) Transcripts of CCL2 and IL-1β genes were amplified by RT-PCR after the treatment. **p<0.01 vs. control. ###p<0.001 vs. FSL-1.
Roles of PI3K-Akt in FSL-1-induced expression of CCL2 and IL-1β
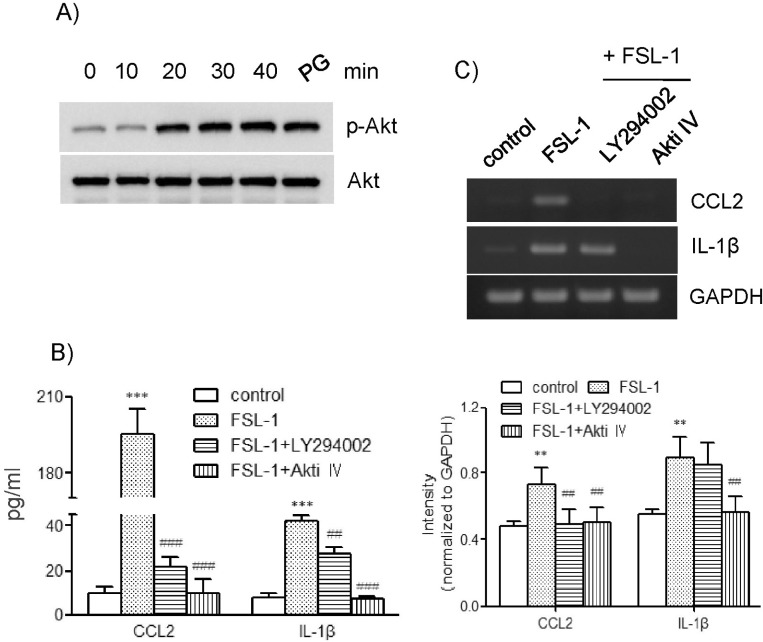
To investigate the effect of FSL-1 in the activity of Akt, we performed western blot analysis in order to examine phosphorylation of Akt. Treatment with FSL-1 resulted in enhanced phosphorylation of Akt. Maximum Akt phosphorylation occurred 20 min post-treatment with FSL-1, and was sustained up to 40 min post-treatment (Fig. 4A). We investigated the roles of Akt in FSL-1-mediated expression of CCL2 and IL-1β using inhibitors of LY294002 and Akti IV. LY294002 inhibited phosphoinositide 3-kinase (PI3K), leading to activation of Akt kinase. Both inhibitors had a significant effect on expression of CCL2 at the messenger and protein levels. Secretion of CCL2 increased by 10.2-fold in response to FSL-1, which was almost completely blocked in the presence of LY294002 and Akti IV (Fig. 4B). In addition, both inhibitors abrogated FSL-1-mediated transcription of CCL2 (Fig. 4C). Treatment with LY294002 and Akti IV also resulted in significantly attenuated secretion of IL-1β (Fig. 4C). Secretion of IL-1β increased by 8.1-fold in response to FSL-1. The increase was attenuated to 3.5-fold and was abrogated in the presence of LY294002 and Akti IV, respectively (Fig. 4B). In addition, treatment with Akti IV, but not LY294002, resulted in abrogated IL-1β gene transcription induced by FSL-1.
Fig. 4.
Effects of LY294002 and Akti IV on secretion and gene transcription of CCL2 and IL-1β. (A) THP-1 cells were exposed to FSL-1 for the indicated time periods; an equal amount of protein was then analyzed by western blotting using antibodies against Akt and phosphorylated Akt. (B) THP-1 cells were stimulated for 9 h with or without FSL-1 (100 ng/ml) after pretreatment for 1 h with LY294002 and Akti IV (10 µM each). The amounts of CCL2 and IL-1β released into the medium were measured by ELISA. ***p <0.001 vs. control. ##p<0.01 vs. FSL-1. ###p<0.001 vs. FSL-1. (C) Transcripts of CCL2 and IL-1β genes were amplified by RT-PCR after the treatment. **p<0.01 vs. control. ##p<0.01 vs. FSL-1.
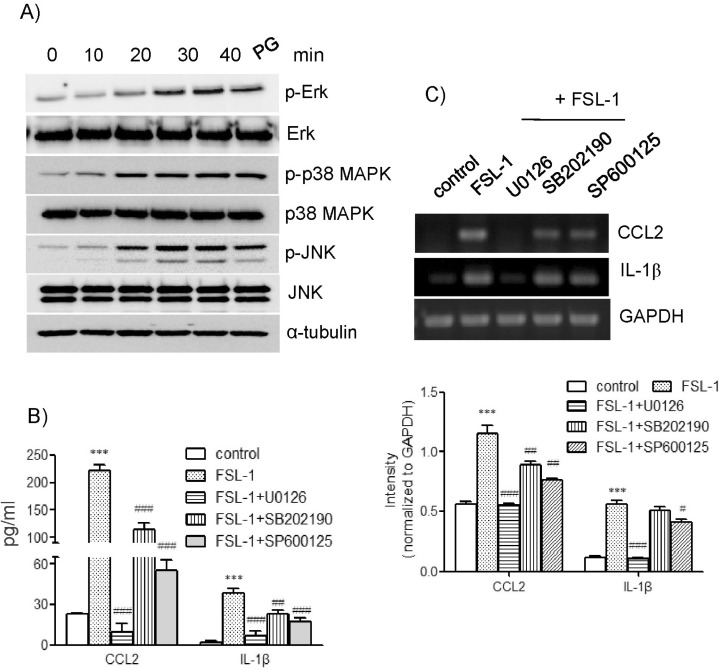
Major roles of the ERK1/2 pathway in FSL-1-induced expression of CCL2 and IL-1β
We attempted to determine whether FSL-1 activated mitogen-activated protein kinases (MAPKs) by detection phosphorylated forms of extracellular signal-regulated kinase (ERK), p38 MAPK, and c-jun N-terminal kinase (JNK) on western blots (Fig. 5A). Treatment with FSL-1 resulted in enhanced phosphorylation of ERK, which was observed 20 min post-treatment and reached maximum 30 min post-treatment with FSL-1. FSL-1 also affected phosphorylation of p38 MAPK and JNK; maximum phosphorylation of the kinases occurred 20 min post-treatment with FSL-1 and was sustained up to 40 min post-treatment with FSL-1. We used inhibitors of SB202190 (a p38 MAPK inhibitor), SP600125 (a JNK inhibitor), and U0126 (an ERK inhibitor) in order to assess the roles of MAPKs in FSL-1-induced upregulation of CCL2 and IL-1β. The three MAPK inhibitors had a significant influence on secretion of CCL2 and IL-1β in a similar pattern. Secretion of CCL2 increased by 9.7-fold in response to treatment with FSL-1, which was abrogated by the treatment with U0126 and reduced to 5.2- and 2.6-fold in the presence of SB202190 and SP600125, respectively (Fig. 5B). Secretion of IL-1β increased by 9.7-fold in response to FSL-1. The increase was lowered to 1.5-, 5.3-, and 4.0-fold in the presence of U0126, SB202190, and SP600125, respectively. The MAPK inhibitors also influenced CCL2 and IL-1β expression at the mRNA level in a pattern similar to that observed with CCL2 and IL-1β secretion (Fig. 5C). Gene transcription of CCL2 and IL-1β induced in the presence of FSL-1 was abolished by treatment with U0126 and attenuated by treatment with SB202190 and SP600125.
Fig. 5.
Effects of MAPKs inhibitors on secretion and gene transcription of CCL2 and IL-1β. (A) THP-1 cells were exposed to FSL-1 for the indicated time periods; an equal amount of protein was then analyzed by western blotting using antibodies against phosphorylated and unphosphorylated forms of ERK, p38 MAPK, and JNK. (B) THP-1 cells were stimulated for 9 h with or without FSL-1 (100 ng/ml) after pretreatment for 1 h with the indicated MAPKs inhibitors (10 µM each). The amounts of CCL2 and IL-1β released into the medium were measured by ELISA. ***p<0.001 vs. control. ##p<0.01 vs. FSL-1. ###p<0.001 vs. FSL-1. (C) Transcripts of CCL2 and IL-1β genes were amplified by RT-PCR. ***p<0.001 vs. control. #p<0.05 vs. FSL-1. ##p<0.01 vs. FSL-1. ###p<0.001 vs. FSL-1.
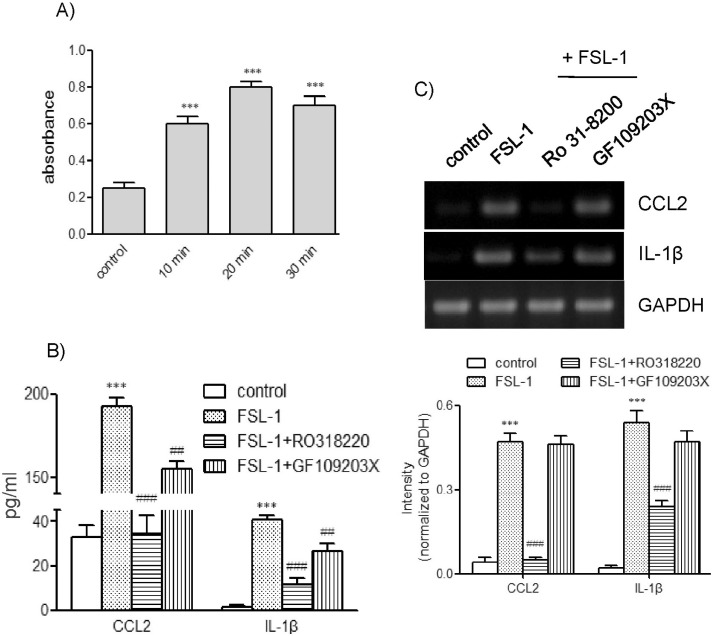
Involvement of PKC in FSL-1-induced expression of CCL2 and IL-1β
Examining the effects of the TLR6 ligand on protein kinase C (PKC), we found that FSL-1 enhanced the activity of PKC (Fig. 6A). Increased PKC activity was observed as early as 5 min post-treatment and remained enhanced up to 30 min post-treatment with FSL-1. To assess the role of PKC in FSL-1-mediated upregulation of CCL2 and IL-1β, we used two PKC inhibitors, GF109203X and RO318220. Both inhibitors affected secretion of FSL-1-mediated CCL2 and IL-1β in a similar pattern. Secretion of CCL2 increased by 9.4-fold in response to the treatment with FSL-1, which was lowered to 1.5- and 5.7-fold in the presence of RO318220 and GF109203X, respectively (Fig. 6B). Secretion of IL-1β was enhanced by 8.1-fold in response to treatment with FSL-1, and was attenuated to 2.1- and 4.9-fold in the presence of RO318220 and GF109203X, respectively. Inhibition of PKC also influenced expression of CCL2 and IL-1β at the mRNA level. FSL-1-mediated CCL2 and IL-1β gene transcription was profoundly inhibited by treatment with RO318220 not by GF109203X (Fig. 6C).
Fig. 6.
Effects of PKC inhibitors on secretion and gene transcription of CCL2 and IL-1β. (A) THP-1 cells were exposed to FSL-1 for the indicated time periods; PKC activity was then determined using the PKC kinase activity assay kit. Data are expressed as mean±SD. ***p<0.01 vs. 0 min. (B) THP-1 cells were stimulated for 9 h with or without FSL-1 (100 ng/ml) after pretreatment with RO318220 (1 µM) and GF109203X (3 µM). The amounts of CCL2 and IL-1β released into the medium were measured by ELISA. ***p<0.001 vs. control. ##p<0.01 vs. FSL-1. ###p<0.001 vs. FSL-1. (C) Transcripts of CCL2 and IL-1β genes were amplified by RT-PCR after the treatment. ***p<0.001 vs. control. ###p<0.001 vs. FSL-1.
DISCUSSION
In the current study, we demonstrated that treatment with FSL-1, a bacterial-derived TLR6 agonist, resulted in upregulated expression of CCL2 and IL-1β both at the mRNA and protein levels in human macrophage THP-1 cells. This finding is consistent with the fact that TLRs cause upregulation of inflammatory gene transcription upon recognition of their ligands [3,20]. We also attempted to identify cellular factors contributing to FSL-1-mediated expression of CCL2 and IL-1β. TLRs can form homodimers or heterodimers. TLR2 forms a heterodimer with TLR-1 or -6 and TLR4 forms a heterodimer with TLR6. Co-expression of them is necessary for gene expression [6-8]. Therefore, we attempted to investigate whether TLR2/4 played roles in FSL-1-induced expression of CCL2 and IL-1β. Treatment with OxPAPC markedly resulted in markedly blocked FSL-1-mediated secretion of CCL2 and abrogated FSL-1-induced CCL2 gene transcription, indicating requirement of TLR2/4 activity. However, OxPAPC did not influence FSL-1-induced IL-1β gene transcription, yet treatment with the inhibitor resulted reduced FSL-1-mediated IL-1β secretion by approximately 50%. The differential regulation of CCL2 and IL-1β expression in the presence of OxPAPC can be explained in conjunction with TLR2/4. FSL-1 is able to activate TLR6 via TLR2/4-dependent and -independent ways. FSL-1 induces CCL2 via TLR2/4-dependent mechanism while TLR2/4-independent signaling pathway seems to play a major role in transcription of IL-1β in response to FSL-1.
The PI3K pathway regulates TLR signaling, leading to either positive or negative effects on signaling, depending on cell type and stimulus [21,22]. We found that FSL-1 enhanced phosphorylation of Akt, suggesting that the TLR6 ligand activated the PI3K pathway, as PI3K activation leads to phosphorylation of the Akt kinase [23,24]. We attempted to determine whether Akt and PI3K, the Akt activator, were involved in expression of CCL2 and IL-1β. Inhibition of Akt and PI3K resulted in complete blockade of FSL-1-mediated secretion of CCL2 as well as CCL2 gene transcription. Inhibition of Akt resulted in blockade of FSL-1-mediated expression of IL-1β at the protein and the mRNA levels, while inhibition of PI3K reduced resulted in FSL-1-mediated secretion of IL-1β by approximately 35% without noticeable decrease of its gene transcription. These findings indicate that both PI3K and Akt are necessary for FSL-1-mediated CCL2 expression, whereas Akt is sufficient for IL-1β expression in response to FSL-1. The failure of LY294002 to blocked transcription of IL-1β might be explained in relation to the mode of action of LY294002 as well as to pattern of IL-1β expression. LY294002 is a reversible PI3Ks inhibitor [25], indicating that inhibition of PI3Ks depends upon relative activity between LY294002 and PI3K activation by treatment with FSL-1. In addition, transcription of IL-1β occurred fast after FSL-1 treatment. It may be possible that signaling pathways activated FSL-1 suppassed inhibitory activity of LY294002 for duration such that IL-1β, but not CCL2, gene transcription was induced.
MAPKs, the serine/threonine-specific protein kinases that respond to extracellular stimuli and regulate various cellular activities, mediate chemokine production in response to activation of TLR-2, -4, and -9 [26-28]. We found that treatment with FSL-1 resulted in elevated phosphorylation of ERK, p38 MAPK, and JNK, indicating that FSL-1 activated the three MAPKs. We investigated the question of whether MAPKs played roles in FSL-1-mediated expression of the chemokine and cytokine using specific pharmacological inhibitors of the kinases. Inhibition of ERK resulted in abrogation of secretion of CCL2 and IL-1β and transcription of the two genes. Inhibition of p38 MAPK and JNK also resulted in significantly attenuated FSL-1-mediated expression of CCL2 and IL-1β primarily at the protein level. Our results indicate that ERK activity is required for FSL-1 mediated expression of CCL2 and IL-1β at the messenger and protein levels and that p38 MAPK and JNK are necessary for maximum expression of CCL2 and IL-1β in response to FSL-1.
THP-1 cells express PKC-βI, -βII, -λ, -η, and -θ subtypes [29]. Pharmacological inhibition of PKC, or its depletion by long-term treatment with phorbol esters, results in decreased secretion of TLR-2 and -4-stimulated cytokines and chemokines [27,30,31]. These reports suggest the possible involvement of PKC in FSL-1-mediated gene expression. We investigated the effects of FSL-1 on PKC activity and found that FSL-1 enhanced PKC activity. Therefore, we attempted to determine whether PKC played roles in FSL-1-mediated expression of CCL2 and IL-1β using PKC inhibitors. FSL-1-mediated expression of CCL2 and IL-1β was profoundly inhibited by RO318220 in comparison with GF109203X. The two inhibitors are bisindolylmaleimide derivatives of staurosporine and inhibit mixed isoforms of PKC [32]. In addition to PKC, RO318220 inhibits Akt, c-Raf, MAPKK-1, and p42 MAPK, however, GF109203X does not [32]. In the current study we demonstrated that activities of Akt and p44/42 MAPK were required for FSL-1-mediated expression of CCL2 and IL-1β. It is possible that the additional inhibition of kinase activity including Akt and p44/42 MAPK by RO318220 contributed to the difference between the two inhibitors in their effects on expression of CCL2 and IL-1β. Our data suggest involvement of PKC activity in FSL-1-induced gene transcription of IL-1β and CCL2. More investigation is necessary to clarify roles of PKCs in the FSL-1 action.
Our results indicate that FSL-1, a TLR6 ligand, upregulates CCL2 or IL-1β expression by activating PI3K-Akt pathway, MAPKs, and PKC in a TLR2/4-dependent and -independent mechanisms. Our results suggest that TLR6 activation contributes to inflammatory responses by upregulation of inflammatory chemokines and cytokines, including CCL2 and IL-1β, via activation of multiple signaling pathways in macrophages.
ACKNOWLEDGEMENTS
This work was supported by a 2-Year Research Grant of Pusan National University.
ABBREVIATIONS
- CCL2
chemokine (C-C motif) ligand 2
- IL-1β
interleukin-1 beta
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- OxPAPC
1-palmitoyl-2-arachidonoyl-sn-glycerol-3-phosphatidylcholine
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- TLR
toll-like receptor
References
- 1.Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138:S534–S536. doi: 10.1016/s0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- 2.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Röschmann K, Jung G, Wiesmüller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2008;83:692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao Y, Funami K, Kikkawa S, Taniguchi M, Nishiguchi M, Fukumori Y, Seya T, Matsumoto M. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J Immunol. 2005;174:1566–1573. doi: 10.4049/jimmunol.174.3.1566. [DOI] [PubMed] [Google Scholar]
- 9.Into T, Fujita M, Okusawa T, Hasebe A, Morita M, Shibata K. Synergic effects of mycoplasmal lipopeptides and extracellular ATP on activation of macrophages. Infect Immun. 2002;70:3586–3591. doi: 10.1128/IAI.70.7.3586-3591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, Gullestad L, Damås JK. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008;28:1909–1919. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 11.Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Med. 2004;36:98–118. doi: 10.1080/07853890310019961. [DOI] [PubMed] [Google Scholar]
- 12.Young JL, Libby P, Schönbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002;88:554–567. [PubMed] [Google Scholar]
- 13.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrader JW, Moyer C, Ziltener HJ, Reinisch CL. Release of the cytokines colony-stimulating factor-1, granulocyte-macrophage colony-stimulating factor, and IL-6 by cloned murine vascular smooth muscle cells. J Immunol. 1991;146:3799–3808. [PubMed] [Google Scholar]
- 16.Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci USA. 2002;99:6280–6285. doi: 10.1073/pnas.092324399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 18.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 19.Kang SH, Lee JH, Choi KH, Rhim BY, Kim K. Roles of ERK and NF-kappaB in interleukin-8 expression in response to heat shock protein 22 in vascular smooth muscle cells. Korean J Physiol Pharmacol. 2008;12:171–176. doi: 10.4196/kjpp.2008.12.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seneviratne AN, Sivagurunathan B, Monaco C. Toll-like receptors and macrophage activation in atherosclerosis. Clin Chim Acta. 2012;413:3–14. doi: 10.1016/j.cca.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 22.Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012;3:185. doi: 10.3389/fimmu.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 24.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 25.El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 2003;17:720–722. doi: 10.1096/fj.02-0802fje. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 27.Lee SA, Kim SM, Son YH, Lee CW, Chung SW, Eo SK, Rhim BY, Kim K. Peptidoglycan enhances secretion of monocyte chemoattractants via multiple signaling pathways. Biochem Biophys Res Commun. 2011;408:132–138. doi: 10.1016/j.bbrc.2011.03.136. [DOI] [PubMed] [Google Scholar]
- 28.Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J Cell Physiol. 2007;210:667–675. doi: 10.1002/jcp.20860. [DOI] [PubMed] [Google Scholar]
- 29.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal. 2005;17:385–394. doi: 10.1016/j.cellsig.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Cuschieri J, Billigren J, Maier RV. Endotoxin tolerance attenuates LPS-induced TLR4 mobilization to lipid rafts: a condition reversed by PKC activation. J Leukoc Biol. 2006;80:1289–1297. doi: 10.1189/jlb.0106053. [DOI] [PubMed] [Google Scholar]
- 31.Fronhofer V, Lennartz MR, Loegering DJ. Role of PKC isoforms in the Fc(gamma)R-mediated inhibition of LPS-stimulated IL-12 secretion by macrophages. J Leukoc Biol. 2006;79:408–415. doi: 10.1189/jlb.0805438. [DOI] [PubMed] [Google Scholar]
- 32.Sipma H, van der Zee L, van den Akker J, den Hertog A, Nelemans A. The effect of the PKC inhibitor GF109203X on the release of Ca2+ from internal stores and Ca2+ entry in DDT1 MF-2 cells. Br J Pharmacol. 1996;119:730–736. doi: 10.1111/j.1476-5381.1996.tb15733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]