Abstract
In mitochondrial myopathies with respiratory chain deficiency impairment of energy cell production may lead to in excess reactive oxygen species generation with consequent oxidative stress and cell damage. Aerobic training has been showed to increase muscle performance in patients with mitochondrial myopathies. Aim of this study has been to evaluate, in 7 patients (6F e 1 M, mean age 44.9 ± 12.1 years) affected by mitochondrial disease, concomitantly to lactate exercise curve, the occurrence of oxidative stress, as indicated by circulating levels of lipoperoxides, in rest condition and as effect of exercise, and also, to verify if an aerobic training program is able to modify, in these patients, ox-redox balance efficiency. At rest and before training blood level of lipoperoxides was 382.4 ± 37.8 AU, compared to controls (318.7 ± 63.8; P < 0.05), this corresponding to a moderate oxidative stress degree according to the adopted scale. During incremental exercise blood level of lipoperoxides did not increase, but maintained significantly higher compared to controls. After an aerobic training of 10 weeks the blood level of lipoperoxides decreased by 13.7% at rest (P < 0.01) and 10.4%, 8.6% and 8.5% respectively at the corresponding times during the exercise test (P = 0.06). These data indicate that, in mitochondrial patients, oxidative stress occurs and that an aerobic training is useful in partially reverting this condition.
Keywords: Mitochondrial diseases, Muscle exercise, Aerobic training, Oxidative stress
1. Introduction
Mitochondrial diseases with defects in respiratory chain are a wide group of disorders resulting from mutations of mitochondrial or nuclear genes encoding for oxidative phosphorylation (OXPHOS) proteins. Mitochondria have a pivotal role in aerobic metabolism, the defect of OXPHOS system leading to insufficient ATP production within the affected cell. At skeletal muscle level there is a precocious activation of anaerobic metabolism in contractile activity, leading to increased production of lactate. Patients with mitochondrial myopathies (MM) often exhibit exercise intolerance, fatigue, lactic acidosis and muscle pain during low-to moderate exercise activity [1]. Exercise intolerance encourages a sedentary lifestyle that promotes further reduction of muscle aerobic capacity through physical deconditioning which has been shown to benefit from aerobic training [2], although mechanisms explaining this are not clear.
Defects of mitochondrial OXPHOS activity lead not only to energy insufficiency but also to abnormal generation of reactive oxygen species (ROS) [3,4]. In fact, in presence of respiratory chain complex defect, electron flow breaks down and electrons can be elapsed to oxygen therefore producing ROS (, , O3, ONOO−). Physiologically the cell contrasts the excess of ROS production by enhancing antioxidant defense system, this including up-regulation of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase. When there is an imbalance between ROS levels and antioxidant defense this realizes the oxidative stress that may lead to cell death with both apoptotic and not apoptotic pathways.
The aim of this study has been to evaluate, in patients affected by mitochondrial myopathy, the occurrence of in vivo oxidative stress in relation to exercise and to asses if aerobic training, other than improve oxidative metabolism, is able to reduce exercise-related ROS production from skeletal muscle. To do that, an indirect marker of oxidative stress, blood lipoperoxides level, measured at rest and during an aerobic exercise test, was considered.
2. Materials and methods
2.1. Patients
The study was performed on a group of 7 patients, 1 male and 6 females (mean age ± standard deviation (SD): 44.9 ± 12.1 years), affected by MM and diagnosed on the grounds of clinical, family-history, muscle biopsy and genetic analysis data.
Four patients were affected by chronic progressive external ophthalmoplegia (CPEO) and harbored a single large-scale mtDNA deletion at muscle biopsy. Three patients presented encephalomyopathy with a limb girdle pattern of skeletal muscle involvement and multiple mtDNA deletions (Table 1). Mean level of blood creatin kinase (CK) was 195.9 ± 126.6 U/L (normal range 25–195 U/L).
Table 1.
Characteristics of mitochondrial patients.
| Patient | Age/sex | Molecular defect | CK (U/L) | PRE-tr rPOmax (% pnPOmax) | PRE-tr rest lactate (mmol/L) | PRE-tr rest lipoperoxides (AU) |
|---|---|---|---|---|---|---|
| 1. | 52/F | Multiple deletion | 104 | 60 | 2.84 | 441 |
| 2. | 55/F | Multiple deletion | 120 | 60 | 1.60 | 405 |
| 3. | 34/F | Multiple deletion | 54 | 70 | 1.93 | 371 |
| 4. | 28/M | Single deletion | 346 | 70 | 2.43 | 377 |
| 5. | 44/F | Single deletion | 284 | 60 | 3.10 | 378 |
| 6. | 39/F | Single deletion | 352 | 60 | 1.70 | 323 |
| 7. | 62/F | Single deletion | 111 | 50 | 1.58 | 389 |
Abbreviations: CK = creatin kinase; PRE-tr = pre-training; rPOmax = real maximum power output; %pnPOmax = percentage of predicted normal maximal power output; AU = arbitrary units.
The clinical criteria for inclusion of these patients in the study had been the following:
-
1.
Mild degree of skeletal myopathy, as established by an activity of daily living score ⩽2 for each items [5] and MRC score ⩾4 in considered muscle groups. The patients could therefore manage an autonomous life and were deemed capable of performing the proposed test exercise;
-
2.
absence of cardiac, respiratory involvement and no evidence for either diabetes or endocrine diseases;
-
3.
absence of joint or bone deformities;
-
4.
body weight not exceeding 20% of the theoretical anthropometric value.
No patient was taking medication at the time of the study. All subjects gave their informed consent after having been explained the purposes and procedures of the study. Approval of the study was obtained by our Institutional Ethical Committee.
2.2. Exercise test
Patients, at least 3–4 h after a normal mixed-diet meal, performed a series of 3 min exercise bouts, at a pedalling rate of 60–70 revolution/min, interspaced with 2 min rest intervals, at increasing workload. According to an already adapted protocol [6], the exercise started at 10% of the predicted normal maximal power output (pnPOmax), defined for each patients on the basis of his/her sex, age and weight [7], and then, through successive increments of 10% of pnPOmax, brought to the highest work level at which cycling could be maintained for 3 min: this work level, expressed in watts, was taken as the real maximum power output (rPOmax).
Consecutive blood samples were collected from antecubital vein for lipoperoxide and lactate dosage in basal conditions, at 40% of pnPOmax, a power level assumed to correspond to anaerobic lactate threshold as previously reported in MM patients [5], at rPOmax and 20 min after the end of the exercise.
Venous lactate levels were assessed spectrophotometrically on an ERIS Analyzer 6170 (Eppendorf Geratebau, Hamburg, Germany), reference values being 0.67–2.47 mmol/l. Lipoperoxides were measured directly using a spin trapping method: the D-Roms test [8]. This method is based on the ability of transition metals to catalyze, in the presence of lipoperoxides, the formation of free radicals that are then trapped by an alchylamine. The alchylamine reacts forming a colored radical detectable at 505 nm through a kinetic reaction that is linear up to 500 arbitrary units (AU), according to the authors. The determination of free radicals can be made with a normal spectrophotometer. The normal range in basal condition has been determined as 250–320 AU. Higher values suggest increasing oxidative stress (320–340 AU mild stress; 340–400 AU moderate stress; 400–500 AU severe stress; >500 AU very severe stress) [8].
Heart and ventilation rate, as well as capillary hemoglobin O2 saturation (radiometer, Copenhagen, Denmark) was assessed under resting condition and during exercise.
A group of healthy volunteers (n = 12), age- and sex-matched, was used as control, by performing the incremental exercise, as described above, till 70% of pnPOmax, a workload level considered to correspond to aerobic threshold in normal untrained people [6].
2.3. Aerobic training
For each patient the exercise test was performed both before (PRE-tr) and after (POST-tr) a 10 week aerobic exercise training program, as previously described [5]. Briefly, the training schedule included 30 min exercise for the first 5 weeks and then 45 min exercise, interrupted half-way by 5 min rest intervals, for the remaining 5 weeks. Exercise training was performed at constant near lactate threshold workload, corresponding to 40% of the pnPOmax. The pedaling rate was maintained constant at a value between 60 and 70 revolution/min.
All the data were compared with those of the 12 healthy untrained subjects.
2.4. Data analysis
All date were reported as means and standard deviations (SD). The T Student test for uncoupled data to differences patients to controls. The T Student test for coupled data to estimate, in MM patients, differences between PRE-tr and POST-tr exercise test results, and the Spearman test to analyse correlations were applied. A significance level of 0.05 was considered.
3. Results
3.1. PRE-tr condition
In MM patients rPOmax ranged from 50% to 70% of the pnPOmax.
The mean level at rest of blood lipoperoxides was 382.4 ± 37.8 AU (318.7 ± 63.8 in controls, P < 0.05) (Table 2), corresponding to a moderate stress on the above-mentioned arbitrary unit scale. When considering individual cases, 5 patients fitted in the moderate stress class and 2 in the severe stress class (Table 1).
Table 2.
Lipoperoxide levels (AU) during PRE-tr and POST-tr exercise test and in controls.
| Basal | 40% of pnPOmax | rPOmax | Recovery | |
|---|---|---|---|---|
| PRE-tr exercise test | ||||
| pt. 1 | 441 | 393 | 403 | 395 |
| pt. 2 | 405 | 393 | 403 | 395 |
| pt. 3 | 371 | 375 | 370 | 362 |
| pt. 4 | 377 | 398 | 394 | 380 |
| pt. 5 | 378 | 365 | 387 | 381 |
| pt. 6 | 316 | 325 | 335 | 322 |
| pt. 7 | 389 | 402 | 415 | 393 |
| Average | 382.4 | 378.7 | 386.7 | 375.4 |
| St. dev. (±) | 37.8 | 27.1 | 26.9 | 26.3 |
| Controls | ||||
| Average | 318.7 | 326.6 | 334.4 | 324.4 |
| St. dev. (±) | 63.8 | 65.9 | 73.1 | 66.5 |
| Reference range 250–320 | ||||
| POST-tr exercise test | ||||
| pt. 1 | 407 | 419 | 398 | 400 |
| pt. 2 | 303 | 311 | 407 | 392 |
| pt. 3 | 310 | 313 | 315 | 305 |
| pt. 4 | 291 | 296 | 298 | 289 |
| pt. 5 | 334 | 341 | 346 | 338 |
| pt. 6 | 323 | 336 | 346 | 325 |
| pt. 7 | 342 | 359 | 364 | 355 |
| Average | 330.0 | 339.3 | 353.4 | 343.4 |
| St. dev. (±) | 38.3 | 41.1 | 40.1 | 41.8 |
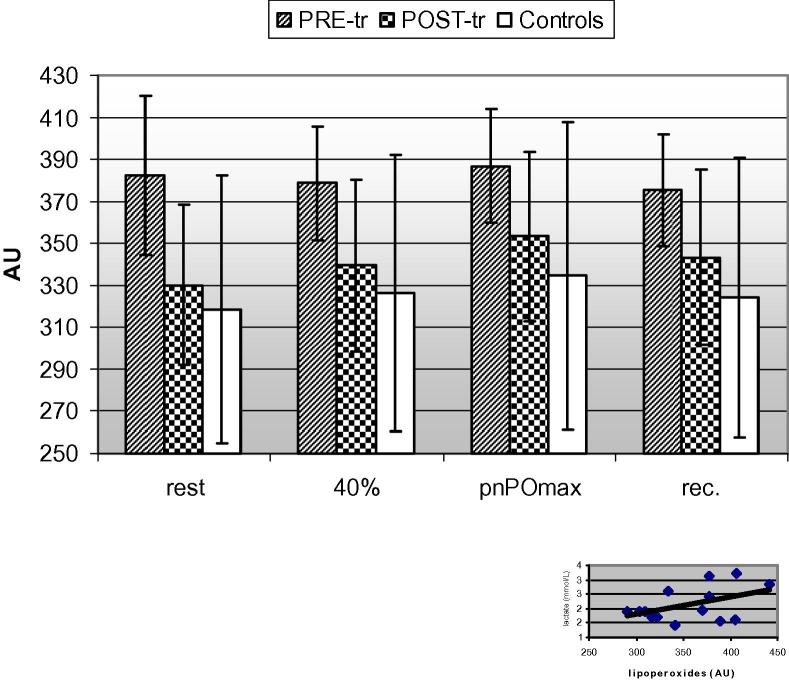
During the exercise test and compared to lactate curve (Fig. 1), this peroxidation marker maintained unchanged at 40% of pnPOmax and at rPOmax (378.7 ± 27.1 AU and 386.7 ± 26.9 AU on average, respectively). The corresponding values for controls were 326.6 ± 65.9 and 334.4 ± 73.1 AU (P < 0.05). After 20 min recovery period the values were 375.4 ± 26.3 AU in MM, 324.4 ± 66.5 AU in controls (P < 0.05) (Fig. 2).
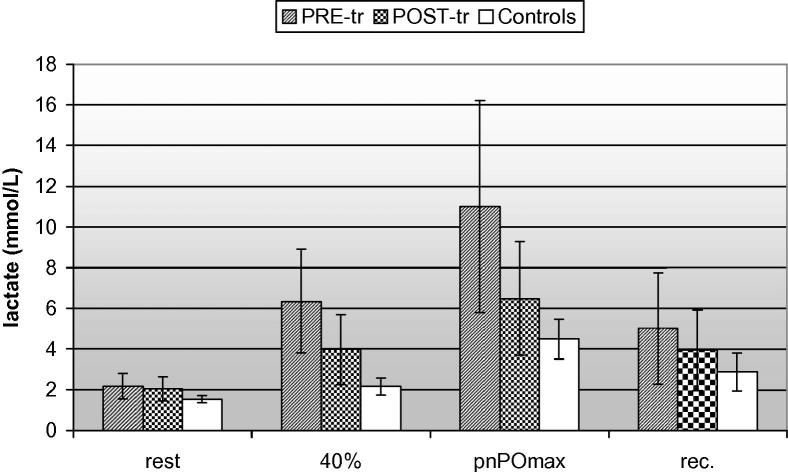
Fig. 1.
Lactate kinetics in MM [at PRE- and POST-tr (training)] and controls during incremental exercise test.
Fig. 2.
Lipoperoxide kinetics in MM [at PRE- and POST-tr (training)] and controls during incremental exercise test and correlation in MM (bottom right corner) between rest values of blood lactate and lipoperoxides, both before and after aerobic training.
The patients mean value of blood lactate at rest was within the laboratory reference range, 2.17 ± 0.62 mmol/l (reference range 0.67–2.47 mmol/l). Only two patients had abnormal basal value [1]. The mean lactate value was 6.34 ± 2.56 mmol/l at 40% of pnPOmax and 11.0 ± 5.21 mmol/l at rPOmax. After 20 min recovery lactate level was 5.01 ± 2.75 mmol/l (Fig. 1). In controls, mean blood lactate at rest was 1.53 ± 0.17 mmol/l (reference range 0.67–2.47 mmol/l). The mean lactate value was 2.15 ± 0.42 mmol/l at 40% of pnPOmax and 4.48 ± 0.99 mmol/l at rPOmax. After 20 min recovery lactate level was 2.88 ± 0.92 mmol/l.
Heart rate was 131.3 ± 14.4 beats/min and the capillary hemoglobin O2 saturation was between 98% and 99% at rPOmax.
3.2. POST-tr condition
At POST-tr test, performed after the 10 weeks training period, rPOmax was increased only in one patient, from 60% to 70% of pnPOmax.
The mean blood lipoperoxide level, at rest, was 330.0 ± 38.0 AU, now corresponding to a mild oxidative stress. At 40% of pnPOmax this value was 339.3 ± 41.1 AU, 353.4 ± 40.1 AU at rPOmax and, after the recovery period, 343.4 ± 41.8 AU. These data, compared to PRE-tr condition, indicated a reduction by 13.7% at rest, by 10.4%, 8.6% and 8.5% respectively at 40% of pnPOmax, at rPOmax and after the recovery period, (Fig. 2). However, the decrease of lipoperoxide level observed in POST-tr test, compared to PRE-tr test, was statistically significant only for rest condition (P < 0.01).
When considering single cases, six patients showed a quite homogeneous reduction of blood lipoperoxides after the training, while only one patient (case no. 6) showed an increment of it (Table 2).
After the 10 weeks training the mean basal level of lactate was not different from PRE-tr condition: 2.09 ± 0.59 mmol/l. On the contrary, a reduction of the increment of lactate during the exercise test was observed: the mean lactate value was 3.97 ± 1.73 mmol/l at 40% of pnPOmax and 6.48 ± 2.80 mmol/l at rPOmax. This difference was not statistically significant because of the high variability of lactate value in patients. After 20 min recovery mean lactate level was 3.95 ± 1.97 mmol/l (Fig. 1).
When pooling altogether exercise data, both before and after aerobic training, it was not observed a significant correlation between serum level of lactate and lipoperoxides, either at rest (Fig. 2) or during exercise.
4. Discussion
MM are an heterogeneous group of diseases due to a defect of mitochondrial oxidative phosphorylation. Respiratory chain dysfunction leads to an increased production of lactate at rest and especially during muscular exercise [9]. The mitochondrial respiratory chain is a major source of free-radicals that are implicated in the pathogenic mechanism of an increasing number of disorders [10].
Physiologically between 1% and 4% of oxygen used by normal mitochondria is converted to and H2O2. is converted to H2O2, the reaction is catalyzed by superoxide dismutase (SOD). H2O2 can generate OH•, the reaction is catalyzed by transition metals like iron (Fe2+) and copper (Cu2+). Peroxinitrite (ONOO−) is formed from reaction between and NO.
Enzymatic and non-enzymatic antioxidants systems defend cells against free radicals generation [4]. Among the major antioxidants there is the SOD in its three isoforms of a cytoplasmic Cu/Zn form (SOD1), a mitochondrial manganese form (SOD2) and an extracellular form (SOD3). Another important antioxidant defense system is represented by glutathione (GSH), capable to oxidize H2O2 and lipid hydroperoxides forming the oxidized GSH which is then reduced to GSH, this reaction being catalyzed by GSH reductase.
Non-enzymatic antioxidants include Vitamins A, C and E. These antioxidants react with and OH•. Metal sequestrating agents are another group of antioxidants which includes transferrin, ferritin, albumin, ceruloplasmin and uric acid; yhey bind metal irons preventing the generation of OH• from H2O2. Reduced ubiquinol, a component of the respiratory chain, is a further important antioxidant. It protects efficiently not only membrane phospholipids from peroxidation, but also mitochondrial DNA and membrane proteins from free radical-induced oxidative damage. In particular, it can prevent the formation of free lipid radicals and remove them directly or by regenerating vitamin E [11].
Free radicals can damage various cell components like proteins, lipids and nucleic acids. When the generation of ROS exceeds the capacity of cellular antioxidant systems accumulation of oxidative cellular damages occurs, this realizing the so called “oxidative stress” condition. At membrane lipid level, hydroxylation of polyinsaturated fatty acids generates, by entrapment of O2, peroxyl radicals, this in turn reacting with further lipid molecules ensuing in a vicious cycle of lipid peroxidation and membrane damage [6]. ROS can also induce DNA strand breaks [12], and oxidation of enzymatic protein with loss of their catalytic activity [13].
Mitochondria at the same time are, due to their essential position along the oxidative cell metabolism, the major source and target of ROS. For this reason they play a pivotal role in the complex interrelated system which governs the delicate equilibrium of oxidant/antioxidant systems within the cell. Recent works indicate novel molecular mechanisms by which mitochondria are involved in the response to oxidative stress pathway in different pathological conditions [14].
As two major respiratory chain complexes implicated in ROS generation are complex I (NADH CoQ reductase) and III (Ubiquinone CytC reductase), tissues containing mutated copies of mtDNA are exposed, due to a defect of electron transport chain, to more severe oxidative stress and cell necrotic damage [15]. Compensatory overexpression of anti-oxidant enzymes can be considered indirect sign of respiratory chain dysfunction in MM, somehow related to the type of mitochondrial enzyme deficiency, as reported for MnSOD activity increase in patients with defect of complex I [16].
ROS are also physiologically implicated in the apoptotic process activating some redox-sensible pathways of programmed cell death while, on the opposite, antioxidants agents and MnSOD hyperexpression can stop or slow down apoptotic process [17]. Programmed cell death is considered one of the ROS-mediated disease-inducing mechanisms in MM. Apoptotic nuclei matching the level of enzymatic reduction in COX deficient myofibers were observed in MM muscle biopsies [18].
Which can be the role of exercise and, more importantly, of aerobic training on oxidative stress in MM is not known. In physiological conditions, there are evidences that exercise can cause increased free radical production in the skeletal muscle and myocardium [19]. On the other hand, it is also known that regular physical activity and training is able to attenuate exercise-induced oxidative stress modifying the prooxidant/antioxidant balance [20,21], increasing endogenous antioxidant activity, and plasmatic lipoprotein resistant to oxidation [22], reducing oxidative-stress induced apoptotic signaling [23].
Benefits of aerobic training on blood lactate kinetics has already been demonstrated study in MM patients [2,5]. The present study was designed to evaluate, in patients with mitochondrial myopathy due to deletion in mtDNA, occurrence of oxidative stress and the effects of an aerobic training program in blood levels of lipid peroxidation markers, compared to venous lactate kinetics as marker of in vivo functional mitochondrial impairment. After a 10 week aerobic training the mean blood lipoperoxide resting level significantly decreased by 13.7%, indicating one-step scaled down score in the four-step conventional scale adopted for oxidative stress [8]. Although not significant, also incremental exercise-related lipoperoxide generation by contracting muscle revealed a quite clear reduction of oxidative stress in post-training compared to pre-training condition, the obtained values approaching those of healthy untrained controls. After aerobic training blood lipoperoxide values paralleled those of both exercise- and training-related lactate, as indirect marker of aerobic mitochondrial functioning level. Interestingly, MM exercise lipoperoxide curve also approached, after aerobic training, the bell-shaped configuration exhibited by healthy controls and already observed for lactate production in the similar experimental conditions both in MM and normals, indicating a more sensible adaptation of lipoperoxide kinetics to exercise intensity.
Altogether, these data confirm that, in MM patients, an aerobic training improves lactate kinetic but also indicate that it can modify the blood level of oxidative stress biochemical markers. Several mechanisms have already been evidenced to explain benefit of aerobic training programs on mitochondrial function at skeletal muscle level in normal subjects, such as increase in myofiber mitochondria number and dimension and enzyme respiratory chain activity, or interstitial capillary proliferation [24]. Some of these mechanisms can also work in MM [25] where, however, additional factors specifically linked to the molecular alterations causing the disease have to be considered, in addition to more general effects induced by the aerobic training at neuroendocrine and neurovegetative level [6]. The burden of mutated mtDNA is generally heteroplasmic in skeletal muscle of MM, the ratio between mutant and wild type genomes appearing crucial for determining phenotypic manifestations of the disease, in a context in which, however, the mitochondrial machine seems to have a certain degree of plasticity in terms of mtDNA replication and transcription [26]. Taivassalo et al. [2] have demonstrated that aerobic training can improve, in patients with complex I and IV deficiency, oxidative capacity in MM together by increase in mitochondrial volume in vastus lateral muscle, suggesting that induced mitochondrial proliferation can be the cellular basis of improved oxidative metabolism in such conditions despite unchanged proportion of mutant/wild-type mtDNA, this excluding a preferential proliferation of wild-type mtDNA genomes as consequence of the training. Analogously, Jeppesen et al. [27] found that increment, with 12 week aerobic training, of VO2max matched with increase in muscle citrate synthase and mtDNA quantity but not mtDNA mutation load, which however resulted reduced after 8 weeks of deconditioning.
Similar and may be more complexes mechanisms of mitochondria adaptation [28] can operate in skeletal muscle in response to aerobic training to increase protective mechanism against oxidative stress within the cell in MM. Recent studies of mRNA expression by real-time PCR or PCR Array techniques in different experimental settings indicate as the mitochondria interplay at various levels with nuclear genome in order to either regulate genes involved in protecting cells from oxidative damages and in free radical detoxification [14] or, conversely depending on the pathological condition, to generate oxidative stress [29], this also suggesting new possibilities to determine the effect of various treatments on the antioxidant response pathway.
5. Conflict of interest
None.
Acknowledgement
Telethon-I (grant GUP 09004) and SpePharm (Milan-I) are gratefully acknowledged for research financement.
References
- 1.Petty R.K.H., Harding A.E., Morgan-Hughes J.A. The clinical features of mitochondrial myopathy. Brain. 1986;109:915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- 2.Taivassalo T., Shoubridge E.A., Chen J. Aerobic conditioning in patients with mitochondrial myopaties: physiological, biochemical, and genetic effects. Ann Neurol. 2001;50:133–141. doi: 10.1002/ana.1050. [DOI] [PubMed] [Google Scholar]
- 3.Lenaz G., Bovina C., D’Aurelio M. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B., Gutterdge J.M.C. 3rd ed. Oxford University Press; New York: 1999. Free radicals in biology and medicine. [Google Scholar]
- 5.Siciliano G., Manca M.L., Renna M., Prontera C., Mercuri A., Murri L. Effects of aerobic training on lactate and catecholaminergic exercise responses in mitochondrial myopathies. Neuromuscul Disord. 2000;10:40–45. doi: 10.1016/s0960-8966(99)00068-1. [DOI] [PubMed] [Google Scholar]
- 6.Siciliano G., Renna M., Manca M.L. The relationship of plasma catecholamine and lactate during anaerobic threshold exercise in mitochondrial myopathies. Neuromuscul Disord. 1999;9:411–416. doi: 10.1016/s0960-8966(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 7.Hansen J.E., Sue D.Y., Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(Suppl):S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 8.Cesarone M.R., Belcaro G., Carratelli M. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 9.Nashef L., Lane R.J. Screening for mitochondrial cytopathies: the sub-anaerobic threshold exercise test (SATET) J Neurol Neurosurg Psychiatry. 1989;52:1090–1094. doi: 10.1136/jnnp.52.9.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nohl H., Jordan W. The mitochondrial site of superoxide formation. Biochem Biophys Res Commun. 1986;138:533–539. doi: 10.1016/s0006-291x(86)80529-0. [DOI] [PubMed] [Google Scholar]
- 11.Pobezhimova T.P., Voinikov V.K. Biochemical and physiological aspects of ubiquinone function. Membr Cell Biol. 2000;13:595–602. [PubMed] [Google Scholar]
- 12.Brawn K., Fridovich I. DNA strand scission by enzimatically generated oxygen free radicals. Arch Biochem Biophys. 1981;206:414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Ann Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 14.Ungvari Z., Bailey-Downs L., Sosnowska D. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y.H., Lu C.Y., Wei C.Y., Ma Y.S., Lee H.C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol. 2001;44:1–11. [PubMed] [Google Scholar]
- 16.Pitkanen S., Robinson B.H. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Invest. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleury C., Mignotte B., Vayssière J.-L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 18.Di Giovanni S., Mirabella M., Papacci M., Odoardi F., Silvestri G., Servidei S. Apoptosis and ROS detoxification enzymes correlate with cytocrome c oxidase deficiency in mitochondrial encephalomyopathies. Mol Cell Neurosci. 2001;17:696–705. doi: 10.1006/mcne.2001.0970. [DOI] [PubMed] [Google Scholar]
- 19.Ji L.L. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. Review. [DOI] [PubMed] [Google Scholar]
- 20.Gomes E.C., Silva A.N., de Oliveira M.R. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. doi: 10.1155/2012/756132. Epub 2012 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers S.K., Nelson W.B., Hudson M.B. Exercise-induced oxidative stress in humans: cause and consequences. Free Radical Biol Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. Review. [DOI] [PubMed] [Google Scholar]
- 22.Ji L.L. Exercise-induced modulation of antioxidant defence. Ann N Y Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 23.Vainshtein A., Kazak L., Hood D.A. Effects of endurance training on apoptotic susceptibility in striated muscle. J Appl Physiol. 2011;110:1638–1645. doi: 10.1152/japplphysiol.00020.2011. [DOI] [PubMed] [Google Scholar]
- 24.Klausen K., Andersen L.B., Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 25.Safdar A., Bourgeois J.M., Ogborn D.I. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci USA. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Shoubridge E.A., Karpati G., Hastings K.E.M. Deletion mutans are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segment in mitochondrial disease. Cell. 1990;62:43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- 27.Jeppesen T.D., Schwartz M., Olsen D.B. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129:3402–3412. doi: 10.1093/brain/awl149. [DOI] [PubMed] [Google Scholar]
- 28.Larsson N.G., Oldfors A., Holme E., Clayton D.A. Low levels of mitochondrial transcription factor A in mitochondrial DNA depletion. Biochem Biophys Res Com. 1994;200:1374–1381. doi: 10.1006/bbrc.1994.1603. [DOI] [PubMed] [Google Scholar]
- 29.Dowling J.J., Arbogast S., Hur J. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain. 2012;135:1115–1127. doi: 10.1093/brain/aws036. [DOI] [PMC free article] [PubMed] [Google Scholar]