Abstract
Cardiac pacemaker cells exhibit spontaneous, rhythmic electrical excitation, termed automaticity. This automatic initiation of action potentials requires spontaneous diastolic depolarisation, whose rate determines normal rhythm generation in the heart. Pacemaker mechanisms have been split recently into: (i) cyclic changes in trans-sarcolemmal ion flows (termed the ‘membrane-clock’), and (ii) rhythmic intracellular calcium cycling (the ‘calcium-clock’). These two ‘clocks’ undoubtedly interact, as trans-sarcolemmal currents involved in pacemaking include calcium-carrying mechanisms, while intracellular calcium cycling requires trans-sarcolemmal ion flux as the mechanism by which it affects membrane potential. The split into separate ‘clocks’ is, therefore, somewhat arbitrary. Nonetheless, the ‘clock’ metaphor has been conceptually stimulating, in particular since there is evidence to support the view that either ‘clock’ could be sufficient in principle to set the rate of pacemaker activation.
Of course, the same has also been shown for sub-sets of ‘membrane-clock’ ion currents, illustrating the redundancy of mechanisms involved in maintaining such basic functionality as the heartbeat, a theme that is common for vital physiological systems.
Following the conceptual path of identifying individual groups of sub-mechanisms, it is important to remember that the heart is able to adapt pacemaker rate to changes in haemodynamic load, even after isolation or transplantation, and on a beat-by-beat basis. Neither the ‘membrane-’ nor the ‘calcium-clock’ do, as such, inherently account for this rapid adaptation to circulatory demand (cellular Ca2+ balance changes over multiple beats, while variation of sarcolemmal ion channel presence takes even longer). This suggests that a third set of mechanisms must be involved in setting the pace. These mechanisms are characterised by their sensitivity to the cyclically changing mechanical environment, and – in analogy to the above terminology – this might be considered a ‘mechanics-clock’.
In this review, we discuss possible roles of mechano-sensitive mechanisms for the entrainment of membrane current dynamics and calcium-handling. This can occur directly via stretch-activation of mechano-sensitive ion channels in the sarcolemma and/or in intracellular membrane compartments, as well as by modulation of ‘standard’ components of the ‘membrane-’ or ‘calcium-clock’. Together, these mechanisms allow rapid adaptation to changes in haemodynamic load, on a beat-by-beat basis.
Additional relevance arises from the fact that mechano-sensitivity of pacemaking may help to explain pacemaker dysfunction in mechanically over- or under-loaded tissue. As the combined contributions of the various underlying oscillatory mechanisms are integrated at the pacemaker cell level into a single output – a train of pacemaker action potentials – we will not adhere to a metaphor that implies separate time-keeping units (‘clocks’), and rather focus on cardiac pacemaking as the result of interactions of a set of coupled oscillators, whose individual contributions vary depending on the pathophysiological context. We conclude by considering the utility and limitations of viewing the pacemaker as a coupled system of voltage-, calcium-, and mechanics-modulated oscillators that, by integrating a multitude of inputs, offers the high level of functional redundancy that is vitally important for cardiac automaticity.
Keywords: Beat-by-beat regulation, Calcium-clock, Coupled oscillators, Electrophysiology, Mechano-electric feedback (or coupling), Membrane-clock, Pacemaker, Rate adaptation, Stretch
Abbreviations: AP, action potential; BR, beating rate; Ca2+, calcium; DD, diastolic depolarisation; HCN, hyperpolarisation-activated, cyclic nucleotide-gated; ICa,L, L-type calcium current; ICa,T, T-type calcium current; If, funny current (hyperpolarisation-activated depolarising current); IK, outward potassium currents; INCX, sodium–calcium exchanger current; K+, potassium; MDP, maximum diastolic potential; MSP, maximum systolic potential; Na+, sodium; Erev, reversal potential; RyR, ryanodine receptor channel; SACNS, cation non-selective stretch-activated channel; SAN, sino-atrial node; SERCA, sarco-/endoplasmic reticulum calcium-ATPase; SR, sarcoplasmic reticulum; Vm, transmembrane potential
1. Introduction
Spontaneous mechanical activity of the heart is reported to have been recognised at least as far back as the second century, when Greek physician Claudius Galenus is credited with having noted that the heart continues to beat for an extended period of time when extracted from the chest (as cited by (Anglo, 1537)). It was not until the 19th century, however, that the seminal work of Gaskell (1882) confirmed the ‘myogenic theory’ of cardiac rhythm generation, originally proposed by Harvey (1651), and later supported by the work of von Haller (1757). Exploring the tortoise heart, Gaskell demonstrated rhythmicity as an intrinsic property of (in his view not fully differentiated) cardiac tissue and he proposed ‘laws’ of cardiac rhythm generation, in which he presaged that rhythmicity decreases with distance from the sinus, and that conductivity is inversely related to rhythmicity. This was followed by recognition of specific anatomical locations of cardiac automaticity in the mammalian heart, which are now known as the sino-atrial node (SAN, the primary structure responsible for initiation of the heartbeat (Keith and Flack, 1907)), and the atrio-ventricular node and Purkinje fibres (secondary and tertiary pacemaker regions (Tawara, 1906)).
A hundred years on, the cellular mechanisms of spontaneous pacemaker activity, and their relative contribution to pacemaking, are still a matter of debate (Brown et al., 1984; Lakatta and DiFrancesco, 2009; Rosen et al., 2012). It is clear, though, that cardiac pacemaking represents a robust and flexible system, integrating multiple contributors that allow adaptation to changes in circulatory demand.
In contrast to working cardiomyocytes in the atria and the ventricles, the action potential (AP) of cardiac pacemaker cells exhibits spontaneous diastolic depolarisation (DD; see Fig. 1). The rate of DD determines the time needed to take the trans-membrane potential (Vm) to the threshold for AP generation. Since Vm is an expression of the charge balance between the in- and out-side of the cell, changes in Vm require an imbalance between inward (depolarising) and outward (re- or hyperpolarising) trans-sarcolemmal currents, i.e., a net-flux of charge across the membrane. DD will result from a ‘surplus’ in inward currents which, in a dynamic setting, may arise either from a relative increase in inward currents, or a decrease in outward currents (in the presence of sufficient inward current).
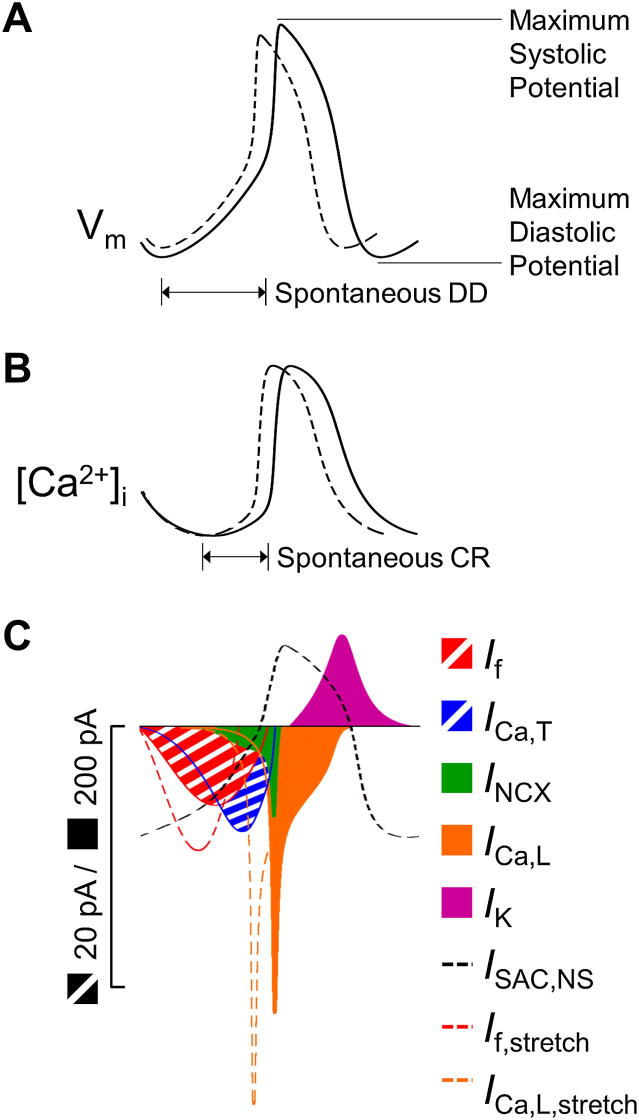
Fig. 1.
The coupled Vm/Ca2+-oscillator system in SAN cells and its modulation by the mechanical environment. SAN (A) Vm and (B) intracellular free Ca2+ concentration ([Ca2+]i) under normal conditions (solid line) and during stretch (dashed line). Time intervals of spontaneous DD and spontaneous Ca2+ release (CR) are indicated. (C) ‘Standard’ transmembrane ionic currents associated with the Vm/Ca2+-oscillator system. Shaded currents (If, ICa,T) and the SACNS current (ISAC,NS) refer to the 20 pA scale, while solid currents (INCX, ICa,L, IK) refer to the 200 pA scale. Vm, [Ca2+]i, and ionic currents generated from (Maltsev and Lakatta, 2008), except for ISAC,NS, which is from (Cooper et al., 2000), and stretch-induced increases in If (If,stretch) and ICa,L (ICa,L,stretch), which represent theoretical predictions.
Early DD is a result of both, as outward potassium (K+) currents (IK) are reducing, in the presence of increasing inward currents, such as the electrogenic sodium–calcium (Na+–Ca2+) exchanger current (INCX), the hyperpolarisation-activated (i.e., increasing in early diastole) ‘funny’ current (If; DiFrancesco, 1985), and any background conductances that carry inward currents, for instance the sodium (Na+) background current (Noble et al., 1992). In contrast to the Na+ background current, whose molecular nature is still under investigation, it is known that If is conducted by hyperpolarisation-activated cyclic nucleotide-gated (HCN) proteins, of which four isoforms have been identified in cardiac and neuronal tissue (Moosmang et al., 2001). Cardiac If is composed primarily of HCN4, and to a lesser degree of HCN2 and HCN1, in a species- and cell-type dependent manner (Barbuti et al., 2011; Scicchitano et al., 2012). Quantitative simulation has shown that these channels, even if one considers them as conductors of monovalent cations only (i.e., K+ and Na+, although there is now evidence that they also conduct calcium, Ca2+; Michels et al., 2008; Yu et al., 2007), are sufficient to give rise to cyclic changes in Vm (Noble et al., 1992). Their importance for pacemaking has been experimentally confirmed as HCN expression in non-pacemaking cells confers spontaneous activity (Li, 2012; Robinson et al., 2006), while pharmacological block significantly slows beating rate (BR; Bucchi et al., 2002; Koncz et al., 2011).
Later on during DD, If is reduced (as Vm increases), and trans-sarcolemmal Ca2+ fluxes become more dominant, involving the transient (T-type) Ca2+ (ICa,T) current and increasing INCX. This raises DD rate. Once Vm crosses the threshold for activation of the long-lasting (L-type) Ca2+ current (ICa,L), a new AP is initiated (Irisawa et al., 1993).
As the combined action of this ensemble of trans-sarcolemmal currents appears sufficient to rhythmically generate spontaneous APs (Noble et al., 1989), it has been dubbed the ‘membrane-clock’ (‘Vm-clock’; Maltsev et al., 2006).
Interestingly, interventions that target intracellular Ca2+ cycling (such as the application of ryanodine to inhibit Ca2+ release from the sarcoplasmic reticulum, SR) affect DD rate in primary (Rigg and Terrar, 1996) and secondary (Rubenstein and Lipsius, 1989) pacemaker cells. This suggests that additional mechanisms are involved, which are thought to require spontaneous cytosolic Ca2+ release from the SR, followed by re-uptake through the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) and extrusion to the cell exterior, primarily via INCX (Ju and Allen, 1998). Since Na+–Ca2+ exchanger activity is electrogenic (one Ca2+ ion leaving the cell is ‘swapped’ for three Na+ ions entering), this gives rise to increased inward INCX. More recently, SR Ca2+ cycling has been identified as rhythmical, even after clamping sarcolemmal Vm (Vinogradova et al., 2004). This has been conceptualised as representing a pacemaker mechanism whose origin is independent of trans-sarcolemmal ion fluxes (its mode of action is not, however, as it requires INCX to cause the membrane depolarisation that drives DD (Sanders et al., 2006)), and has been referred to as the ‘Ca2+-clock’ (Maltsev et al., 2006).
Both of these ‘clocks’ could theoretically drive pacemaker activity on their own. In reality, of course, they do not operate in isolation (as mentioned, the ‘Ca2+-clock’ relies on a trans-sarcolemmal ion flux to alter Vm, and the ‘Vm-clock’ includes Ca2+ cycling). It is more appropriate, therefore, to think of them as coupled oscillators, rather than separate ‘clocks’. This is especially true as their interaction at the level of normal whole-cell pacemaker function involves mutual entrainment (Noble et al., 2010), resulting in a robust and stable system (the Vm/Ca2+-oscillator, see Fig. 1). This finds representation in conceptual and computational models, and can explain a vast range of experimentally observed aspects of cardiac pacemaker function (Maltsev and Lakatta, 2008; Severi et al., 2012).
Of course, the mere plausibility of an explanation does not necessarily confirm that the mechanisms identified as causal are indeed major contributors, or even involved at all (Hunter et al., 2001; Kohl et al., 2010). To avoid falling victim to this ‘plausibility trap’ (Quinn and Kohl, 2011) requires continuous questioning, even of what may seem ‘obvious’, and for pacemaker function this continues to generate interesting and productive discussion (DiFrancesco and Noble, 2012; Maltsev and Lakatta, 2012; Rosen et al., 2012).
One shortfall of our current understanding of pacemaker function is that it has been developed based largely on investigations of molecular mechanisms in isolated cells. Pacemaking in vivo is influenced by factors not generally represented in these preparations, including biochemical influences, such as from the metabolic and autonomic systems, para- and endocrine signalling, as well as biophysical parameters, such as temperature and cyclically changing levels of stretch (Quinn et al., 2011). This review identifies the relevance of mechanical modulation of pacemaker function, explores putative mechanisms underlying adaptation of pacemaker activity to the mechanical environment, and presents the foundations for conceptual consideration of cardiac pacemaking as a coupled Vm-, Ca2+-, and mechanics-oscillator, whose integration is relevant for heart function in health and disease. Other aspects of pacemaker control will not be covered, as they have been discussed extensively in numerous recent reviews (Boyden et al., 2010; DiFrancesco and Noble, 2012; Dobrzynski et al., 2007; Maltsev and Lakatta, 2012; Mighiu and Heximer, 2012; Rosen et al., 2012; Stewart et al., 2012).
2. Direct mechanical effects on cardiac pacemaker activity
2.1. Whole animal observations
Effects of stretch on pacemaker activity were recognised soon after the identification of the SAN. In 1915, Francis Arthur Bainbridge observed that intravenous fluid injection in anaesthetised dogs resulted in right-atrial distension and an increase in BR (Bainbridge, 1915). He described this as an “Acceleration of heart rate […] caused by impulses arising within the heart.” Similar effects have since been observed in various vertebrate phyla, including Amphibia, Reptilia, Aves, and Mammals (Pathak, 1973). Confirming this finding in humans proved difficult, initially, as most non-invasive interventions that raise central venous pressure in human (such as tilt-table studies) also increase arterial pressure. The latter triggers a (dominant) baroreceptor response, the so called ‘depressor reflex’ (von Bezold and Hirt, 1867; Jarisch and Richter, 1939), which gives rise to a reduction in BR. It was not until 1978 that David Donald and John Shepherd resolved this experimental problem by passively elevating the legs of volunteers in the supine position (Donald and Shepherd, 1978). This favours venous return to the trunk of the body, raising central venous pressure with no concurrent rise on the arterial side. As a result, BR was seen to increase, establishing the presence of the ‘Bainbridge response’ in humans.
The positive correlation between right atrial volume loading and BR was originally attributed to a predominantly vagal autonomic reflex (Bainbridge, 1915). More recent research showed, however, that near instantaneous increases in BR occur in open-chest, anaesthetised dogs upon SAN-specific distension (Brooks et al., 1966), in a response that is insensitive to denervation, as well as adrenergic and cholinergic blockade. It has been confirmed that a positive chronotropic response to stretch can be observed in a host of denervated preparations, such as isolated hearts (Blinks, 1956), right atrial tissue (Blinks, 1956), and the SAN (Deck, 1964b), as well as isolated Purkinje fibres (Kaufmann and Theophile, 1967) and even single SAN cells (Cooper et al., 2000). This demonstrates that intracardiac ‘myogenic’ mechanisms contribute to mechanical modulation of pacemaker rate, beyond the originally implied vagal reflex response.
An interesting physiological phenomenon, which may in part be caused by stretch-induced changes in pacemaker rate, has been well documented in humans: heart rate variability synchronised with the respiratory cycle (in which BR rises during inspiration, and declines during expiration). This phenomenon is known as ‘respiratory sinus arrhythmia’ (even though it is a physiological fluctuation in BR). While generally considered to be a consequence (and, hence, useful indicator) of autonomic nervous input, variability continues to exist, albeit at a reduced magnitude, in the transplanted and, hence, denervated heart (Bernardi et al., 1989). This supports a role of intracardiac mechanical BR modulation. This may be explained by the fact that during inspiration, low intrathoracic pressure aids venous return (loading the right atrium), while during expiration, the opposite is true. Observed changes in BR are, thus, compatible with stretch effects on SAN function.
These early observations of stretch-effects on pacemaker electrophysiology have been followed up by studies examining underlying mechanisms at the tissue level.
2.2. Tissue level insight
Electrical and mechanical activity in the heart are linked via an intrinsic regulatory loop (mechano-electric coupling (Kohl et al., 2011)), involving both a feed-forward flow of information from electrical excitation to mechanical contraction (excitation–contraction coupling (Bers, 2002)) and feed-back from the mechanical environment to the origin and spread of excitation (mechano-electric feedback (Lab, 1982)). This can be observed at all levels of cardiac structural integration, from (sub-)cellular and tissue levels, to whole organ and body, including stretch effects on pacemaker electrophysiology.
In canine isolated hearts and atria isolated from various mammalian species subjected to progressive increases in atrial pressure at constant flow, BR responses similar to those in the intact animal have been observed (Blinks, 1956). In canine (Chiba, 1977) and rat (Wilson and Bolter, 2002) isolated right atrial preparations, and feline SAN tissue (Lange et al., 1966), it has been shown that adrenergic and cholinergic blockade with propranolol and atropine, respectively, do not abolish stretch-induced changes in BR, excluding intramural neural plexi as necessary contributors. Similarly, application of tetrodotoxin (a blocker of fast Na+ channels required for neuronal activation (Chiba, 1977; Wilson and Bolter, 2002)) or neonatal capsaicin injections (to ablate intracardiac neurons (Wilson and Bolter, 2002)) do not remove the positive chronotropic response to stretch. There is some evidence that application of dichlorosisoproterenol, a non-selective β-adrenergic antagonist (Lange et al., 1966) can reduce stretch-induced changes in BR, but the mechanisms of action for this have not been identified. Finally, the near-instantaneous response of pacemaker rate to stretch, as well as the high solution flow-rates used in isolated heart preparations, suggest that humoural factors are also unlikely to be key contributors to the mechanical modulation of pacemaking.
Combined, these results suggest that neither humoural, nor extra- or intracardiac neuronal signalling, are pre-required for the intrinsic BR response to stretch. There is evidence, however, supporting an interaction between mechanical and autonomic BR modulation. In intact rabbit (Bolter, 1994; Bolter and Wilson, 1999), as well as in isolated atria of rabbit (Bolter, 1996), guinea-pig (Wilson and Bolter, 2001) and rat (Barrett et al., 1998), an increase in right atrial pressure induces both BR acceleration and a significant reduction in the percentage-response to vagal stimulation, effectively reducing the influence of ‘vagal tone’ on the heart. Vice versa, when BR is reduced by vagal stimulation, the BR response to stretch is augmented. This effect may be mediated by the repolarising muscarinic K+ current, as an increase in pressure in rat isolated right atria has been shown to close these channels, and application of Tertiapin-Q appears to eliminate their mechano-sensitivity (Han et al., 2010). It is speculated that mechanical regulation of muscarinic K+ current may at least partly be explained by a rapid change in RGS4 (regulator of G protein signalling 4) activity via a stretch-induced increase in intracellular Ca2+ (Mighiu and Heximer, 2012). Thus, parasympathetic control of BR may be continuously modulated by atrial loading, contributing to efficient stretch-induced adjustment of BR to fluctuations in venous return. Similarly, when BR is reduced by carbamylcholine (a cholinergic agonist), the BR response to stretch is amplified, and when increased with isoprenaline (a non-selective β-adrenergic agonist), the response is diminished (and in the extreme case reversed, such that stretch decreases already elevated BR, similar to the response seen in smaller mammals with a normally high resting BR (Cooper and Kohl, 2005)). The extent to which this is a consequence of basal BR per se, however, is a matter of continued investigation.
In contrast, it has been observed that stretch, caused by increased pressure in isolated rabbit right atria, potentiates the dose-dependent decrease in BR upon application of the muscarinic agonist β-homobetaine methylester, a structural isomer of acetylcholine (Rossberg et al., 1985). Thus, intrinsic and systemic BR modulations occur in conjunction, and may amplify, or dampen, each other’s effect (part of this may be indicative of possible differences between neural and humoural effects). In addition, as previously mentioned, haemodynamic changes that affect both venous return and arterial pressure will give rise to potentially competing regulatory responses (e.g., stretch-induced acceleration vs. the ‘depressor reflex’).
2.3. Cell level findings
In 1964, Klaus Deck reported intracellular ‘sharp electrode’ recordings of SAN pacemaker cell Vm during linear and equi-biaxial stretch of feline and rabbit isolated atrial tissue containing the SAN (Deck, 1964b). Along with an instantaneous increase in BR, he found a reduction in the absolute values of both maximum diastolic and maximum systolic potentials (MDP and MSP, respectively). The extent of positive chronotropic change was inversely related to background BR (altered by acetylcholine, pH, temperature, or hypoxia). At about the same time, similar effects were demonstrated in Rhesus-monkey isolated Purkinje fibres, where stretch moved MDP in a positive direction and increased DD rate (Kaufmann and Theophile, 1967). These electrophysiological observations help in narrowing the range of plausible molecular mechanisms involved in the Vm response to stretch, as the most intuitive explanation would invoke a trans-sarcolemmal whole-cell net current with a reversal potential (Erev) somewhere between the MDP and MSP of primary or secondary pacemaker cells.
Early single cell studies into the mechanisms underlying mechanical modulation of pacemaker BR used positive pressure cell inflation as a mechanical stimulus. In rabbit isolated SAN cells, this activates a cell swelling related chloride current with an Erev near 0 mV, which could in theory account for observed changes in pacemaker electrophysiology (Hagiwara et al., 1992). However, this current does not activate instantaneously upon cell swelling (lag times usually exceed 1 min in cardiac cells), rendering it too slow for acute beat-by-beat changes. Furthermore, cell inflation is micro-mechanically different from stretch, being associated with an increase in cell diameter and negligible changes in length (which may even reduce). In contrast, stretch causes cell lengthening and (as cell volume remains constant) a reduction in diameter. Indeed, subsequent studies using hypo-osmotic swelling of spontaneously beating rabbit isolated SAN cells showed a reduction, rather than the anticipated increase, in BR (Lei and Kohl, 1998). Computational modelling of SAN cell swelling effects suggested that the reduction in BR was driven by dilution of the cytosol which, via a decrease in intracellular K+ concentration, reduced rapid delayed rectifier IK, shifting MDP towards more depolarised levels and reducing If. The proposed swelling-induced reduction in rapid delayed rectifier IK (also seen in guinea-pig isolated ventricular myocytes (Rees et al., 1995)) has been confirmed in rabbit SAN cells (Lei and Kohl, 1998).
Cell volume manipulation has been shown to affect other pacemaking-relevant currents in isolated myocytes, such as INCX, ICa,L, ICa,T, slow delayed rectifier IK, the Na+–K+ pump, and muscarinic K+ currents (for a review, see (Baumgarten et al., 2011)). Differences in micro-mechanical characteristics, patho-physiological context, response times, and electrophysiological outcomes suggest, however, that cell volume manipulation is not a suitable intervention to experimentally explore the effects of stretch, such as encountered by pacemaker tissue in situ on a beat-by-beat basis during the cardiac cycle.
Just over a decade ago, spontaneously beating SAN cells were axially stretched using a modified version (Iribe et al., 2007) of the carbon fibre technique (Le Guennec et al., 1990), while simultaneously collecting patch-clamp recordings of their Vm dynamics (Cooper et al., 2000). In this study, a significant increase in BR during 5–10% stretch was observed, which was accompanied by a reduction in the absolute values of MDP and MSP, as previously reported in native SAN tissue (Deck, 1964b). Vm-clamp studies revealed that this response was caused by a stretch-activated whole-cell current with a Erev of −11 mV (Cooper et al., 2000).
This current is similar to that carried by cation non-selective stretch-activated channels (SACNS), reported in many other eukaryotic cells. SACNS are rapidly activating, with a Erev between 0 and −20 mV in cardiac myocytes (Craelius et al., 1988; Guharay and Sachs, 1984). SACNS opening will therefore depolarise diastolic, and repolarise systolic, Vm, potentially explaining the observed changes in SAN BR during stretch. Over a slightly slower time scale, SACNS may also affect intracellular Ca2+ dynamics, such as through direct Ca2+ influx, or effects on INCX that are secondary to Na+ influx (Gannier et al., 1996).
Interestingly, BR responses to stretch can differ between species. Most medium- and large-sized mammals (with inherently slow background BR) respond to sustained stretch of the SAN with BR acceleration, while smaller mammals (with resting BR exceeding 200 bpm) show BR deceleration (Cooper and Kohl, 2005). It would appear, nonetheless, that both responses are caused by SACNS activation. Larger animals with slower baseline BR have SAN AP shapes that are characterised by a relatively slow AP upstroke and a relatively prominent plateau-like early repolarisation phase, while smaller mammals with high BR exhibit faster upstrokes (often carried by a mix of Na+ and Ca2+ currents (Lei et al., 2007)) and swift initial repolarisation, giving rise to a more spike-like AP shape (see Fig. 2). As a consequence, ‘slow’ SAN APs spend the majority of each cycle moving their Vm towards the Erev of SACNS, while, in contrast, ‘fast’ SAN APs spend a larger proportion of time moving their Vm away from the Erev of SACNS. This can explain why activation of SACNS (which will act to pull Vm in the direction of Erev) may increase slow, yet reduce fast, BR (Cooper and Kohl, 2005).
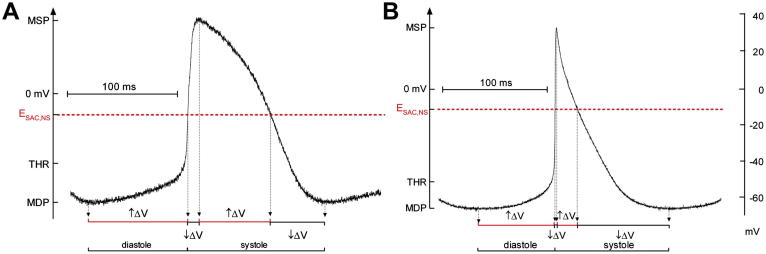
Fig. 2.
Schematic illustration of theoretical SACNS effects on (A) rabbit and (B) murine isolated SAN cell AP. Experimental Vm recordings from both species show the relation of electrophysiological parameters (MDP, MSP, threshold for AP excitation, THR) and SACNS reversal potential (ESAC,NS). The time periods during which SACNS activity would either accelerate (↑ΔV) or slow (↓ΔV) intrinsic changes in Vm are indicated. In rabbit, SACNS would accelerate intrinsic changes in Vm during 70.8% of AP duration, while in mouse this would occur over only 46.4% of AP duration.
From Cooper and Ravens (2011), with permission.
The plausibility of SACNS as a key contributor to the chronotropic response of SAN pacemaking to stretch is corroborated by experimental studies which have shown reversible block of stretch-induced BR changes in guinea pig and murine SAN tissue using GsMTx-4 (Grammostola spatulata mechanotoxin-4), the most specific blocker of SACNS available to date (Cooper and Kohl, 2005). This now requires confirmation in mechanically loaded isolated primary and lower order pacemaker cells.
Interestingly, SACNS might also affect BR if they are located not in pacemaker cells, but in electrically coupled non-myocytes, such as fibroblasts. These usually are relatively depolarised cells, possess SACNS (Stockbridge and French, 1988), and their heterotypic electrical coupling to cardiac myocytes has been confirmed in rabbit SAN tissue (Camelliti et al., 2004). Stretch has been shown to cause fibroblast depolarisation (Kohl et al., 1994; Kohl and Noble, 1996), a response that is sensitive to pharmacological depletion or buffering of fibroblast intracellular Ca2+ (Kiseleva et al., 1996). In computational models, this is quantitatively sufficient to increase BR in electrically coupled SAN cells on a beat-by-beat basis (Kohl et al., 1994). Experimental validation of the importance of fibroblast- (and other non-myocyte-) mediated stretch-effects on BR is another important target for further research.
Therefore, stretch of pacemaker tissue and cells has a pronounced effect on their electrophysiology, acting at least in part via activation of specific mechano-sensitive ion channels in the sarcolemma of pacemaker (and possibly other) cells. Mechanically induced changes demonstrably affect pacemaking rate, and they do so with a time-course sufficient to explain beat-by-beat responses. This may tune the Vm/Ca2+-oscillator, in keeping with circulatory system requirements. However, quantitative plausibility is no substitute for validation. For instance, SACNS-based computational models do not currently reproduce certain cycle-dependent effects on BR (such as deceleration upon systolic stretch in murine SAN models). This suggests that other mechanisms must be involved. In fact, mechano-sensitivity is known to be wide-spread in cardiac electrophysiology and Ca2+-handling. The evidence for direct effects of the mechanical environment on components of the Vm/Ca2+-oscillator will be considered next.
3. Mechanical effects on components of the Vm/Ca2+-oscillator
3.1. Effects on the Vm-oscillator
Effects of mechanical stimulation on components involved in driving the Vm-oscillator have been reported (see Fig. 1). For instance, it has been shown in oocytes that current through HCN2 channels is reversibly increased by hypo-osmotic swelling and by intracellular fluid injection (Calloe et al., 2005). In addition, in cell-attached oocyte patches, it has been demonstrated that membrane deformation accelerates both hyperpolarisation-induced activation and depolarisation-induced deactivation of HCN2 channels, essentially ‘accelerating the dynamics’ of this channel (Lin et al., 2007). To determine how this might affect automaticity, oocyte membrane patches were AP-clamped to cyclic SAN and Purkinje cell AP waveforms. Whether membrane deformation caused an increase or a decrease in HCN2 current depended on BR: at higher rates HCN2 current was increased by stretch, and at lower rates it was decreased. If HCN mechano-sensitivity is also present in axially stretched pacemaker cells, which is yet to be tested, it could have complex and potentially species-dependent effects on BR responses to stretch.
Mechano-sensitivity has been demonstrated as well in recombinant human L-type (but interestingly not T-type) Ca2+ channels expressed in HEK cells (Calabrese et al., 2002; Lyford et al., 2002), as well as for various Vm-gated Na+ (NaV1.5 and NaV1.6) and K+ (Kv1, Kv3, Kv7, KvCa) channels (for review, see (Morris, 2011)).
Thus, several components of the Vm-oscillator are directly mechano-sensitive, at least in various expressions systems.
3.2. Effects on the Ca2+-oscillator
Stretch has been shown to directly affect intracellular Ca2+ handling in cardiac cells (Allen and Kentish, 1985; Calaghan and White, 1999; ter Keurs, 2012). The pacemaker BR response to stretch, in particular, is reduced by lowering extracellular Ca2+ concentration, and by interference with normal SR Ca2+ handling using ryanodine or thapsigargin (Arai et al., 1996), but it is insensitive to ICa,L block with either nifedipine (Arai et al., 1996) or verapamil (Chiba, 1977). As BR responses are decreased by interventions which either reduce SR Ca2+ content (lowered extracellular Ca2+ or block of SERCA activity), or reduce SR Ca2+ release (by interfering with ryanodine receptor channels, RyR), this suggests an involvement of intracellular Ca2+ mobilisation in the stretch-induced enhancement of pacemaker activity. Of note, stretch has been shown to acutely increase SR Ca2+ release in guinea-pig (Iribe and Kohl, 2008) and rat (Gamble et al., 1992; Iribe et al., 2009) ventricular myocytes (see Fig. 3). This appears to occur by an acute and transient increase in Ca2+ spark rate (attributed to augmented RyR open probability (Iribe et al., 2009)). In rat (which shows diastolic gain in SR Ca2+ content in resting cells), stretch slows diastolic SR Ca2+ accumulation (Gamble et al., 1992), while in guinea pig (which shows diastolic loss of SR Ca2+ content), stretch enhances the rate of loss (Iribe and Kohl, 2008) – i.e., in both cases, stretch favours Ca2+ extrusion from otherwise well-loaded SR. This may point towards a more general applicability of stretch-induced increase in RyR open probability. At the same time, the mechanism of the acute stretch-induced increase in Ca2+ spark rate is unknown: RyR may themselves be mechano-sensitive, or they may be locally activated due to release of Ca2+ from mitochondria upon mechanical stimulation (possibly involving mitochondrial INCX (Belmonte and Morad, 2008)). The fact that mitochondrial Ca2+ flux can alter BR by affecting spontaneous SR Ca2+ release has recently been corroborated in rabbit isolated SAN cells (Yaniv et al., 2012). Stretch, maintained over several minutes, additionally increases Ca2+ spark rate via a nitric oxide-mediated pathway (Petroff et al., 2001). This mechanism is not involved, however, in the acute stretch-induced increase in Ca2+ spark rate seen in rat ventricular myocytes (Iribe et al., 2009).
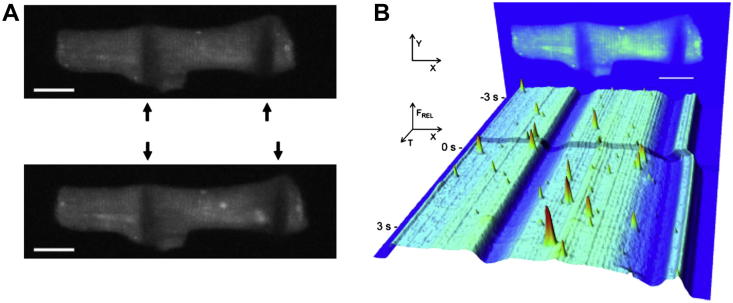
Fig. 3.
(A) Confocal images of intracellular Ca2+ concentration showing an acute increase in Ca2+ spark activity with axial stretch (change in sarcomere length of 8% from resting) applied by carbon fibres to only one half (right side) of a rat isolated myocyte. (B) Time course of relative intracellular Ca2+ concentration signal intensity, illustrating dynamic local changes in spatially resolved Ca2+ concentration during half-cell stretch (time progressing from back to front) and an acute increase in spark rate in the distended part of the cell only (individual spark dynamics remain unchanged).
From Iribe et al. (2009), with permission.
Intracellular free Ca2+ concentration in contracting cardiac myocytes is also affected by stretch-induced changes in Ca2+ buffering capacity, due to length-dependent modulation of the affinity of troponin-C to Ca2+ (Allen and Kentish, 1988). As a result, mismatched electrical and mechanical activity can give rise to mechanically induced Ca2+ waves, for example in ventricular myocardium (ter Keurs et al., 2008).
If mechanisms similar to those described above for acute and sustained changes in Ca2+ handling in response to stretch are present in pacemaker cells, they would be relevant for modulation of pacemaker activity through effects on the Ca2+-oscillator. The potential importance of this is supported by recent evidence showing that acute changes in SAN intracellular Ca2+ concentration can result in immediate changes in BR (Yaniv et al., 2011). In addition, altered Ca2+ handling may affect the store-operated Ca2+ channel current (an inward Ca2+ current which depends on the level of depletion of intracellular Ca2+ stores). While not generally included as a ‘standard’ component of the Ca2+-oscillator, this current has been implicated in modulation of pacemaker BR (Ju et al., 2007). This is thought to occur either directly via a background inward current that is modulated by beat-by-beat changes in SR Ca2+ content (Ju et al., 2007), or through interactions with INCX (Ju and Allen, 1998). Moreover, it has been suggested that this channel may be accounted for by transient receptor potential cation channel expression (Ju et al., 2007), and since type 1 of this protein may underlie SACNS (Maroto et al., 2005), it could also play a role in direct mechanical effects on cardiac pacemaker activity.
It is evident, then, that stretch of pacemaker cells alters automaticity, not only by activation of specialised mechano-sensitive ion channels in the sarcolemma and in sub-cellular membrane compartments, but also by affecting components of the Vm- and Ca2+-oscillators.
4. The mechanics-oscillator and its patho/physiological relevance
4.1. Physiological loading
4.1.1. Chamber loading dynamics
The cardiac cycle involves a complex sequence of atrial and ventricular electro-mechanical activity. During atrial diastole, the tissue containing the SAN is stretched, initially by ventricular contraction that pulls the atrio-ventricular valve plane in an apical direction, promoting ventricular ejection by the development of significant positive pressure gradients compared to outflow arteries, and atrial filling at negative pressure gradients relative to inflow veins. This atrio-ventricular valve plane shift is a crucial determinant of the dual pressure-suction pump activity of ventricular contraction. Upon ventricular relaxation (and still during atrial diastole), the ventricles are passively re-filled to about 85% of their end-diastolic volume, simply by the return of the atrio-ventricular valves to their more basal resting position, resulting in stretch of the Purkinje network. After ‘diastasis’ (the pause between the end of ventricular, and the onset of atrial, activity), atrial contraction increases ventricular volume to its final end-diastolic level, prior to the next ventricular activation (thanks to the atrio-ventricular conduction delay).
As chamber dimensions are determined by volume (largely a function of venous return in the healthy heart), the above gives rise to loading dynamics which differ for the atria and ventricles (and, less prominently in terms of timing, for the right and left side of the heart). This is associated with different stretch cycles of pacemaker regions, such as the SAN and Purkinje system. Stretch effects on Vm dynamics of pacemaker tissue will therefore vary with time and with location. Pre-load-induced effects are maximal in the respective chamber-specific diastole – i.e., at the very time when Vm in pacemaker cells is moving towards initiation of the next AP. This means that physiological stretch will predominantly affect MDP and DD rate (rather than MSP, which is often altered during experimental application of stretch, such as during interventions sustained throughout multiple cardiac cycles). Any additional change in driving forces for venous return (such as alterations in posture, or modulation of thorax-abdomen pressure gradients caused by respiratory activity, coughing, or defecation straining) will modulate the effect on DD. This has been employed, for example by using the Valsalva manoeuvre in heart transplant recipients (Ambrosi et al., 1995), to demonstrate the link between reduction in pre-load and termination of ventricular tachycardia.
Mechanical ‘priming’ of pacemaker cell electrophysiology by mechanical factors (the mechanics-oscillator) would act, therefore, in addition to and in combination with the Vm/Ca2+-oscillator, to adjust the pacemaking system on a beat-by-beat basis to diastolic load.
4.1.2. Atrial effects
An increased right-atrial preload can accelerate SAN excitation, contributing to the matching of cardiac output (which is the product of stroke volume and BR) to venous return. Interestingly, as mentioned above, mechanically induced increases in BR are proportionally greater when stretch is applied at low rates, such as during vagus nerve activation, or pharmacological cholinergic stimulation (Barrett et al., 1998; Bolter, 1996; Deck, 1964b; Wilson and Bolter, 2001). This may help to prevent excessive slowing and diastolic (over-)distension, while maintaining cardiac output and adequate circulation (Brooks and Lange, 1977).
In contrast, appropriately maintained ‘minimal’ diastolic distension may be important for stabilisation of rhythm. In feline isolated SAN tissue preparations with irregular or no rhythm, it has been demonstrated that application of physiological levels of stretch can restore regular activity (Lange et al., 1966), an effect accompanied by a shift of MDP towards less negative values (see Fig. 4). In this setting, it was apparent that missed beats were due to the failure of other pacemaker oscillators to sustain spontaneous DD in the absence of a sufficient mechanical preload. As shown in Fig. 4A, stretch restored function by decreasing the gap between MDP and AP threshold. Likewise, stretch was seen to initiate spontaneous firing in quiescent SAN tissue by progressively reducing MDP, which resulted initially in the appearance of sub-threshold oscillations of Vm, followed by spontaneous AP generation (see Fig. 4B). This pre-requirement for a minimum mechanical background stimulus is also apparent from observations on ontogenetic initiation of the first heartbeat. This has been linked to fluid pressure build-up in the quiescent cardiac tube, which is required not only for structurally competent pacemaker formation (Sankova et al., 2010), but even for the very initiation of spontaneous activity (Rajala et al., 1977).
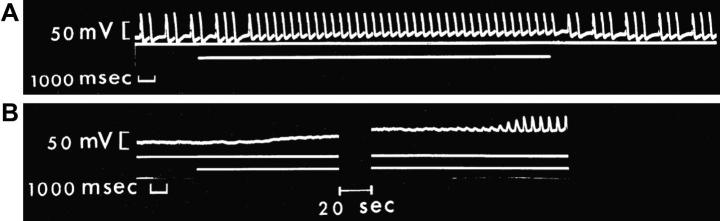
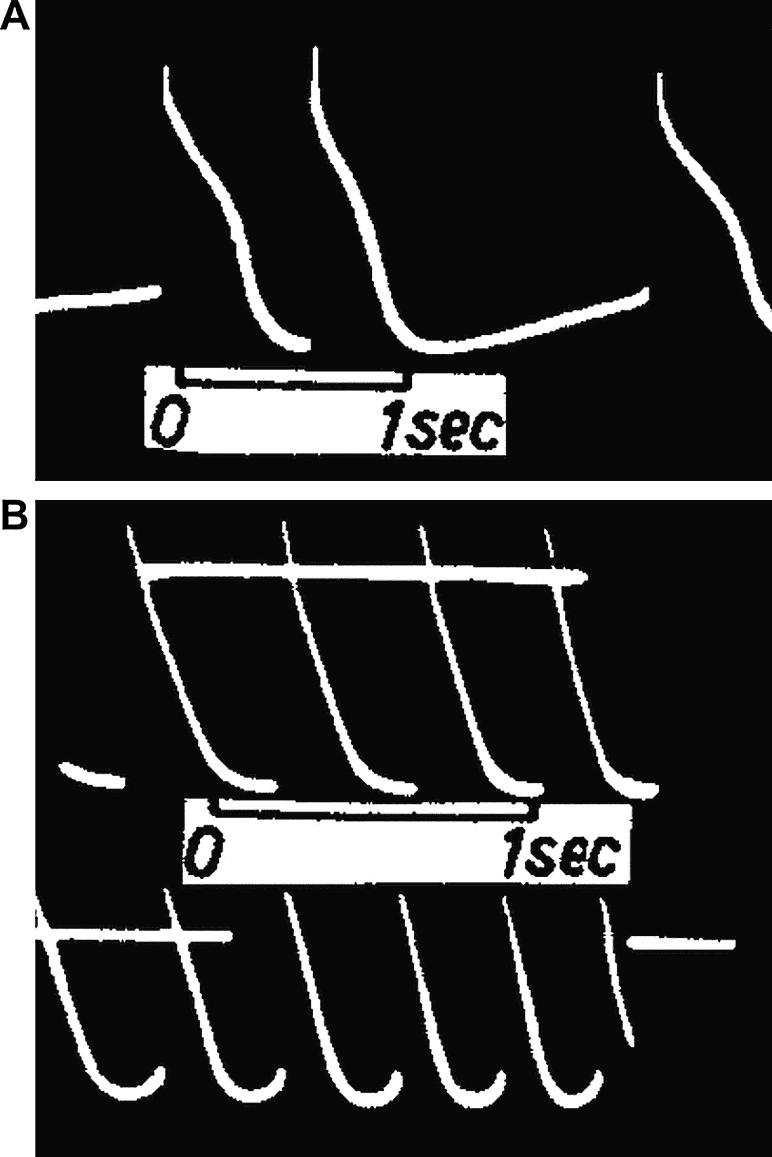
Fig. 4.
Floating microelectrode recordings of Vm in cat isolated SAN showing a stretch-induced shift of MDP towards less negative values resulting in (A) restoration of regular activity in a preparation with an irregular rhythm and (B) initiation of spontaneous discharge in an otherwise quiescent preparation. In both examples, tissue length was increased by ∼40% from resting, with periods of stretch indicated by the lower horizontal lines.
From Lange et al. (1966), with permission.
These data demonstrate that a physiological mechanical environment is necessary for the initiation and maintenance of normal pacemaker function. Absence of physiological preloads affects pacemaking and can result in abnormal activity. Such lack of mechanical control is, however, common for experimental models used to investigate cardiac pacemaker mechanisms ex vivo.
Apart from the technical challenges associated with controlling the mechanical environment of isolated cardiac cells and tissue, it is not entirely clear what determines a physiological mechanical environment. Aspects that need to be addressed include: (i) whether the key mechanical parameter for changes in pacemaker function is stretch (Kamiyama et al., 1984; Sanders et al., 1979), tension (Arai et al., 1996; Brooks et al., 1966; Chiba, 1977), or a combination of both (Lange et al., 1966); (ii) whether there is an effect of the application rate of mechanical load (Brooks et al., 1966; Lange et al., 1966) or not (Chiba, 1977); and (iii) whether particular stretch patterns (linear, biaxial, concentric) are more appropriate than others (Deck, 1964b). Additionally, as stretch-responses (at least in ventricular myocytes) are AP phase-dependent (Calkins et al., 1991; Franz et al., 1992; Hansen et al., 1990; Nishimura et al., 2008), the timing of load application must be controlled to mimic desired patho/physiological states. Conceptual models of pacemaker function suggest that, late diastolic stretch (e.g., mimicking physiological preload) should speed-up BR, while systolic/early diastolic stretch (e.g., such as during pathophysiologically increased afterload or in dyssynchronous contraction) should cause slowing. Thus far, responses have been studied largely with non-physiological (sustained, often excessive) stretch. In this setting, responses may be inconclusive, in part because of background BR-dependent effects (Cooper and Kohl, 2005).
4.1.3. Ventricular effects
In the ventricles, physiologically increased preload may support faster spread of excitation via the Purkinje system by a stretch-induced increase in Purkinje fibre conduction velocity (Deck, 1964a; Dominguez and Fozzard, 1979; Rosen et al., 1981) and enhanced automaticity (Sanders et al., 1979). That Purkinje fibres are indeed stretched during diastole (and buckle in systole) has been confirmed in sheep hearts fixed in the two different mechanical states (Canale et al., 1983). At the level of the whole heart, increased conduction velocity in the Purkinje system may be important in counter-acting the stretch-induced reduction in ventricular tissue conduction velocity reported upon increased ventricular filling (Sung et al., 2003), maintaining rapid and coordinated activation of the ventricles as preload is raised.
4.2. Pathophysiological loading
In diseases associated with atrial or ventricular volume- or pressure-overload, pathophysiological stretch-related effects may unbalance pacemaker mechanisms.
4.2.1. Atrial effects
In isolated SAN preparations, it has been observed that after an initial acceleration in BR, excessive stretch induces irregular rhythms (Lange et al., 1966) and multifocal activity (Hoffman and Cranefield, 1960), which can lead to the induction and sustenance of cardiac arrhythmias. In the whole body, SAN stretch (such as in patients with pulmonary arterial hypertension (McGowan et al., 2009), or in anesthetised pigs with targeted right-atrial distension (Horner et al., 1996)) reduces normal heart rate variability. This has been correlated with an increased risk of cardiac arrhythmias and sudden cardiac death (Chattipakorn et al., 2007; Sandercock and Brodie, 2006). Currently, relative contributions of stretch to heart rate variability per se, or of the stretch-induced increase in BR (which, in its own right, is associated with reduced variability), are unknown. This constitutes another important target of further investigation, especially in diseases associated with right atrial overload.
Pathophysiological states such as chronic atrial volume overload (Morton et al., 2003; Sanders et al., 2003; Sparks et al., 1999) and atrial fibrillation (Elvan et al., 1996; Kumagai et al., 1991), or advanced age (Mohler and Anderson, 2008; Rubenstein et al., 1972), are often associated with fibrosis and tissue remodelling, and may be accompanied by SAN dysfunction. The validation of causal links in this setting is difficult, so the following discussion is largely hypothetical, to guide consideration of further investigations in this area. Changes in tissue characteristics will alter tissue mechanical properties and, potentially, restrict changes in chamber volume (while possibly increasing tension, a potentially important modulator of the stretch response (Arai et al., 1996; Brooks et al., 1966; Chiba, 1977; Lange et al., 1966)) during diastolic filling. At the same time, as overload of the atria increases chamber radii, similar absolute changes in volume will result in smaller changes in surface area, if the chambers are already enlarged (as a consequence of the surface area : volume ratio, which for a sphere is 3 × radius−1 (Humphrey, 2002)). Alterations in tissue stiffness may be heterogeneous, introducing regional stretch differences, and thus the extent of changes in mechanical load experienced by cells in different areas of the tissue. The importance of tissue mechanics for the stretch-response is demonstrated by the observation that, for similar levels of stretch, changes in BR are greater in SAN preparations from younger animals (Deck, 1964b), where generally less fibrosis is present. The possible relevance of mechanical heterogeneity is corroborated by the observation that the central SAN is less extensible than the perinodal region, while changes in BR during externally applied force correlate best with maximum tissue strain in the periphery of the SAN (Kamiyama et al., 1984). This might be explained by the fact that the SAN periphery is where the (stretch-sensitive) If is thought to play the largest role in pacemaker activity (due to a more negative MDP (Kreitner, 1985; Nikmaram et al., 1997), which will also increase the driving force for SACNS). Both If and SACNS furthermore affect transmission of electrical activity from SAN to atrium (which opposes SAN periphery depolarisation (Garny et al., 2003)). This could make electrical invasion of the atria more secure or faster during physiological levels of stretch (while disease-related changes in regional tissue mechanics could have deleterious effects). This will depend greatly on the pre-existing structural complexity of the SAN, which will result in regional differences in stretch sensing, and variation across species (Nikolaidou et al., 2012). An additional mechanism worth considering is a possible change in the inherent mechano-sensitivity of SAN cells that may occur with disease or age, due to changes in expression and activity of mechano-sensitive channels (Boyett, 2009; Stones et al., 2008; Tellez et al., 2011). To determine the role and relative influence of the various factors potentially contributing to mechanically induced changes in SAN function will require experiments examining responses in isolated cells under mechanical load, from chronically volume- or pressure-overloaded hearts, as well as studies at different developmental stages.
In cases where disease-related changes in mechanics are accompanied by myocardial ischaemia, an additional current may be important for mechanical modulation of pacemaker function, the ATP-sensitive K+ current (Billman, 2008). This current, whose activity is increased by stretch in atrial and ventricular myocytes (Van Wagoner, 1993), has been shown to be present in rabbit isolated SAN cells (Han et al., 1996). By allowing K+ to exit the cell, activation of this current should hyperpolarise Vm, and therefore slow BR in the ischaemic heart.
4.2.2. Ventricular effects
Excessive loading of the ventricles may over-stretch Purkinje fibres, which, in isolated preparations, has been shown to result in reduced conduction velocity (Rosen et al., 1981) and eventual loss of AP activation (Dudel and Trautwein, 1954; Kaufmann and Theophile, 1967). In addition, as a consequence of increased DD rate and reduced MDP, focal ectopy (see Fig. 5A; Dudel and Trautwein, 1954; Hoffman and Cranefield, 1960) and tachycardia (see Fig. 5B; Dudel and Trautwein, 1954) have been observed. These effects have been proposed as mechanisms of ventricular bigeminy (Reynolds et al., 1975). So, like excessive stretch of the SAN, Purkinje fibre overload may play an important role in the induction and sustenance of cardiac arrhythmias.
Fig. 5.
Microelectrode recordings of Vm in cat isolated Purkinje fibres showing stretch-induced (A) focal ectopic excitation (first and last AP − middle AP represents normal conducted excitation, as evidenced by faster AP upstroke), induced by a change in fibre length of 10% from resting, and (B) increase in DD rate, which resulted in focal tachycardia when the change in fibre length reached 30% (lower tracing). In both examples, measurements were made from the point of focal activity.
From Dudel and Trautwein (1954), with permission.
The pathophysiological effects of Purkinje fibre network stretch are further complicated in chronic disease settings. Changes in chamber dimensions and tissue composition, structure, electrophysiology, and cellular coupling (such as are associated with heart failure, cardiomyopathies, ischaemia, and infarction) alter ventricular geometry, regional mechanical properties, and electro-mechanical function of myocardial tissue. This can result in altered regional ventricular stretch distributions, which may lead to increased dispersion of Purkinje fibre excitation. Distension of mechanically inactive regions by contraction of healthy myocardium will stretch attached and intramural Purkinje fibres, which may result in sub-threshold after-depolarisations (Ferrier, 1976), potentially promoting focal arrhythmias (Dudel and Trautwein, 1954; Hoffman and Cranefield, 1960). In patients with coronary artery disease, for instance, an increase in systolic aortic pressure with metaraminol infusion causes ventricular ectopy, which shows a strong correlation with the presence of segmental wall motion abnormalities (Siogas et al., 1998). Similarly, focal stretch of the Purkinje fibre network has been implicated as a cause of idiopathic left ventricular tachycardia in patients with Purkinje system branches interconnecting the interventricular septum and postero-inferior free wall (Thakur et al., 1996).
4.2.3. Suggested mechanisms
Thus, cardiac disease is often associated with changes in the magnitude and distribution of stretch and tension in the heart. This can lead to mechanically induced pathophysiological effects on primary and lower order pacemaker function. Abnormal activation leads to non-physiological excitation rates, reduction in heart rate variability, and increased dispersion of pacemaker electrophysiology, all of which may increase the risk of arrhythmias. These clinically relevant effects may occur directly via SACNS, or through Vm/Ca2+-oscillator pathways. For instance, both ICa,L (Lyford et al., 2002) and the Na+ window current (Beyder et al., 2012) have been shown to be mechano-sensitive, and they can be involved in the generation of early after-depolarisations and ectopy in the SAN (Matsuda and Kurata, 1999; Miyamae et al., 1991) and Purkinje fibres (Fedida et al., 2006; January and Riddle, 1989; Nattel and Quantz, 1988; Orth et al., 2006). Importantly, both of these currents become increasingly relevant with depolarisation of MDP, a well-established consequence of SACNS activation.
5. Conclusion
The cardiac pacemaker control system is sensitive to the beat-by-beat changes in the mechanical environment that are present in vivo (the mechanics-oscillator). This affects pacemaking directly via stretch-activation of specialised mechano-sensitive ion channels in sarcolemmal and in intracellular membrane compartments, as well as by interaction with ‘standard components’ of the Vm- and Ca2+-oscillators.
The pacemaker mechanisms that are currently conceptualised as forming the Vm/Ca2+-oscillator do not, as such, account for beat-by-beat adaptation in pacemaker activity: cellular Ca2+ balance changes measurably over the course of multiple beats (Vinogradova et al., 2005), while modification of sarcolemmal ion channel expression takes even longer. This is a dynamic system, with pronounced circadian dynamics, such as was shown initially for Na+–Ca2+ exchanger surface-to-internalised ratio that doubles during thenightly activity cycle of mice (Shen et al., 2007), and more recently for a whole host of key players underlying cardiac cellular electrophysiology (Jeyaraj et al., 2012). The ability to adjust BR to diastolic filling, however, is required to adapt primary and lower order pacemaker function to varying loading conditions on a beat-by-beat basis, and involves contributions by mechano-sensitive mechanisms to DD. While stretch is associated with the very beginning of cardiac rhythmic activity (Rajala et al., 1977) and remains a regulator of BR throughout life, in disease this system can destabilise pacemaker function, promoting irregular rhythms and the induction or sustenance of arrhythmias. All this calls for an improved understanding of the mechanical modulation of pacemaker electrophysiology.
This is an area which deserves more attention in basic research, as isolated cells – the main source of insight into the molecular mechanisms underlying Vm/Ca2+-oscillator function – are not normally subjected to controlled mechanical environments. In vivo, however, pacemaker cells are continuously subjected to cyclic variations in haemodynamic load. Therefore, improved experimental approaches are needed to explore the complex interrelations between Vm, Ca2+, and mechanical oscillators.
As highlighted before, the notion of separate ‘clocks’ is not helpful for a systematic understanding of integrated pacemaker function, as under normal conditions none of them work in isolation. Cyclic stretch, Ca2+-handling, and Vm-dynamics represent coupled systems. Their interaction prevents uncontrolled changes in BR, even when individual mechanisms are impeded. Such reciprocity of mechanisms involved in cardiac pacemaking was first demonstrated on the example of individual components of the Vm-oscillator, where If and Na+ background current behave in an inversely related manner. This prevents major alteration in BR when one or the other of the currents is subjected to increasing levels of block (Noble et al., 1992).
Thus, the entire pacemaker system is probably best considered as a Vm/Ca2+/mechanics-oscillator, which integrates at the cellular level a multitude of inputs with a high degree of redundancy, generating a single output – automaticity. This integrative approach will improve our ability to conceptualise and interpret normal and disease-related changes in primary and lower order pacemaker function.
Editors’ note
Please see also related communications in this issue by Markhasin et al. (2012) and Morris et al. (2012).
Acknowledgements
Work of the authors is supported by the Engineering and Physical Science Research Council of the United Kingdom, the British Heart Foundation, the Magdi Yacoub Institute, and the European Commission FP7 Virtual Physiological Human Initiative (Network of Excellence, preDiCT, euHeart).
References
- Allen D.G., Kentish J.C. The cellular basis of the length–tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 1985;17:821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D.G., Kentish J.C. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J. Physiol. 1988;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi P., Habib G., Kreitmann B., Faugere G., Metras D. Valsalva manoeuvre for supraventricular tachycardia in transplanted heart recipient. Lancet. 1995;346:713. doi: 10.1016/s0140-6736(95)92331-4. [DOI] [PubMed] [Google Scholar]
- Anglo L.T. first ed. Ex officina Christiani Wecheli; Paris: 1537. Claudii Galeni Pergameni De naturalibus facultatibus libri tres. [Google Scholar]
- Arai A., Kodama I., Toyama J. Roles of Cl− channels and Ca2+ mobilization in stretch-induced increase of SA node pacemaker activity. Am. J. Physiol. 1996;270:H1726–H1735. doi: 10.1152/ajpheart.1996.270.5.H1726. [DOI] [PubMed] [Google Scholar]
- Bainbridge F.A. The influence of venous filling upon the rate of the heart. J. Physiol. 1915;50:65–84. doi: 10.1113/jphysiol.1915.sp001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbuti A., Bucchi A., Milanesi R., Bottelli G., Crespi A., DiFrancesco D. The “funny” pacemaker current. In: Tripathi O.N., Ravens U., Sanguinetti M.C., editors. Heart Rate and Rhythm: Molecular Basis, Pharmacological Modulation and Clinical Implications. Springer; Heidelberg: 2011. pp. 59–81. [Google Scholar]
- Barrett C.J., Bolter C.P., Wilson S.J. The intrinsic rate response of the isolated right atrium of the rat, Rattus norvegicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998;120:391–397. doi: 10.1016/s1095-6433(98)10077-6. [DOI] [PubMed] [Google Scholar]
- Baumgarten C.M., Deng W., Raucci F.J., Jr. Cell volume-sensitive ion channels and transporters in cardiac myocytes. In: Kohl P., Sachs F., Franz M.R., editors. Cardiac Mechano-Electric Coupling and Arrhythmias. Oxford University Press; Oxford: 2011. pp. 27–34. [Google Scholar]
- Belmonte S., Morad M. ‘Pressure-flow’-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J. Physiol. 2008;586:1379–1397. doi: 10.1113/jphysiol.2007.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L., Keller F., Sanders M., Reddy P.S., Griffith B., Meno F., Pinsky M.R. Respiratory sinus arrhythmia in the denervated human heart. J. Appl. Physiol. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- Bers D.M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Beyder A., Strege P.R., Reyes S., Bernard C.E., Terzic A., Makielski J., Ackerman M.J., Farrugia G. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel NaV1.5: a novel mechanism of drug action. Circulation. 2012;125:2698–2706. doi: 10.1161/CIRCULATIONAHA.112.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman G.E. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacol. Ther. 2008;120:54–70. doi: 10.1016/j.pharmthera.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Blinks J.R. Positive chronotropic effect of increasing right atrial pressure in the isolated mammalian heart. Am. J. Physiol. 1956;186:299–303. doi: 10.1152/ajplegacy.1956.186.2.299. [DOI] [PubMed] [Google Scholar]
- Bolter C.P. Intrinsic cardiac rate regulation in the anaesthetized rabbit. Acta Physiol. Scand. 1994;151:421–428. doi: 10.1111/j.1748-1716.1994.tb09764.x. [DOI] [PubMed] [Google Scholar]
- Bolter C.P. Effect of changes in transmural pressure on contraction frequency of the isolated right atrium of the rabbit. Acta Physiol. Scand. 1996;156:45–50. doi: 10.1046/j.1365-201X.1996.430151000.x. [DOI] [PubMed] [Google Scholar]
- Bolter C.P., Wilson S.J. Influence of right atrial pressure on the cardiac pacemaker response to vagal stimulation. Am. J. Physiol. 1999;276:R1112–R1117. doi: 10.1152/ajpregu.1999.276.4.R1112. [DOI] [PubMed] [Google Scholar]
- Boyden P.A., Hirose M., Dun W. Cardiac Purkinje cells. Heart Rhythm. 2010;7:127–135. doi: 10.1016/j.hrthm.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Boyett M.R. ‘And the beat goes on.’ The cardiac conduction system: the wiring system of the heart. Exp. Physiol. 2009;94:1035–1049. doi: 10.1113/expphysiol.2009.046920. [DOI] [PubMed] [Google Scholar]
- Brooks C.M., Lange G. Interaction of myogenic and neurogenic mechanisms that control heart rate. Proc. Natl. Acad. Sci. U. S. A. 1977;74:1761–1762. doi: 10.1073/pnas.74.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C.M., Lu H.H., Lange G., Mangi R., Shaw R.B., Geoly K. Effects of localized stretch of the sinoatrial node region of the dog heart. Am. J. Physiol. 1966;211:1197–1202. doi: 10.1152/ajplegacy.1966.211.5.1197. [DOI] [PubMed] [Google Scholar]
- Brown H.F., Kimura J., Noble D., Noble S.J., Taupignon A. The ionic currents underlying pacemaker activity in rabbit sino-atrial node: experimental results and computer simulations. Proc. Roy. Soc. Lond. B Biol. Sci. 1984;222:329–347. doi: 10.1098/rspb.1984.0067. [DOI] [PubMed] [Google Scholar]
- Bucchi A., Baruscotti M., DiFrancesco D. Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J. Gen. Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese B., Tabarean I.V., Juranka P., Morris C.E. Mechanosensitivity of N-type calcium channel currents. Biophys. J. 2002;83:2560–2574. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaghan S.C., White E. The role of calcium in the response of cardiac muscle to stretch. Prog. Biophys. Mol. Biol. 1999;71:59–90. doi: 10.1016/s0079-6107(98)00037-6. [DOI] [PubMed] [Google Scholar]
- Calkins H., Levine J.H., Kass D.A. Electrophysiological effect of varied rate and extent of acute in vivo left ventricular load increase. Cardiovasc. Res. 1991;25:637–644. doi: 10.1093/cvr/25.8.637. [DOI] [PubMed] [Google Scholar]
- Calloe K., Elmedyb P., Olesen S.P., Jorgensen N.K., Grunnet M. Hypoosmotic cell swelling as a novel mechanism for modulation of cloned HCN2 channels. Biophys. J. 2005;89:2159–2169. doi: 10.1529/biophysj.105.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelliti P., Green C.R., LeGrice I., Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ. Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- Canale E., Campbell G.R., Uehara Y., Fujiwara T., Smolich J.J. Sheep cardiac Purkinje fibers: configurational changes during the cardiac cycle. Cell Tissue Res. 1983;232:97–110. doi: 10.1007/BF00222376. [DOI] [PubMed] [Google Scholar]
- Chattipakorn N., Incharoen T., Kanlop N., Chattipakorn S. Heart rate variability in myocardial infarction and heart failure. Int. J. Cardiol. 2007;120:289–296. doi: 10.1016/j.ijcard.2006.11.221. [DOI] [PubMed] [Google Scholar]
- Chiba S. Pharmacologic analysis of stretch-induced sinus acceleration of the isolated dog atrium. Jpn. Heart J. 1977;18:398–405. doi: 10.1536/ihj.18.398. [DOI] [PubMed] [Google Scholar]
- Cooper P.J., Kohl P. Species- and preparation-dependence of stretch effects on sino-atrial node pacemaking. Ann. N. Y. Acad. Sci. 2005;1047:324–335. doi: 10.1196/annals.1341.029. [DOI] [PubMed] [Google Scholar]
- Cooper P.J., Ravens U. Mechanical modulation of pacemaker electrophysiology. In: Kohl P., Sachs F., Franz M.R., editors. Cardiac Mechano-Electric Coupling and Arrhythmias. Oxford University Press; Oxford: 2011. pp. 95–102. [Google Scholar]
- Cooper P.J., Lei M., Cheng L.X., Kohl P. Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. J. Appl. Physiol. 2000;89:2099–2104. doi: 10.1152/jappl.2000.89.5.2099. [DOI] [PubMed] [Google Scholar]
- Craelius W., Chen V., el-Sherif N. Stretch activated ion channels in ventricular myocytes. Biosci. Rep. 1988;8:407–414. doi: 10.1007/BF01121637. [DOI] [PubMed] [Google Scholar]
- Deck K.A. Änderungen des Ruhepotentials und der Kabeleigenschaften von Purkinje-Fäden bei der Dehnung. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1964;280:131–140. [PubMed] [Google Scholar]
- Deck K.A. Dehnungseffekte am spontanschlagenden, isolierten Sinusknoten. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1964;280:120–130. [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog. Biophys. Mol. Biol. 1985;46:163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. The funny current has a major pacemaking role in the sinus node. Heart Rhythm. 2012;9:299–301. doi: 10.1016/j.hrthm.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Dobrzynski H., Boyett M.R., Anderson R.H. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- Dominguez G., Fozzard H.A. Effect of stretch on conduction velocity and cable properties of cardiac Purkinje fibers. Am. J. Physiol. 1979;237:C119–C124. doi: 10.1152/ajpcell.1979.237.3.C119. [DOI] [PubMed] [Google Scholar]
- Donald D.E., Shepherd J.T. Reflexes from the heart and lungs: physiological curiosities or important regulatory mechanisms. Cardiovasc. Res. 1978;12:446–469. [PubMed] [Google Scholar]
- Dudel J., Trautwein W. Das Aktionspotential und Mechanogramm des Herzmuskels unter dem Einfluss der Dehnung. Cardiologia. 1954;25:344–362. [PubMed] [Google Scholar]
- Elvan A., Wylie K., Zipes D.P. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling. Circulation. 1996;94:2953–2960. doi: 10.1161/01.cir.94.11.2953. [DOI] [PubMed] [Google Scholar]
- Fedida D., Orth P.M., Hesketh J.C., Ezrin A.M. The role of late INa and antiarrhythmic drugs in EAD formation and termination in Purkinje fibers. J. Cardiovasc. Electrophysiol. 2006;17(Suppl. 1):S71–S78. doi: 10.1111/j.1540-8167.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Ferrier G.R. The effects of tension on acetylstrophanthidin-induced transient depolarizations and after contractions in canine myocardial and Purkinje tissues. Circ. Res. 1976;38:156–162. doi: 10.1161/01.res.38.3.156. [DOI] [PubMed] [Google Scholar]
- Franz M.R., Cima R., Wang D., Profitt D., Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992;86:968–978. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- Gamble J., Taylor P.B., Kenno K.A. Myocardial stretch alters twitch characteristics and Ca2+ loading of sarcoplasmic reticulum in rat ventricular muscle. Cardiovasc. Res. 1992;26:865–870. doi: 10.1093/cvr/26.9.865. [DOI] [PubMed] [Google Scholar]
- Gannier F., White E., Garnier, Le Guennec J.Y. A possible mechanism for large stretch-induced increase in [Ca2+]i in isolated guinea-pig ventricular myocytes. Cardiovasc. Res. 1996;32:158–167. [PubMed] [Google Scholar]
- Garny A., Kohl P., Hunter P.J., Boyett M.R., Noble D. One-dimensional rabbit sinoatrial node models: benefits and limitations. J. Cardiovasc. Electrophysiol. 2003;14:S121–S132. doi: 10.1046/j.1540.8167.90301.x. [DOI] [PubMed] [Google Scholar]
- Gaskell W.H. The Croonian lecture. On the rhythm of the heart of the frog, and on the nature of the action of the vagus nerve. Philos. Trans. Roy. Soc. 1882;173:993–1033. [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Masuda H., Shoda M., Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J. Physiol. 1992;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Light P.E., Giles W.R., French R.J. Identification and properties of an ATP-sensitive K+ current in rabbit sino-atrial node pacemaker cells. J. Physiol. 1996;490(Pt 2):337–350. doi: 10.1113/jphysiol.1996.sp021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Wilson S.J., Bolter C.P. Tertiapin-Q removes a mechanosensitive component of muscarinic control of the sinoatrial pacemaker in the rat. Clin. Exp. Pharmacol. Physiol. 2010;37:900–904. doi: 10.1111/j.1440-1681.2010.05408.x. [DOI] [PubMed] [Google Scholar]
- Hansen D.E., Craig C.S., Hondeghem L.M. Stretch-induced arrhythmias in the isolated canine ventricle. Evidence for the importance of mechanoelectrical feedback. Circulation. 1990;81:1094–1105. doi: 10.1161/01.cir.81.3.1094. [DOI] [PubMed] [Google Scholar]
- Harvey W. first ed. Typis Du-Gardianis; Impensis O. Pulleyn; London: 1651. Exercitationes de Generatione Animalium. Quibus accedunt quaedam de Partu; de Membranis ac Humoribus de Uteri; & de Conceptione. [Google Scholar]
- Hoffman B.F., Cranefield P.F. first ed. McGraw-Hill; New York: 1960. Electrophysiology of the Heart. [Google Scholar]
- Horner S.M., Murphy C.F., Coen B., Dick D.J., Harrison F.G., Vespalcova Z., Lab M.J. Contribution to heart rate variability by mechanoelectric feedback. Stretch of the sinoatrial node reduces heart rate variability. Circulation. 1996;94:1762–1767. doi: 10.1161/01.cir.94.7.1762. [DOI] [PubMed] [Google Scholar]
- Humphrey J.D. first ed. Springer; New York: 2002. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. [Google Scholar]
- Hunter P.J., Kohl P., Noble D. Integrative models of the heart: achievements and limitations. Philos. Trans. A Math. Phys. Eng. Sci. 2001;359:1049–1054. [Google Scholar]
- Iribe G., Kohl P. Axial stretch enhances sarcoplasmic reticulum Ca2+ leak and cellular Ca2+ reuptake in guinea pig ventricular myocytes: experiments and models. Prog. Biophys. Mol. Biol. 2008;97:298–311. doi: 10.1016/j.pbiomolbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Iribe G., Helmes M., Kohl P. Force–length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1487–H1497. doi: 10.1152/ajpheart.00909.2006. [DOI] [PubMed] [Google Scholar]
- Iribe G., Ward C.W., Camelliti P., Bollensdorff C., Mason F., Burton R.A., Garny A., Morphew M.K., Hoenger A., Lederer W.J., Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ. Res. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H.F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- January C.T., Riddle J.M. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ. Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- Jarisch A., Richter H. Die afferenten bahnen des veratrine effektes in den herznerven. Arch. Exp. Pathol. Pharmacol. 1939;193:355–371. [Google Scholar]
- Jeyaraj D., Haldar S.M., Wan X., McCauley M.D., Ripperger J.A., Hu K., Lu Y., Eapen B.L., Sharma N., Ficker E., Cutler M.J., Gulick J., Sanbe A., Robbins J., Demolombe S., Kondratov R.V., Shea S.A., Albrecht U., Wehrens X.H., Rosenbaum D.S., Jain M.K. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.K., Allen D.G. Intracellular calcium and Na+–Ca2+ exchange current in isolated toad pacemaker cells. J. Physiol. 1998;508(Pt 1):153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.K., Chu Y., Chaulet H., Lai D., Gervasio O.L., Graham R.M., Cannell M.B., Allen D.G. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ. Res. 2007;100:1605–1614. doi: 10.1161/CIRCRESAHA.107.152181. [DOI] [PubMed] [Google Scholar]
- Kamiyama A., Niimura I., Sugi H. Length-dependent changes of pacemaker frequency in the isolated rabbit sinoatrial node. Jpn. J. Physiol. 1984;34:153–165. doi: 10.2170/jjphysiol.34.153. [DOI] [PubMed] [Google Scholar]
- Kaufmann R., Theophile U. Automatic-fördernde Dehnungseffekte an Purkinje-Fäden, Pappillarmuskeln und Vorhoftrabekeln von Rhesus-Affen. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1967;297:174–189. [PubMed] [Google Scholar]
- Keith A., Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J. Anat. Physiol. 1907;41:172–189. [PMC free article] [PubMed] [Google Scholar]
- Kiseleva I., Kamkin A., Kohl P., Lab M.J. Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc. Res. 1996;32:98–111. [PubMed] [Google Scholar]
- Kohl P., Noble D. Mechanosensitive connective tissue: potential influence on heart rhythm. Cardiovasc. Res. 1996;32:62–68. [PubMed] [Google Scholar]
- Kohl P., Crampin E.J., Quinn T.A., Noble D. Systems biology: an approach. Clin. Pharmacol. Ther. 2010;88:25–33. doi: 10.1038/clpt.2010.92. [DOI] [PubMed] [Google Scholar]
- Kohl P., Kamkin A.G., Kiseleva I.S., Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp. Physiol. 1994;79:943–956. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- Kohl P., Sachs F., Franz M.R. second ed. Oxford University Press; Oxford: 2011. Cardiac Mechano-Electric Coupling and Arrhythmias. [Google Scholar]
- Koncz I., Szel T., Jaeger K., Baczko I., Cerbai E., Romanelli M.N., Gy Papp J., Varro A. Selective pharmacological inhibition of the pacemaker channel isoforms (HCN1–4) as new possible therapeutical targets. Curr. Med. Chem. 2011;18:3662–3674. doi: 10.2174/092986711796642427. [DOI] [PubMed] [Google Scholar]
- Kreitner D. Electrophysiological study of the two main pacemaker mechanisms in the rabbit sinus node. Cardiovasc. Res. 1985;19:304–318. doi: 10.1093/cvr/19.5.304. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Akimitsu S., Kawahira K., Kawanami F., Yamanouchi Y., Hiroki T., Arakawa K. Electrophysiological properties in chronic lone atrial fibrillation. Circulation. 1991;84:1662–1668. doi: 10.1161/01.cir.84.4.1662. [DOI] [PubMed] [Google Scholar]
- Lab M.J. Contraction–excitation feedback in myocardium. Physiological basis and clinical relevance. Circ. Res. 1982;50:757–766. doi: 10.1161/01.res.50.6.757. [DOI] [PubMed] [Google Scholar]
- Lakatta E.G., DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J. Mol. Cell. Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange G., Lu H.H., Chang A., Brooks C.M. Effect of stretch on the isolated cat sinoatrial node. Am. J. Physiol. 1966;211:1192–1196. doi: 10.1152/ajplegacy.1966.211.5.1192. [DOI] [PubMed] [Google Scholar]
- Le Guennec J.Y., Peineau N., Argibay J.A., Mongo K.G., Garnier D. A new method of attachment of isolated mammalian ventricular myocytes for tension recording: length dependence of passive and active tension. J. Mol. Cell. Cardiol. 1990;22:1083–1093. doi: 10.1016/0022-2828(90)90072-a. [DOI] [PubMed] [Google Scholar]
- Lei M., Kohl P. Swelling-induced decrease in spontaneous pacemaker activity of rabbit isolated sino-atrial node cells. Acta Physiol. Scand. 1998;164:1–12. doi: 10.1046/j.1365-201X.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- Lei M., Zhang H., Grace A.A., Huang C.L. SCN5A and sinoatrial node pacemaker function. Cardiovasc. Res. 2007;74:356–365. doi: 10.1016/j.cardiores.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Li R.A. Gene- and cell-based bio-artificial pacemaker: what basic and translational lessons have we learned? Gene Ther. 2012;19:588–595. doi: 10.1038/gt.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Laitko U., Juranka P.F., Morris C.E. Dual stretch responses of mHCN2 pacemaker channels: accelerated activation, accelerated deactivation. Biophys. J. 2007;92:1559–1572. doi: 10.1529/biophysj.106.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford G.L., Strege P.R., Shepard A., Ou Y., Ermilov L., Miller S.M., Gibbons S.J., Rae J.L., Szurszewski J.H., Farrugia G. α1C (CaV1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am. J. Physiol. Cell. Physiol. 2002;283:C1001–C1008. doi: 10.1152/ajpcell.00140.2002. [DOI] [PubMed] [Google Scholar]
- Maltsev V.A., Lakatta E.G. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc. Res. 2008;77:274–284. doi: 10.1093/cvr/cvm058. [DOI] [PubMed] [Google Scholar]
- Maltsev V.A., Lakatta E.G. The funny current in the context of the coupled-clock pacemaker cell system. Heart Rhythm. 2012;9:302–307. doi: 10.1016/j.hrthm.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev V.A., Vinogradova T.M., Lakatta E.G. The emergence of a general theory of the initiation and strength of the heartbeat. J. Pharmacol. Sci. 2006;100:338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- Markhasin V.S., Balakin A., Katsnelson L.B., Konovalov P., Lookin O., Protsenko Y., Solovyova O. Slow force response and auto-regulation of contractility in heterogeneous myocardium. Prog. Biophys. Mol. Biol. 2012;110(2–3):305–318. doi: 10.1016/j.pbiomolbio.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Maroto R., Raso A., Wood T.G., Kurosky A., Martinac B., Hamill O.P. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell. Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Kurata Y. Effects of nicardipine and bupivacaine on early after depolarization in rabbit sinoatrial node cells: a possible mechanism of bupivacaine-induced arrhythmias. Gen. Pharmacol. 1999;33:115–125. doi: 10.1016/s0306-3623(99)00004-x. [DOI] [PubMed] [Google Scholar]
- McGowan C.L., Swiston J.S., Notarius C.F., Mak S., Morris B.L., Picton P.E., Granton J.T., Floras J.S. Discordance between microneurographic and heart-rate spectral indices of sympathetic activity in pulmonary arterial hypertension. Heart. 2009;95:754–758. doi: 10.1136/hrt.2008.157115. [DOI] [PubMed] [Google Scholar]
- Michels G., Brandt M.C., Zagidullin N., Khan I.F., Larbig R., van Aaken S., Wippermann J., Hoppe U.C. Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc. Res. 2008;78:466–475. doi: 10.1093/cvr/cvn032. [DOI] [PubMed] [Google Scholar]
- Mighiu A.S., Heximer S.P. Controlling parasympathetic regulation of heart rate: a gatekeeper role for RGS proteins in the sinoatrial node. Front. Physiol. 2012;3:204. doi: 10.3389/fphys.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamae S., Matsuda T., Goto K., Mori H. Effects of lidocaine and verapamil on early afterdepolarizations in isolated rabbit sinoatrial node. J. Anesth. 1991;5:213–220. doi: 10.1007/s0054010050213. [DOI] [PubMed] [Google Scholar]
- Mohler P.J., Anderson M.E. New insights into genetic causes of sinus node disease and atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008;19:516–518. doi: 10.1111/j.1540-8167.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Moosmang S., Stieber J., Zong X., Biel M., Hofmann F., Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur. J. Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- Morris C.E. Pacemaker, potassium, calcium, sodium: stretch modulation of the voltage-gated channels. In: Kohl P., Sachs F., Franz M.R., editors. Cardiac Mechano-Electric Coupling and Arrhythmias. Oxford University Press; Oxford: 2011. pp. 42–49. [Google Scholar]
- Morris C.E., Juranka P.F., Joós B. Perturbed voltage-gated channel activity in perturbed bilayers: implications for ectopic arrhythmias arising from damaged membrane. Prog. Biophys. Mol. Biol. 2012;110(2–3):245–256. doi: 10.1016/j.pbiomolbio.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Morton J.B., Sanders P., Vohra J.K., Sparks P.B., Morgan J.G., Spence S.J., Grigg L.E., Kalman J.M. Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation. 2003;107:1775–1782. doi: 10.1161/01.CIR.0000058164.68127.F2. [DOI] [PubMed] [Google Scholar]
- Nattel S., Quantz M.A. Pharmacological response of quinidine induced early afterdepolarisations in canine cardiac Purkinje fibres: insights into underlying ionic mechanisms. Cardiovasc. Res. 1988;22:808–817. doi: 10.1093/cvr/22.11.808. [DOI] [PubMed] [Google Scholar]
- Nikmaram M.R., Boyett M.R., Kodama I., Suzuki R., Honjo H. Variation in effects of Cs+, UL-FS-49, and ZD-7288 within sinoatrial node. Am. J. Physiol. 1997;272:H2782–H2792. doi: 10.1152/ajpheart.1997.272.6.H2782. [DOI] [PubMed] [Google Scholar]
- Nikolaidou T., Aslanidi O.V., Zhang H., Efimov I.R. Structure–function relationship in the sinus and atrioventricular nodes. Pediatr. Cardiol. 2012;33:890–899. doi: 10.1007/s00246-012-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Seo K., Nagasaki M., Hosoya Y., Yamashita H., Fujita H., Nagai R., Sugiura S. Responses of single-ventricular myocytes to dynamic axial stretching. Prog. Biophys. Mol. Biol. 2008;97:282–297. doi: 10.1016/j.pbiomolbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Noble D., Denyer J.C., Brown H.F., DiFrancesco D. Reciprocal role of the inward currents ib, Na and if in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proc. Biol. Sci. 1992;250:199–207. doi: 10.1098/rspb.1992.0150. [DOI] [PubMed] [Google Scholar]
- Noble D., DiFrancesco D., Denyer J.C. Ionic mechanisms in normal and abnormal cardiac pacemaker activity. In: Jacklet J.W., editor. Cellular and Neuronal Oscillators. Dekker; New York: 1989. pp. 59–85. [Google Scholar]
- Noble D., Noble P.J., Fink M. Competing oscillators in cardiac pacemaking: historical background. Circ. Res. 2010;106:1791–1797. doi: 10.1161/CIRCRESAHA.110.218875. [DOI] [PubMed] [Google Scholar]
- Orth P.M., Hesketh J.C., Mak C.K., Yang Y., Lin S., Beatch G.N., Ezrin A.M., Fedida D. RSD1235 blocks late INa and suppresses early afterdepolarizations and torsades de pointes induced by class III agents. Cardiovasc. Res. 2006;70:486–496. doi: 10.1016/j.cardiores.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Pathak C.L. Autoregulation of chronotropic response of the heart through pacemaker stretch. Cardiology. 1973;58:45–64. doi: 10.1159/000169618. [DOI] [PubMed] [Google Scholar]
- Petroff M.G., Kim S.H., Pepe S., Dessy C., Marban E., Balligand J.L., Sollott S.J. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell. Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Quinn T.A., Kohl P. Systems biology of the heart: hype or hope? Ann. N. Y. Acad. Sci. 2011;1245:40–43. doi: 10.1111/j.1749-6632.2011.06327.x. [DOI] [PubMed] [Google Scholar]
- Quinn T.A., Bayliss R.A., Kohl P. Mechano-electric feedback in the heart: effects on heart rate and rhythm. In: Tripathi O.N., Ravens U., Sanguinetti M.C., editors. Heart Rate and Rhythm: Molecular Basis, Pharmacological Modulation and Clinical Implications. Springer; Heidelberg: 2011. pp. 133–151. [Google Scholar]
- Rajala G.M., Pinter M.J., Kaplan S. Response of the quiescent heart tube to mechanical stretch in the intact chick embryo. Dev. Biol. 1977;61:330–337. doi: 10.1016/0012-1606(77)90302-5. [DOI] [PubMed] [Google Scholar]
- Rees S.A., Vandenberg J.I., Wright A.R., Yoshida A., Powell T. Cell swelling has differential effects on the rapid and slow components of delayed rectifier potassium current in guinea pig cardiac myocytes. J. Gen. Physiol. 1995;106:1151–1170. doi: 10.1085/jgp.106.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.K., Chiz J.F., Tanikella T.K. On the mechanisms of coupling in adrenaline-induced bigeminy in sensitized hearts. Can. J. Physiol. Pharmacol. 1975;53:1158–1171. doi: 10.1139/y75-161. [DOI] [PubMed] [Google Scholar]
- Rigg L., Terrar D.A. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp. Physiol. 1996;81:877–880. doi: 10.1113/expphysiol.1996.sp003983. [DOI] [PubMed] [Google Scholar]
- Robinson R.B., Brink P.R., Cohen I.S., Rosen M.R. If and the biological pacemaker. Pharmacol. Res. 2006;53:407–415. doi: 10.1016/j.phrs.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rosen M.R., Legato M.J., Weiss R.M. Developmental changes in impulse conduction in the canine heart. Am. J. Physiol. 1981;240:H546–H554. doi: 10.1152/ajpheart.1981.240.4.H546. [DOI] [PubMed] [Google Scholar]
- Rosen M.R., Nargeot J., Salama G. The case for the funny current and the calcium clock. Heart Rhythm. 2012;9:616–618. doi: 10.1016/j.hrthm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Rossberg F., Seim H., Strack E. Chronotropic effects of the reversed carboxyl (RC) analogue of acetylcholine (β-homobetaine methylester) at defined intraluminal pressures on isolated right rabbit atria. Res. Exp. Med. (Berl.) 1985;185:139–144. doi: 10.1007/BF01854899. [DOI] [PubMed] [Google Scholar]
- Rubenstein D.S., Lipsius S.L. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ. Res. 1989;64:648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- Rubenstein J.J., Schulman C.L., Yurchak P.M., DeSanctis R.W. Clinical spectrum of the sick sinus syndrome. Circulation. 1972;46:5–13. doi: 10.1161/01.cir.46.1.5. [DOI] [PubMed] [Google Scholar]
- Sandercock G.R., Brodie D.A. The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin. Electrophysiol. 2006;29:892–904. doi: 10.1111/j.1540-8159.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- Sanders R., Myerburg R.J., Gelband H., Bassett A.L. Dissimilar length–tension relations of canine ventricular muscle and false tendon: electrophysiologic alterations accompanying deformation. J. Mol. Cell. Cardiol. 1979;11:209–219. doi: 10.1016/0022-2828(79)90465-6. [DOI] [PubMed] [Google Scholar]
- Sanders P., Morton J.B., Davidson N.C., Spence S.J., Vohra J.K., Sparks P.B., Kalman J.M. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- Sanders L., Rakovic S., Lowe M., Mattick P.A., Terrar D.A. Fundamental importance of Na+–Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J. Physiol. 2006;571:639–649. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankova B., Machalek J., Sedmera D. Effects of mechanical loading on early conduction system differentiation in the chick. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1571–H1576. doi: 10.1152/ajpheart.00721.2009. [DOI] [PubMed] [Google Scholar]
- Scicchitano P., Carbonara S., Ricci G., Mandurino C., Locorotondo M., Bulzis G., Gesualdo M., Zito A., Carbonara R., Dentamaro I., Riccioni G., Ciccone M.M. HCN channels and heart rate. Molecules. 2012;17:4225–4235. doi: 10.3390/molecules17044225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severi S., Fantini M., Charawi L.A., Difrancesco D. An updated computational model of rabbit sinoatrial action potential to investigate the mechanisms of heart rate modulation. J. Physiol. 2012 doi: 10.1113/jphysiol.2012.229435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Lin M.J., Yaradanakul A., Lariccia V., Hill J.A., Hilgemann D.W. Dual control of cardiac Na+–Ca2+ exchange by PIP2: analysis of the surface membrane fraction by extracellular cysteine PEGylation. J. Physiol. 2007;582:1011–1026. doi: 10.1113/jphysiol.2007.132720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siogas K., Pappas S., Graekas G., Goudevenos J., Liapi G., Sideris D.A. Segmental wall motion abnormalities alter vulnerability to ventricular ectopic beats associated with acute increases in aortic pressure in patients with underlying coronary artery disease. Heart. 1998;79:268–273. doi: 10.1136/hrt.79.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks P.B., Mond H.G., Vohra J.K., Jayaprakash S., Kalman J.M. Electrical remodeling of the atria following loss of atrioventricular synchrony: a long-term study in humans. Circulation. 1999;100:1894–1900. doi: 10.1161/01.cir.100.18.1894. [DOI] [PubMed] [Google Scholar]
- Stewart A., Huang J., Fisher R.A. RGS proteins in heart: brakes on the vagus. Front. Physiol. 2012;3:95. doi: 10.3389/fphys.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge L.L., French A.S. Stretch-activated cation channels in human fibroblasts. Biophys. J. 1988;54:187–190. doi: 10.1016/S0006-3495(88)82944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stones R., Gilbert S.H., Benoist D., White E. Inhomogeneity in the response to mechanical stimulation: cardiac muscle function and gene expression. Prog. Biophys. Mol. Biol. 2008;97:268–281. doi: 10.1016/j.pbiomolbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Sung D., Mills R.W., Schettler J., Narayan S.M., Omens J.H., McCulloch A.D. Ventricular filling slows epicardial conduction and increases action potential duration in an optical mapping study of the isolated rabbit heart. J. Cardiovasc. Electrophysiol. 2003;14:739–749. doi: 10.1046/j.1540-8167.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- Tawara S. first ed. Verlag von Gustav Fischer; Jena: 1906. Das Reizleitungssystem des Säugetierherzens; eine anatomisch-histologische Studie über das Atrioventrikularbündel und die Purkinjeschen Fäden. [Google Scholar]
- Tellez J.O., McZewski M., Yanni J., Sutyagin P., Mackiewicz U., Atkinson A., Inada S., Beresewicz A., Billeter R., Dobrzynski H., Boyett M.R. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp. Physiol. 2011;96:1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- ter Keurs H.E. The interaction of Ca2+ with sarcomeric proteins: role in function and dysfunction of the heart. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H38–H50. doi: 10.1152/ajpheart.00219.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs H.E., Shinozaki T., Zhang Y.M., Zhang M.L., Wakayama Y., Sugai Y., Kagaya Y., Miura M., Boyden P.A., Stuyvers B.D., Landesberg A. Sarcomere mechanics in uniform and non-uniform cardiac muscle: a link between pump function and arrhythmias. Prog. Biophys. Mol. Biol. 2008;97:312–331. doi: 10.1016/j.pbiomolbio.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Thakur R.K., Klein G.J., Sivaram C.A., Zardini M., Schleinkofer D.E., Nakagawa H., Yee R., Jackman W.M. Anatomic substrate for idiopathic left ventricular tachycardia. Circulation. 1996;93:497–501. doi: 10.1161/01.cir.93.3.497. [DOI] [PubMed] [Google Scholar]
- Van Wagoner D.R. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ. Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- Vinogradova T.M., Maltsev V.A., Bogdanov K.Y., Lyashkov A.E., Lakatta E.G. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann. N. Y. Acad. Sci. 2005;1047:138–156. doi: 10.1196/annals.1341.013. [DOI] [PubMed] [Google Scholar]
- Vinogradova T.M., Zhou Y.Y., Maltsev V., Lyashkov A., Stern M., Lakatta E.G. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ. Res. 2004;94:802–809. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- von Bezold A., Hirt L. Über die physiologischen Wirkungen des essigsauren Veratrins. Untersuchungen aus dem Physiologischen Laboratorium Wurzburg. 1867;1:75–156. [Google Scholar]
- von Haller A. first ed. Sumptibus M. M. Bousquet et Sociorum; Lausannae: 1757. Elementa Physiologiae Corporis Humani. [Google Scholar]
- Wilson S.J., Bolter C.P. Interaction of the autonomic nervous system with intrinsic cardiac rate regulation in the guinea-pig, Cavia porcellus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;130:723–730. doi: 10.1016/s1095-6433(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Wilson S.J., Bolter C.P. Do cardiac neurons play a role in the intrinsic control of heart rate in the rat? Exp. Physiol. 2002;87:675–682. doi: 10.1113/eph8702364. [DOI] [PubMed] [Google Scholar]
- Yaniv Y., Maltsev V.A., Escobar A.L., Spurgeon H.A., Ziman B.D., Stern M.D., Lakatta E.G. Beat-to-beat Ca2+-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. J. Mol. Cell. Cardiol. 2011;51:902–905. doi: 10.1016/j.yjmcc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv Y., Spurgeon H.A., Lyashkov A.E., Yang D., Ziman B.D., Maltsev V.A., Lakatta E.G. Crosstalk between mitochondrial and sarcoplasmic reticulum Ca2+ cycling modulates cardiac pacemaker cell automaticity. PLoS ONE. 2012;7:e37582. doi: 10.1371/journal.pone.0037582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Chen X.W., Zhou P., Yao L., Liu T., Zhang B., Li Y., Zheng H., Zheng L.H., Zhang C.X., Bruce I., Ge J.B., Wang S.Q., Hu Z.A., Yu H.G., Zhou Z. Calcium influx through If channels in rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 2007;292:C1147–C1155. doi: 10.1152/ajpcell.00598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]